Organic Chemistry Organic Synthesis via Enolates Dr P
- Slides: 45

Organic Chemistry Organic Synthesis via Enolates Dr. P. S. Bhale 1 © 2011 Pearson Education, Inc.

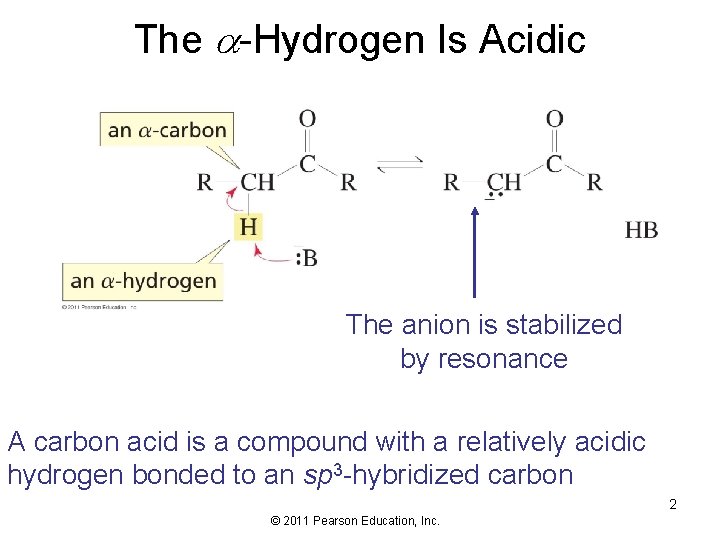
The -Hydrogen Is Acidic The anion is stabilized by resonance A carbon acid is a compound with a relatively acidic hydrogen bonded to an sp 3 -hybridized carbon 2 © 2011 Pearson Education, Inc.

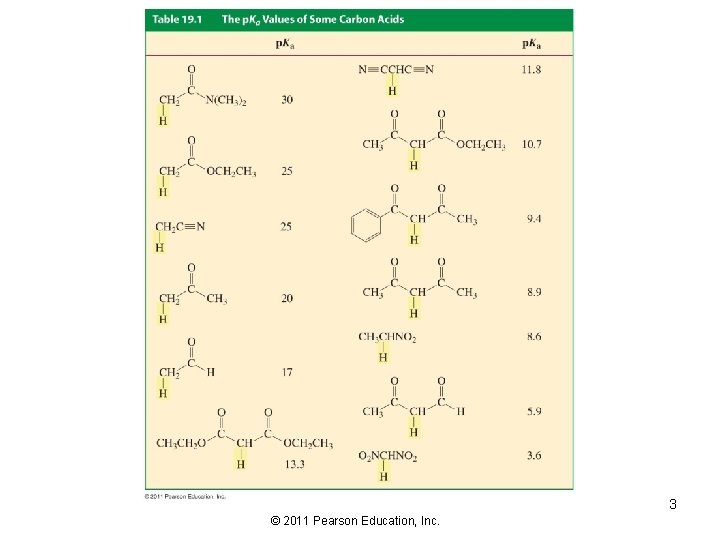
3 © 2011 Pearson Education, Inc.

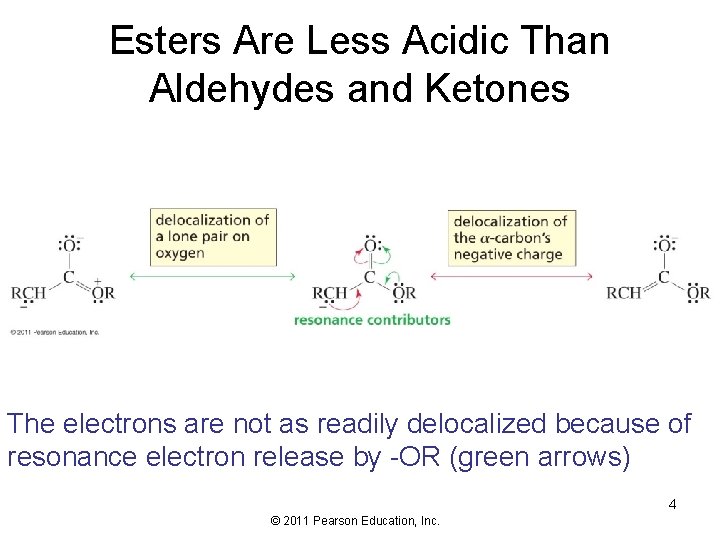
Esters Are Less Acidic Than Aldehydes and Ketones The electrons are not as readily delocalized because of resonance electron release by -OR (green arrows) 4 © 2011 Pearson Education, Inc.

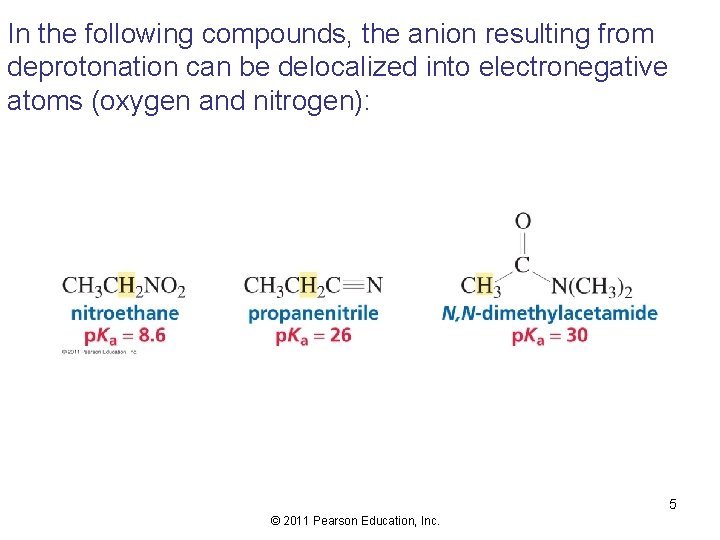
In the following compounds, the anion resulting from deprotonation can be delocalized into electronegative atoms (oxygen and nitrogen): 5 © 2011 Pearson Education, Inc.

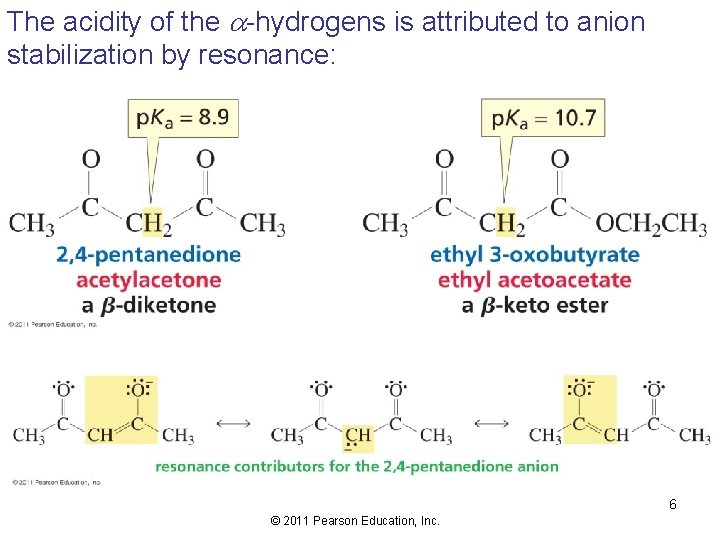
The acidity of the -hydrogens is attributed to anion stabilization by resonance: 6 © 2011 Pearson Education, Inc.

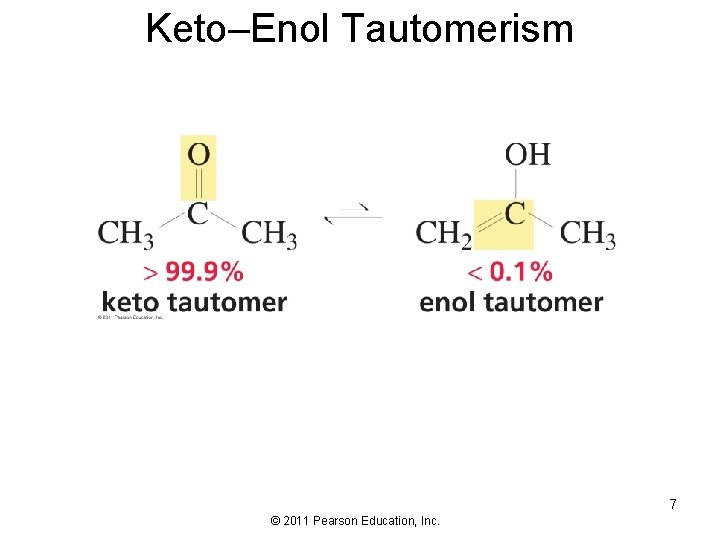
Keto–Enol Tautomerism 7 © 2011 Pearson Education, Inc.

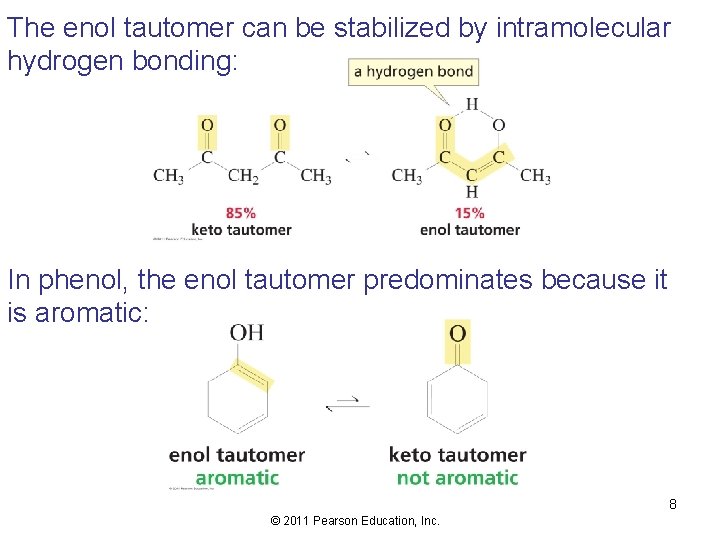
The enol tautomer can be stabilized by intramolecular hydrogen bonding: In phenol, the enol tautomer predominates because it is aromatic: 8 © 2011 Pearson Education, Inc.

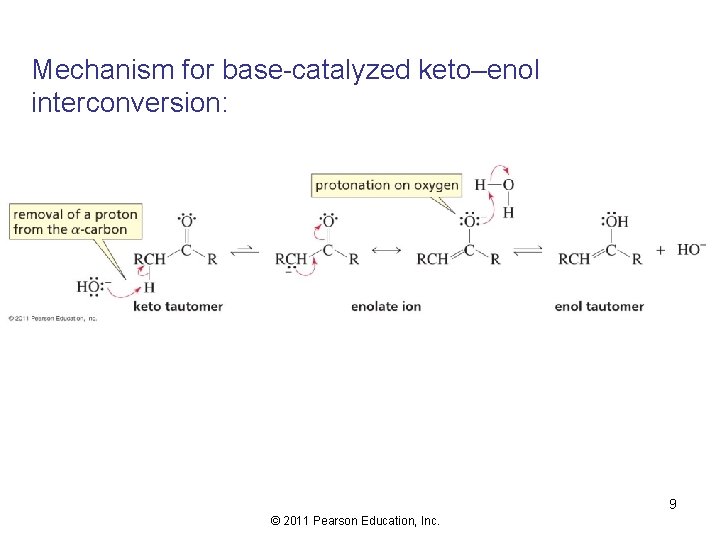
Mechanism for base-catalyzed keto–enol interconversion: 9 © 2011 Pearson Education, Inc.

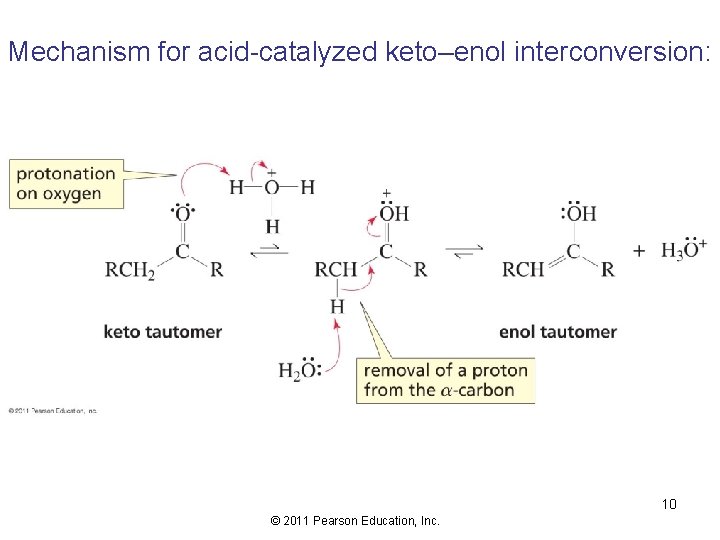
Mechanism for acid-catalyzed keto–enol interconversion: 10 © 2011 Pearson Education, Inc.

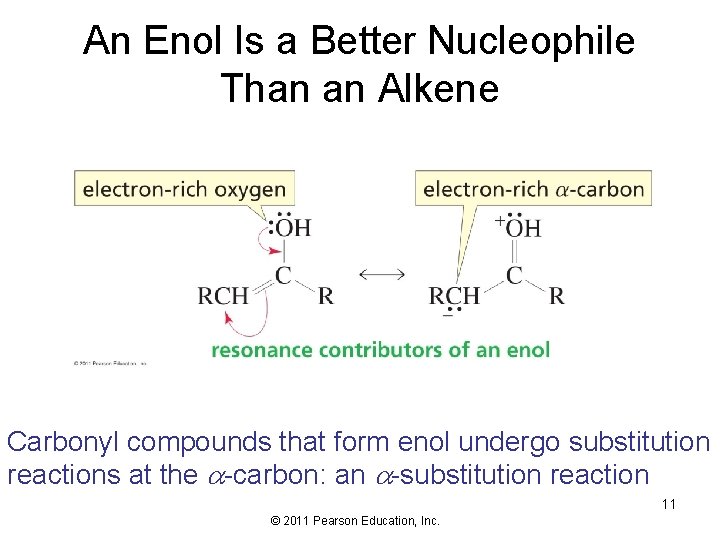
An Enol Is a Better Nucleophile Than an Alkene Carbonyl compounds that form enol undergo substitution reactions at the -carbon: an -substitution reaction 11 © 2011 Pearson Education, Inc.

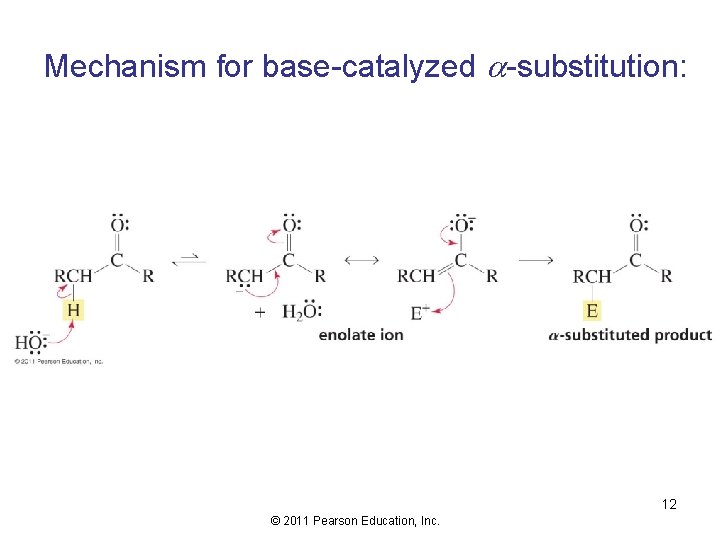
Mechanism for base-catalyzed -substitution: 12 © 2011 Pearson Education, Inc.

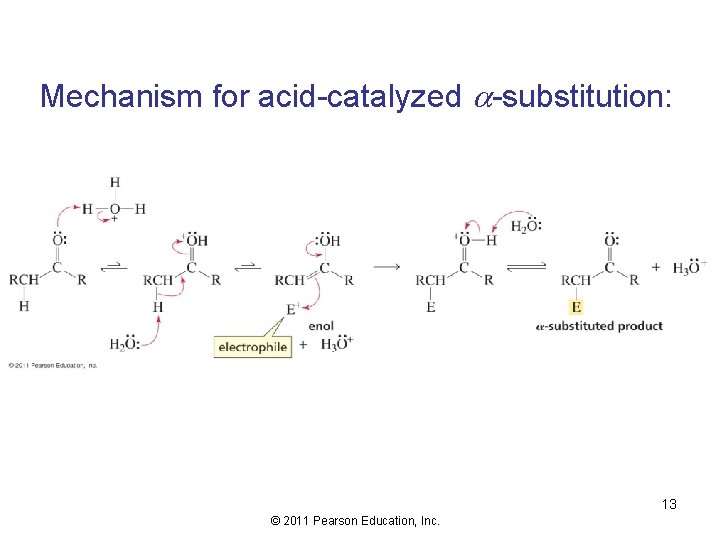
Mechanism for acid-catalyzed -substitution: 13 © 2011 Pearson Education, Inc.

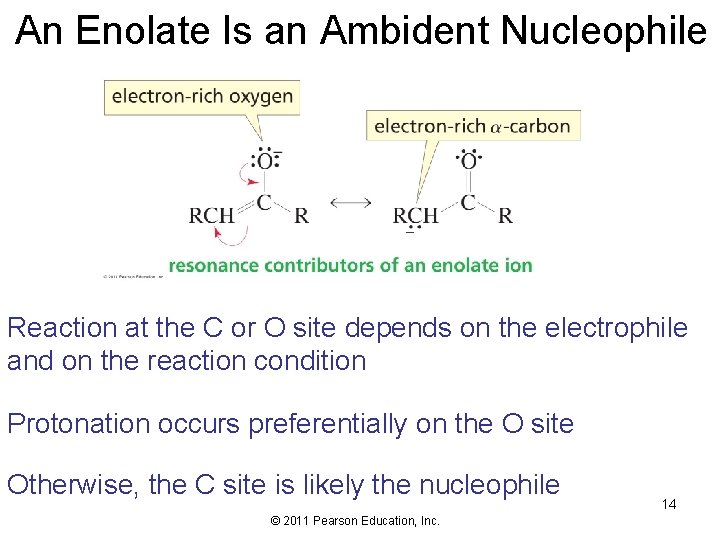
An Enolate Is an Ambident Nucleophile Reaction at the C or O site depends on the electrophile and on the reaction condition Protonation occurs preferentially on the O site Otherwise, the C site is likely the nucleophile © 2011 Pearson Education, Inc. 14

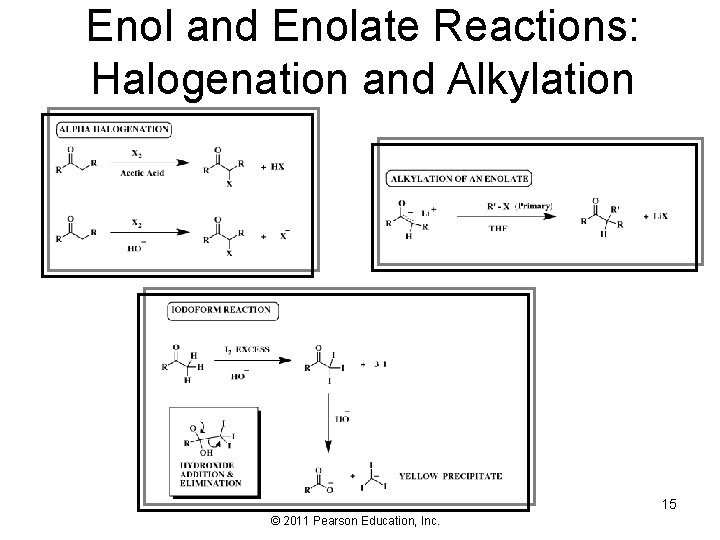
Enol and Enolate Reactions: Halogenation and Alkylation 15 © 2011 Pearson Education, Inc.

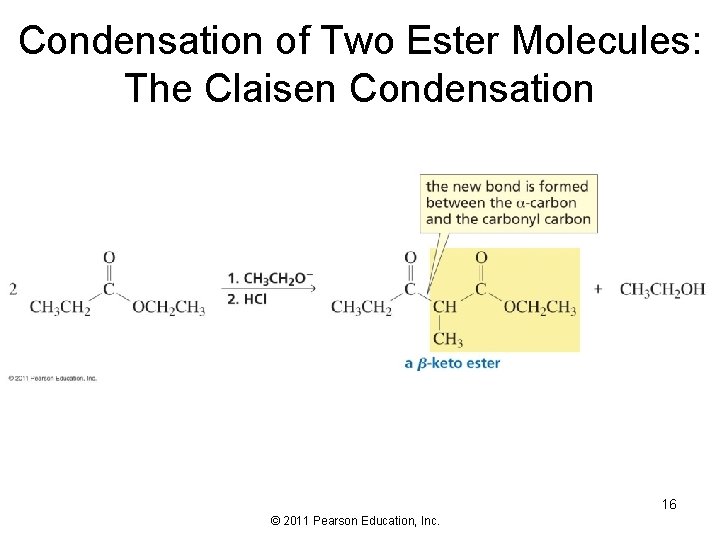
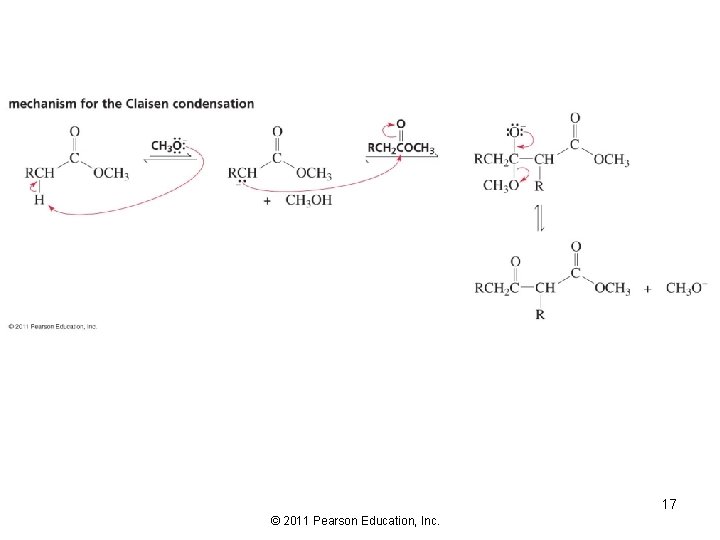
Condensation of Two Ester Molecules: The Claisen Condensation 16 © 2011 Pearson Education, Inc.

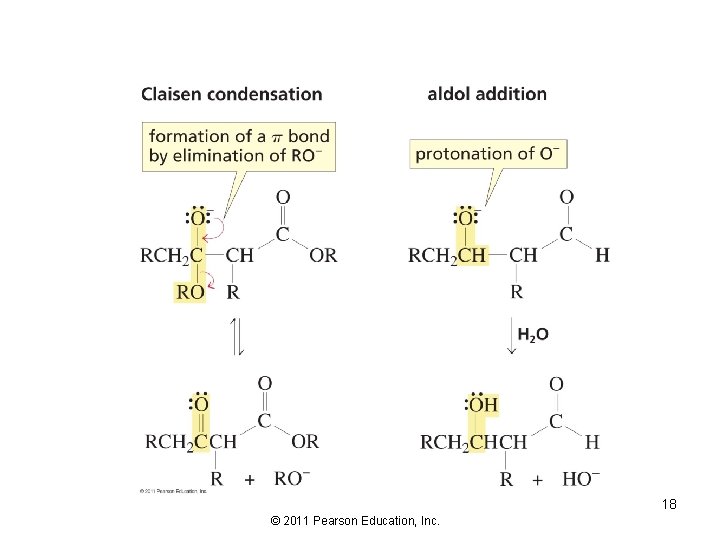
17 © 2011 Pearson Education, Inc.

18 © 2011 Pearson Education, Inc.

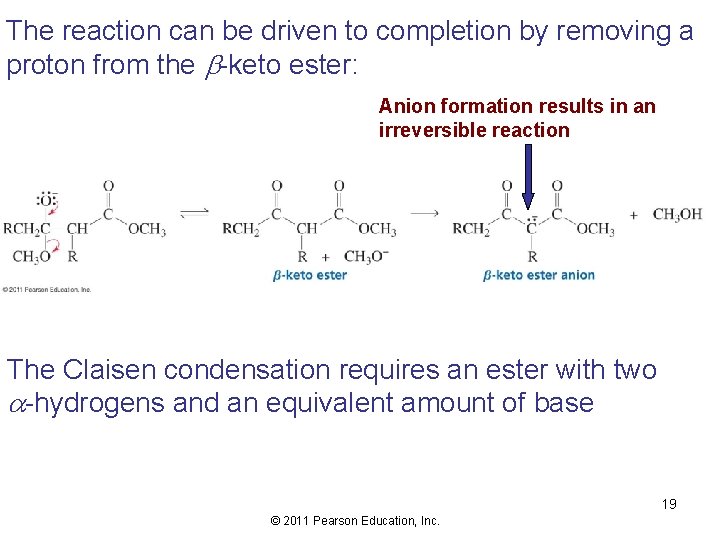
The reaction can be driven to completion by removing a proton from the b-keto ester: Anion formation results in an irreversible reaction The Claisen condensation requires an ester with two -hydrogens and an equivalent amount of base 19 © 2011 Pearson Education, Inc.

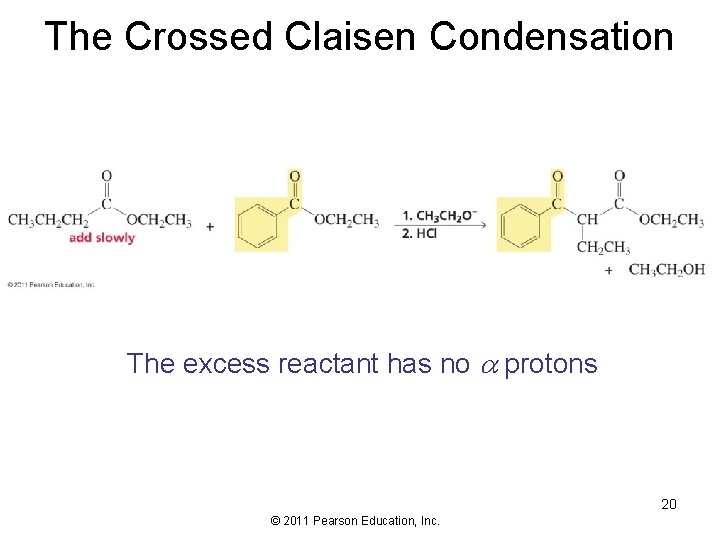
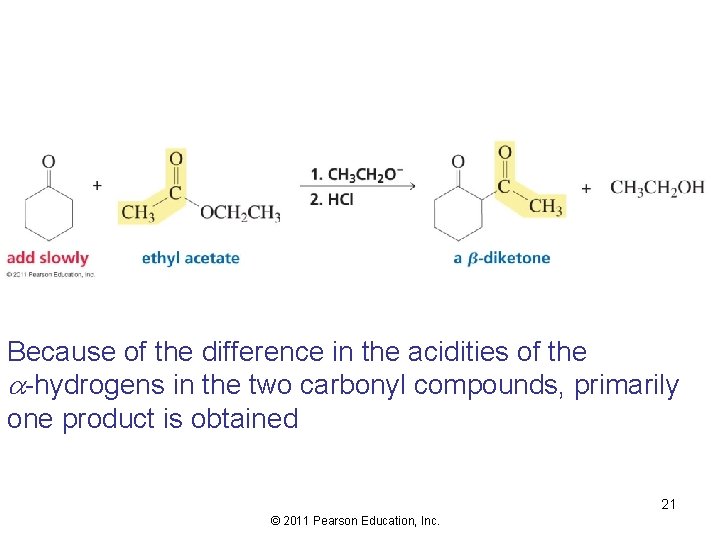
The Crossed Claisen Condensation The excess reactant has no protons 20 © 2011 Pearson Education, Inc.

Because of the difference in the acidities of the -hydrogens in the two carbonyl compounds, primarily one product is obtained 21 © 2011 Pearson Education, Inc.

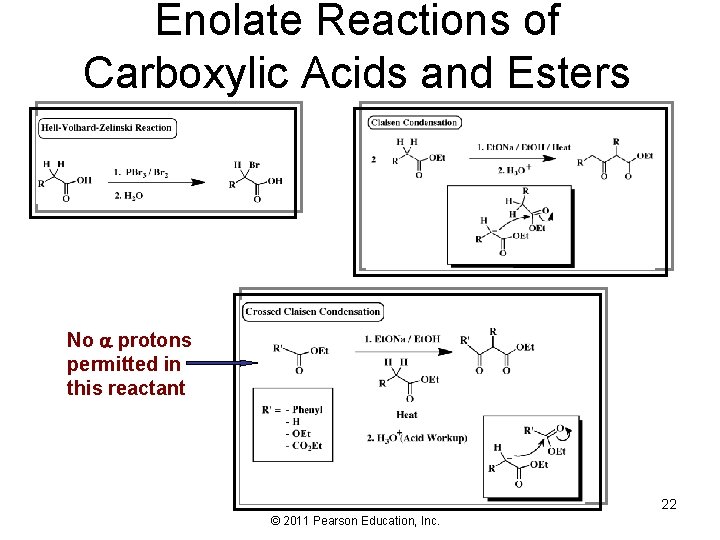
Enolate Reactions of Carboxylic Acids and Esters No protons permitted in this reactant 22 © 2011 Pearson Education, Inc.

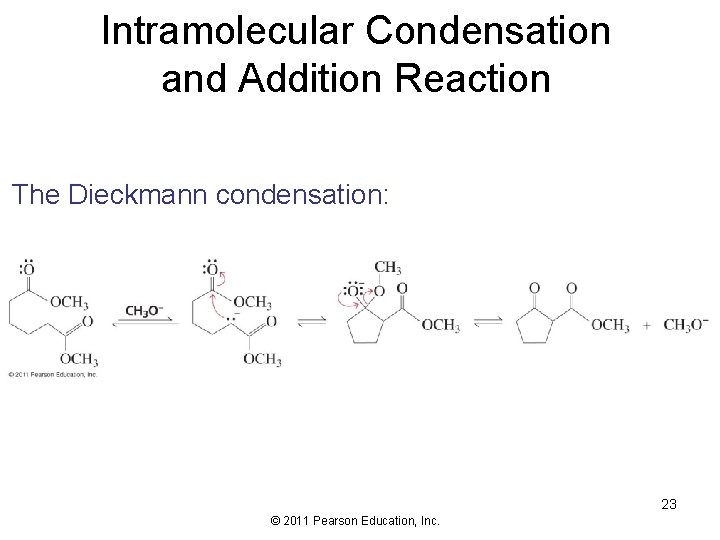
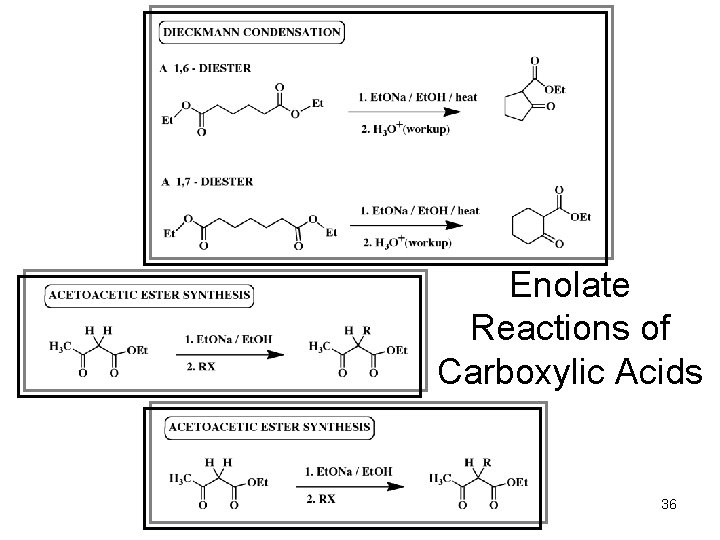
Intramolecular Condensation and Addition Reaction The Dieckmann condensation: 23 © 2011 Pearson Education, Inc.

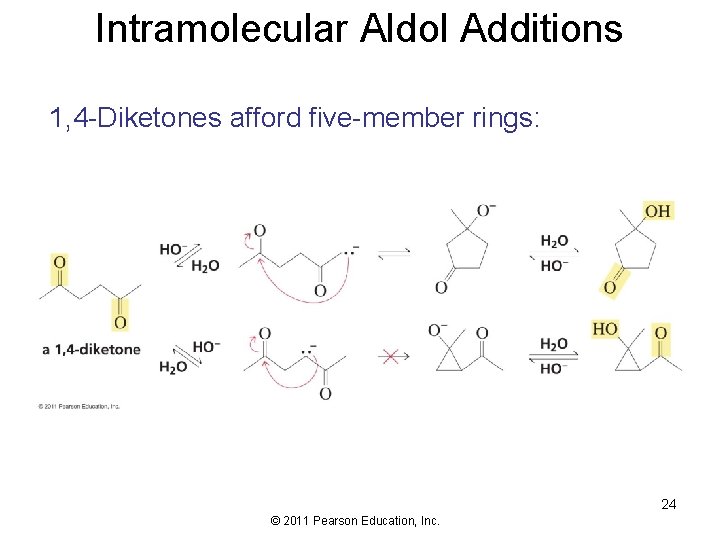
Intramolecular Aldol Additions 1, 4 -Diketones afford five-member rings: 24 © 2011 Pearson Education, Inc.

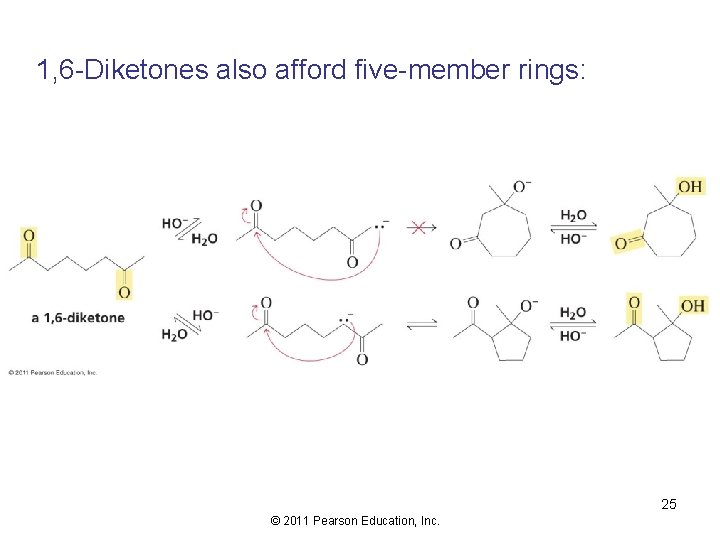
1, 6 -Diketones also afford five-member rings: 25 © 2011 Pearson Education, Inc.

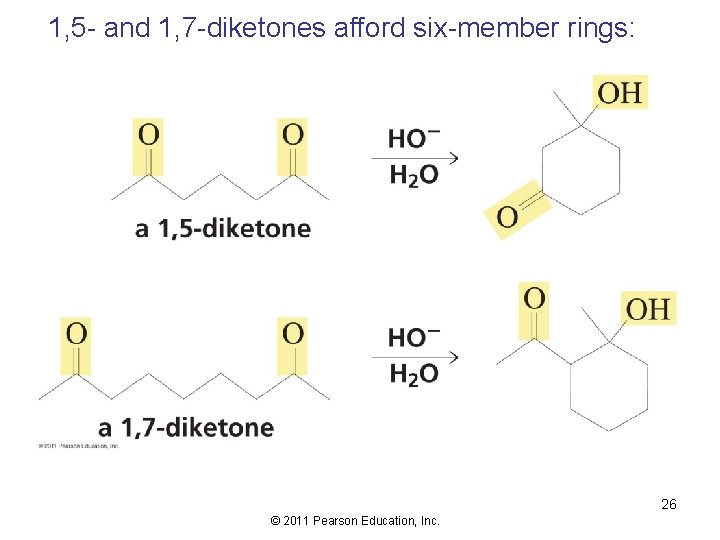
1, 5 - and 1, 7 -diketones afford six-member rings: 26 © 2011 Pearson Education, Inc.

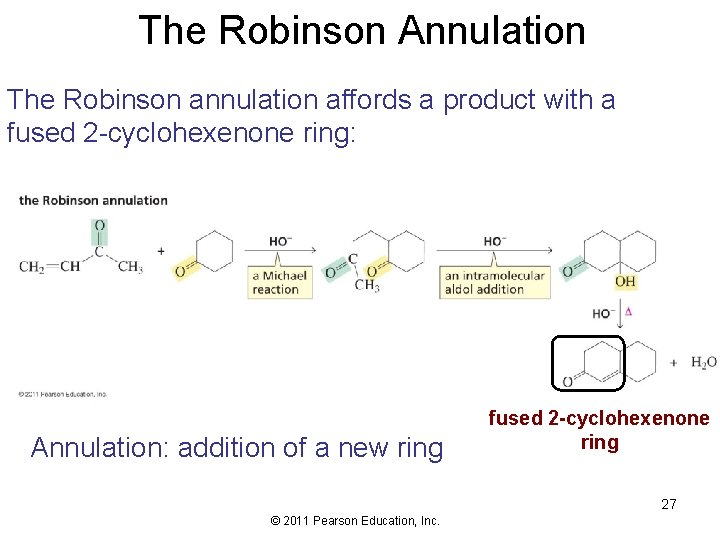
The Robinson Annulation The Robinson annulation affords a product with a fused 2 -cyclohexenone ring: Annulation: addition of a new ring fused 2 -cyclohexenone ring 27 © 2011 Pearson Education, Inc.

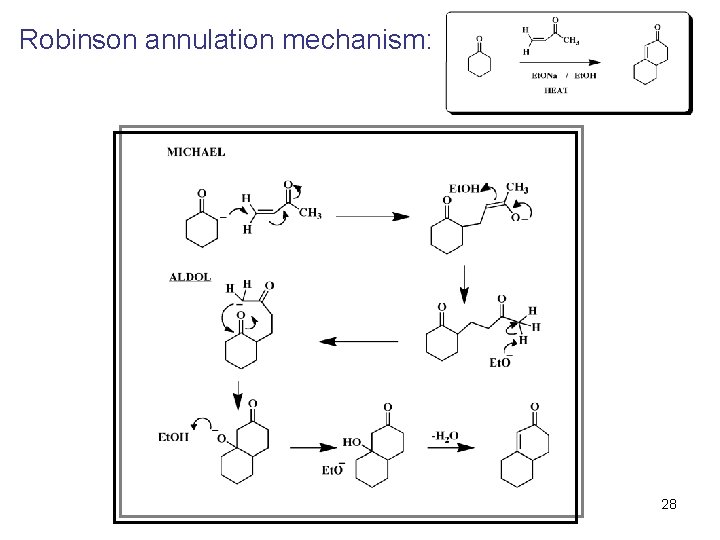
Robinson annulation mechanism: 28 © 2011 Pearson Education, Inc.

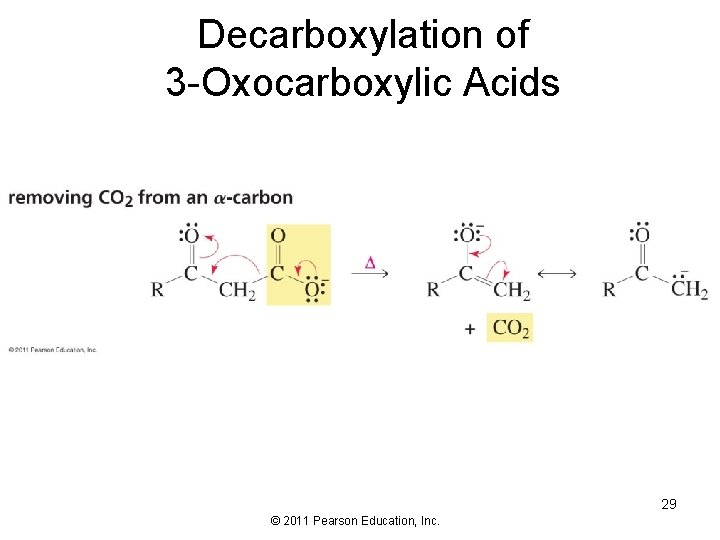
Decarboxylation of 3 -Oxocarboxylic Acids 29 © 2011 Pearson Education, Inc.

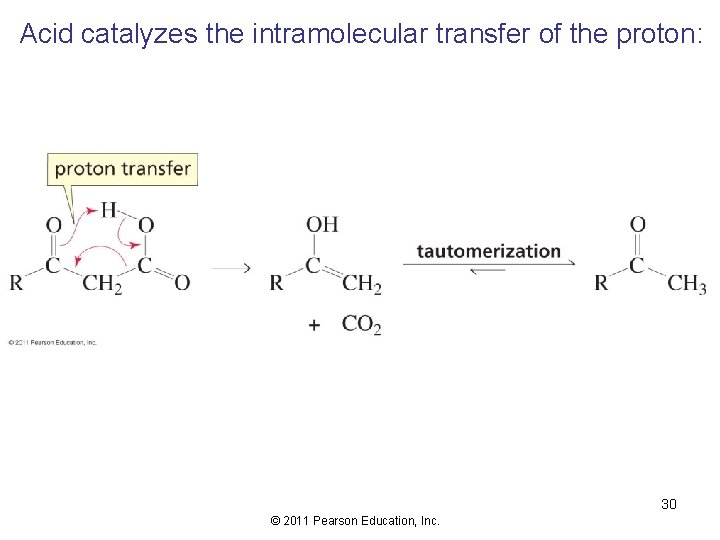
Acid catalyzes the intramolecular transfer of the proton: 30 © 2011 Pearson Education, Inc.

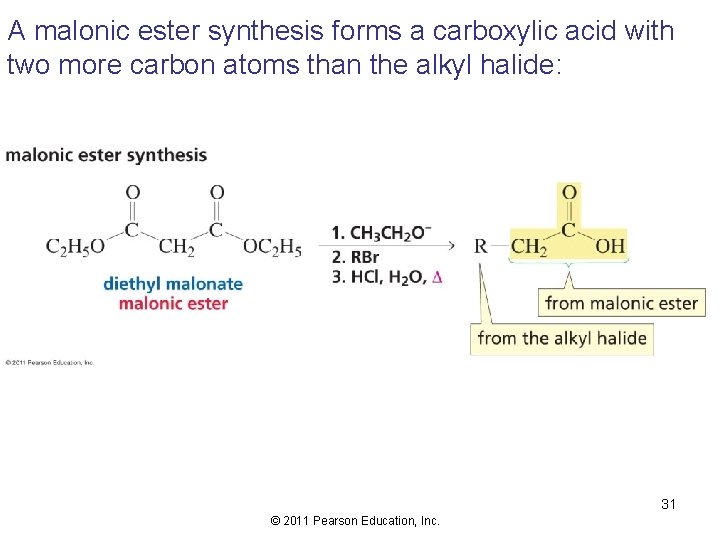
A malonic ester synthesis forms a carboxylic acid with two more carbon atoms than the alkyl halide: 31 © 2011 Pearson Education, Inc.

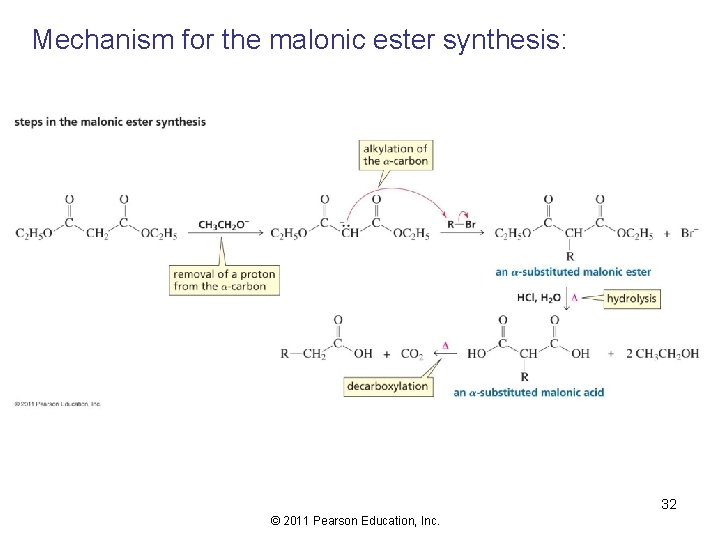
Mechanism for the malonic ester synthesis: 32 © 2011 Pearson Education, Inc.

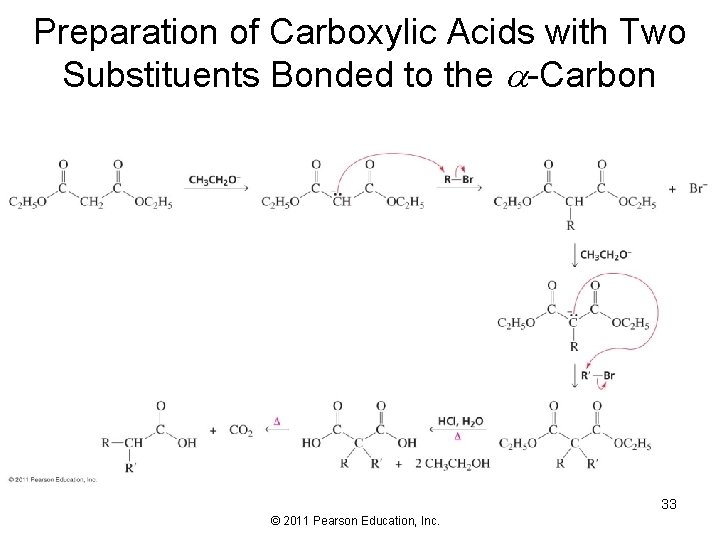
Preparation of Carboxylic Acids with Two Substituents Bonded to the -Carbon 33 © 2011 Pearson Education, Inc.

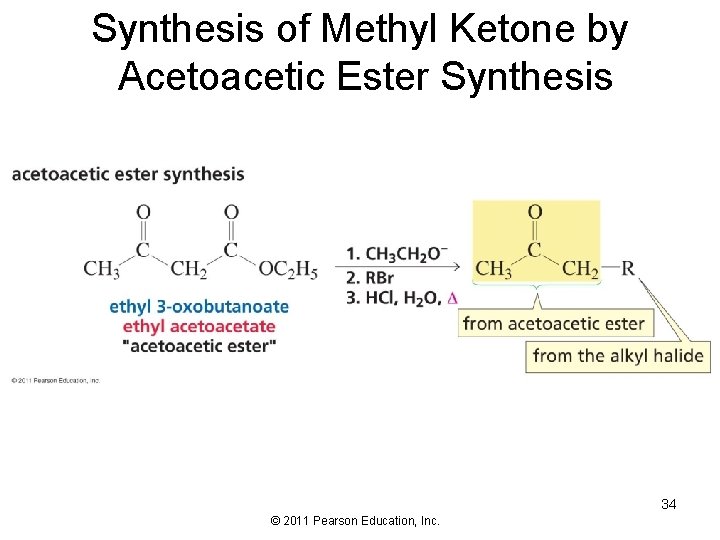
Synthesis of Methyl Ketone by Acetoacetic Ester Synthesis 34 © 2011 Pearson Education, Inc.

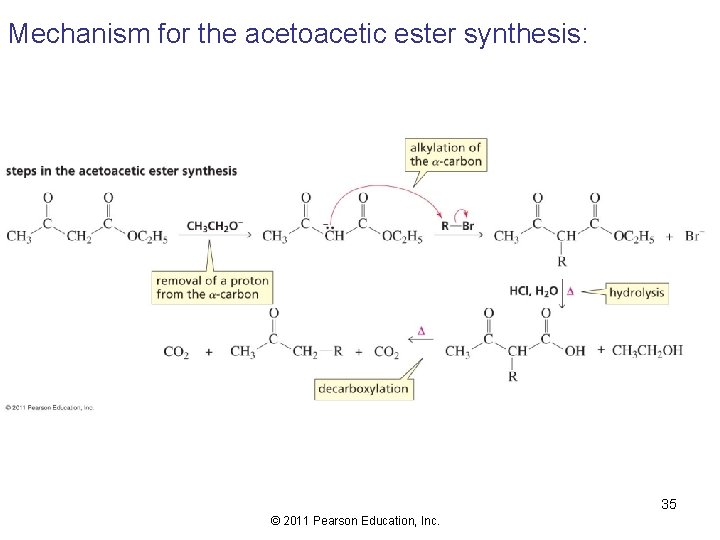
Mechanism for the acetoacetic ester synthesis: 35 © 2011 Pearson Education, Inc.

Enolate Reactions of Carboxylic Acids 36 © 2011 Pearson Education, Inc.

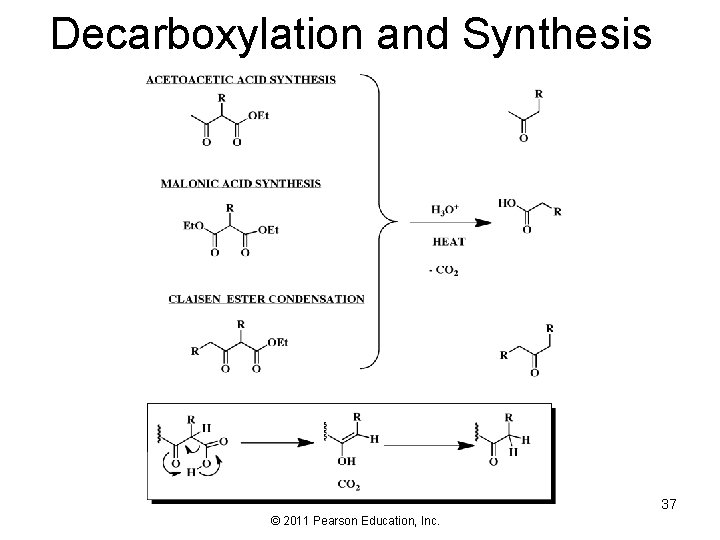
Decarboxylation and Synthesis 37 © 2011 Pearson Education, Inc.

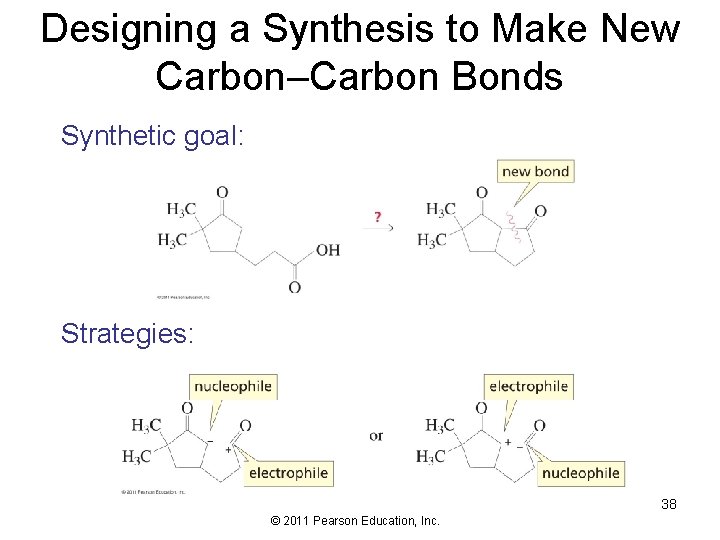
Designing a Synthesis to Make New Carbon–Carbon Bonds Synthetic goal: Strategies: 38 © 2011 Pearson Education, Inc.

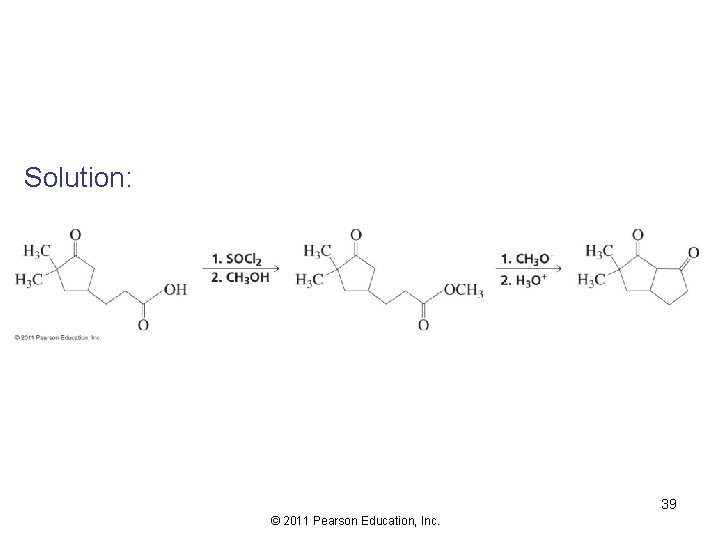
Solution: 39 © 2011 Pearson Education, Inc.

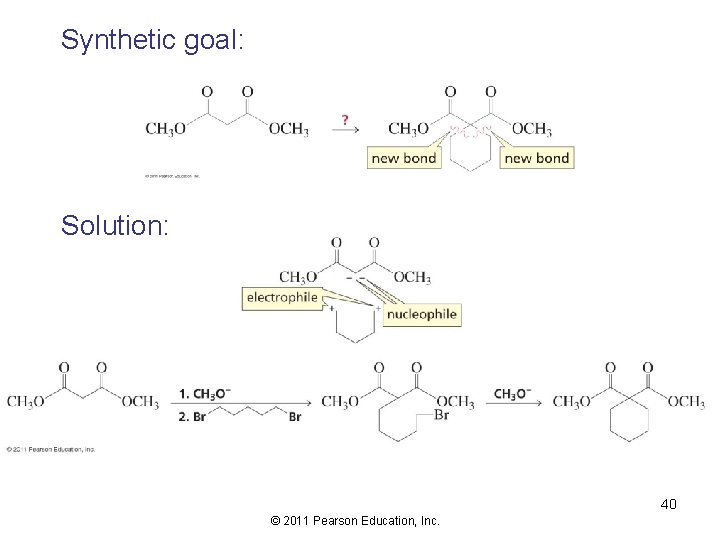
Synthetic goal: Solution: 40 © 2011 Pearson Education, Inc.

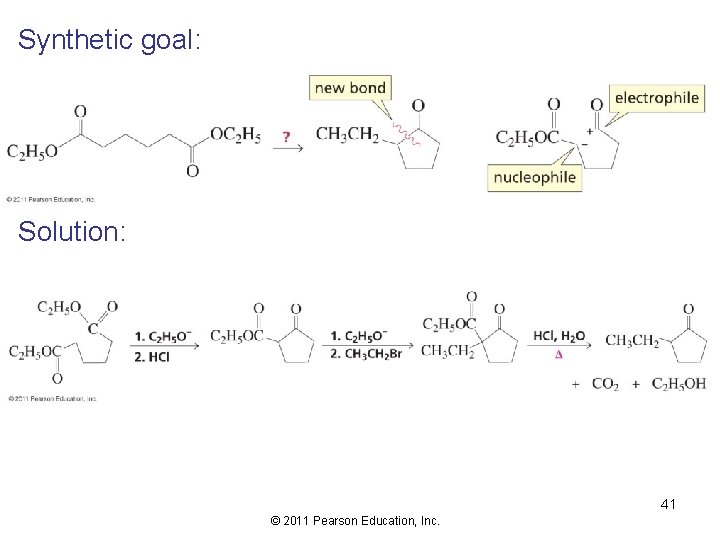
Synthetic goal: Solution: 41 © 2011 Pearson Education, Inc.

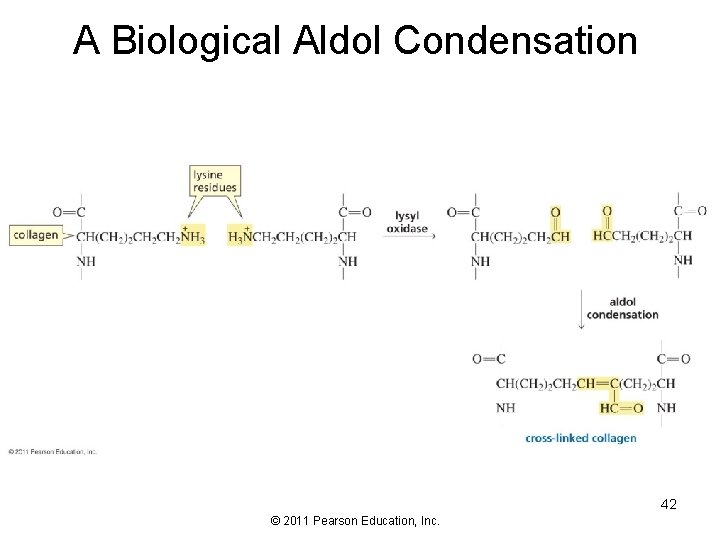
A Biological Aldol Condensation 42 © 2011 Pearson Education, Inc.

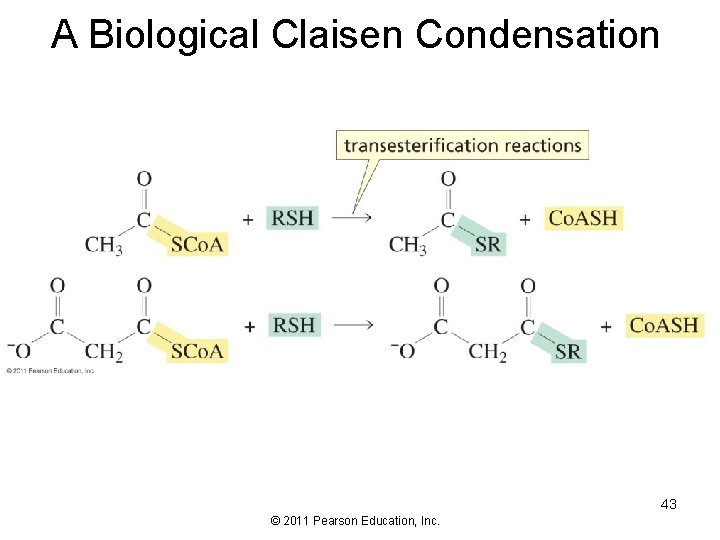
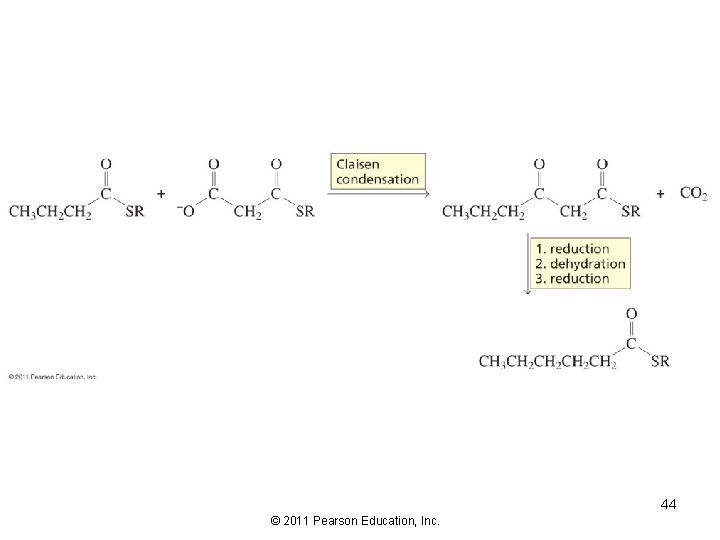
A Biological Claisen Condensation 43 © 2011 Pearson Education, Inc.

44 © 2011 Pearson Education, Inc.

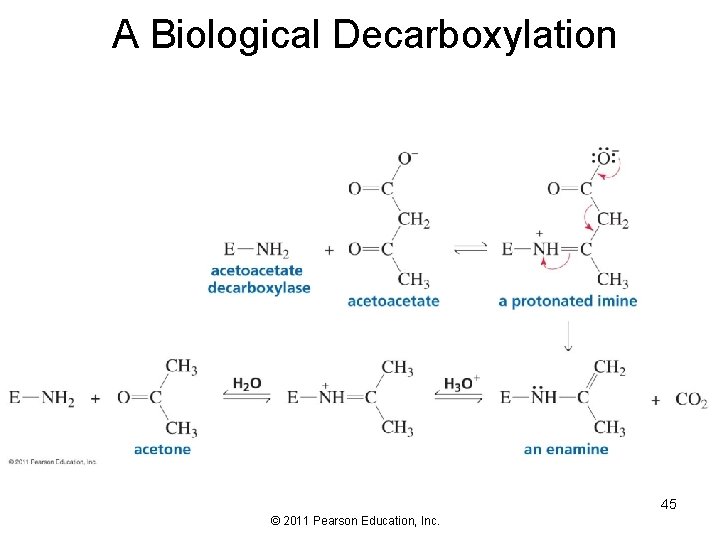
A Biological Decarboxylation 45 © 2011 Pearson Education, Inc.