Organic Chemistry Organic compounds Compounds must contain carbon
Organic Chemistry

Organic compounds • Compounds must contain carbon – will also contain hydrogen – may contain oxygen, nitrogen, sulfur and/or phosphorus There are only ~ 60, 000 inorganic compounds BUT millions of organic compounds

HOW? • Carbon has 4 unpaired valence electrons and needs 4 more e- to complete its outer shell. • Carbon makes a total of 4 bonds – Simplest: CH 4 • Carbon can bond with itself to form chains of many lengths

Counting in the language of organic • • • 1 2 3 4 5 meth prop but pent • • • 6 hex 7 hept 8 oct 9 non 10 dec Organic names directly related to # of carbons
Characteristics of Organics. . . • Forces of attraction – molecules contain covalent bonds – molecules tend to be non-polar – intermolecular attractions tend to be weak • van Der Waals • The longer the molecule is the stronger the forces are

• So. . . – High vapor pressure • evaporate quickly & have strong odors – low boiling points & melting points when compared to ionic compounds

• Conductivity – organic molecules do not ionize – do not conduct electricity • Solubility – because organic molecules are non-polar they are soluble in non-polar substances • miscible – insoluble in polar substances • immiscible Few organic substances are miscible in water sugar, acetic acid, alcohols

• Reactivity – flammable / highly combustible • Rate of reaction – because of the covalent bonding, reactivity rates tend to be slow • Strong covalent bonding doesn’t allow activated complexes to form easily • random collisions don’t provide enough KE to overcome activation energy. Many collisions aren’t effective
Different types of formulas • molecular formula – tells you how many of each element are in the molecule – ex. C 6 H 12 O 6 • empirical formula – gives you lowest possible ratio of atoms in the molecule – ex. CH 2 O
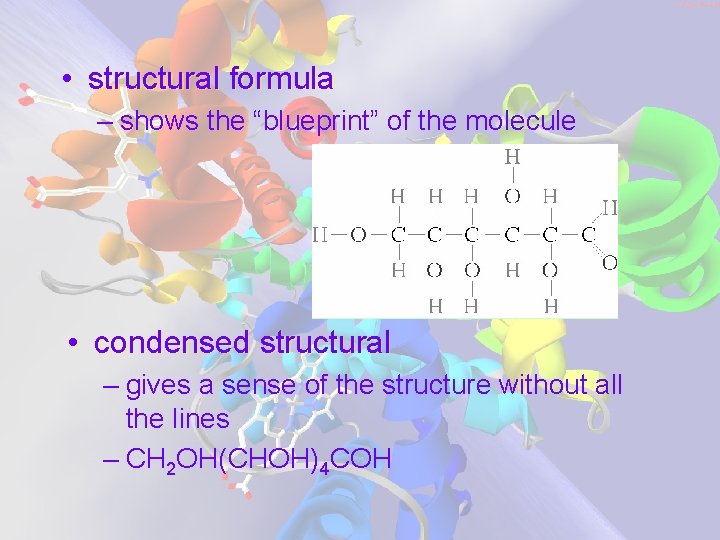
• structural formula – shows the “blueprint” of the molecule • condensed structural – gives a sense of the structure without all the lines – CH 2 OH(CHOH)4 COH

Hydrocarbons • Type of organic molecule that contains only hydrogen and carbon • Shapes – straight chain – branched – cyclic • closed • not necessarily circular
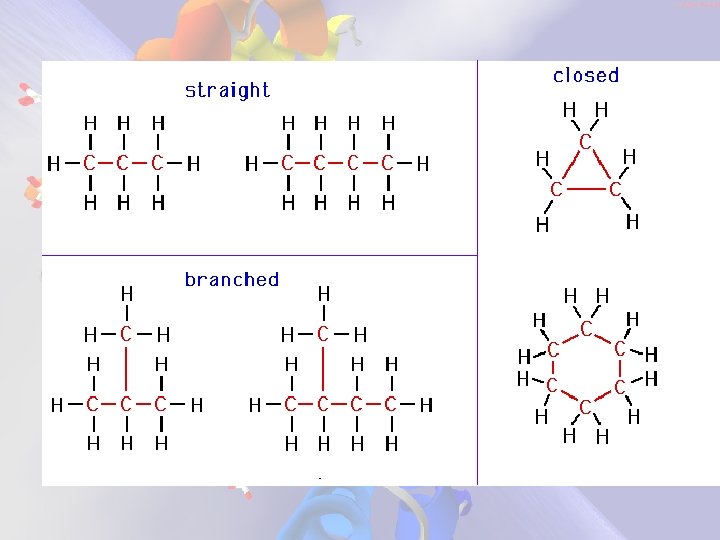

H-C characteristics • Vary greatly in size – smallest tend to be gases – largest tend to be solids • As H-C chains become longer the b. p. and m. p. increase due to increased van der Waals forces Larger molecules are attracted to each other (more than smaller molecules) because they contain more p+ and e-
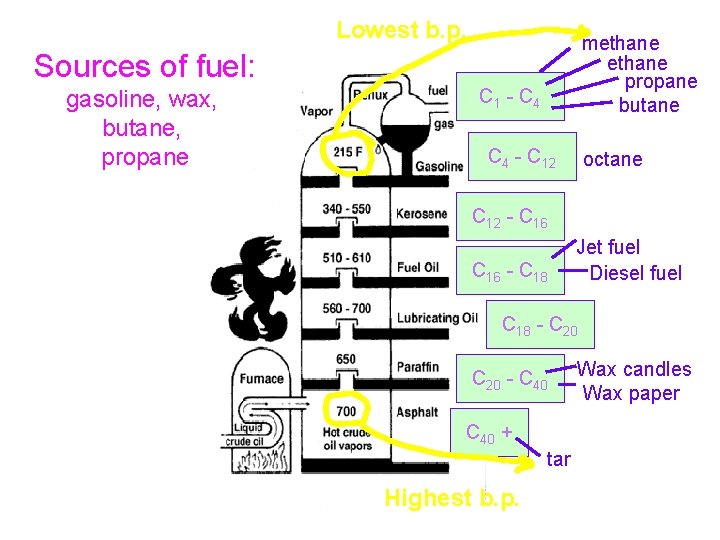
Lowest b. p. methane propane butane Sources of fuel: gasoline, wax, butane, propane C 1 - C 4 - C 12 octane C 12 - C 16 - C 18 Jet fuel Diesel fuel C 18 - C 20 - C 40 + tar Highest b. p. Wax candles Wax paper

Naming Simple Hydrocarbons 1. Count the longest continuous chain of Carbons – this is your “parent chain” – use organic “language” as your prefix – meth, prop, but. . . 2. Determine suffix according to which homologous series it belongs to • alkane or alkene or alkyne

Series of Hydrocarbons • Alkanes – contain only single bonds BETWEEN Cs • ane suffix to hydrocarbons Cn H(2 n + 2) • a. k. a. Saturated – every carbon is bonded to 4 other atoms
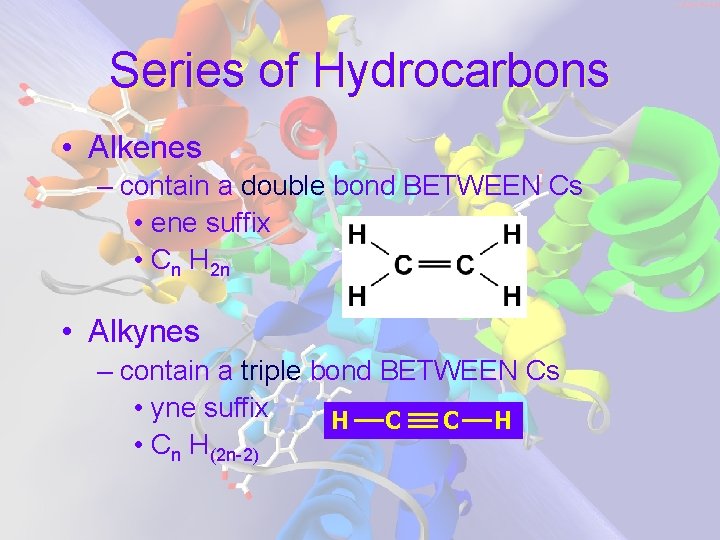
Series of Hydrocarbons • Alkenes – contain a double bond BETWEEN Cs • ene suffix • Cn H 2 n • Alkynes – contain a triple bond BETWEEN Cs • yne suffix H C C H • Cn H(2 n-2)

Alkenes & Alkynes • a. k. a. Unsaturated – multiple bonds between Cs • tend to be more reactive than saturated H-Cs because double and triple bonds easily broken • LAST RULE FOR NAMING 3. Where is the double or triple bond? • Always use the lowest possible number!
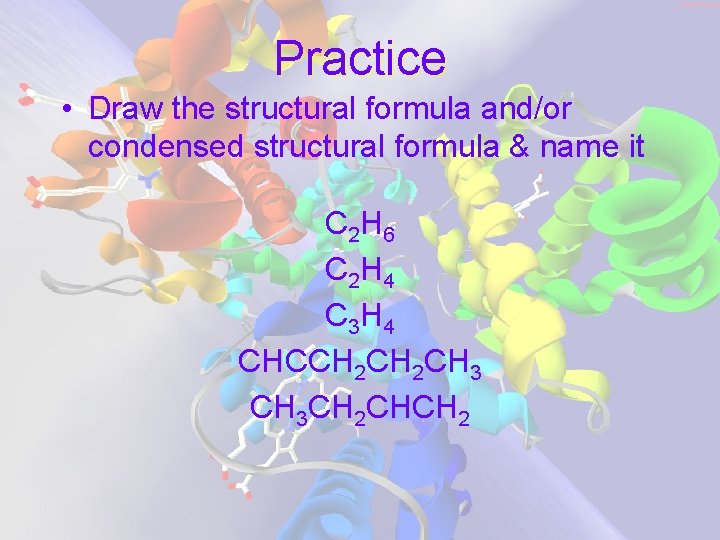
Practice • Draw the structural formula and/or condensed structural formula & name it C 2 H 6 C 2 H 4 C 3 H 4 CHCCH 2 CH 3 CH 2 CHCH 2

How do you name more complex Organic Molecules? IUPAC naming system

Halogenated H-Cs • A halide has been substituted for a H Group 17 Fluorine Chlorine Bromine Iodine Prefix Fluoro. Chloro. Bromo. Iodo-
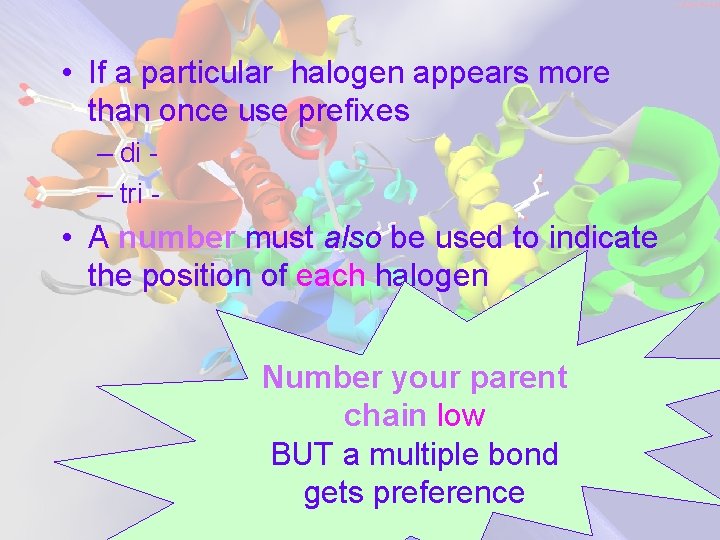
• If a particular halogen appears more than once use prefixes – di – tri - • A number must also be used to indicate the position of each halogen Number your parent chain low BUT a multiple bond gets preference
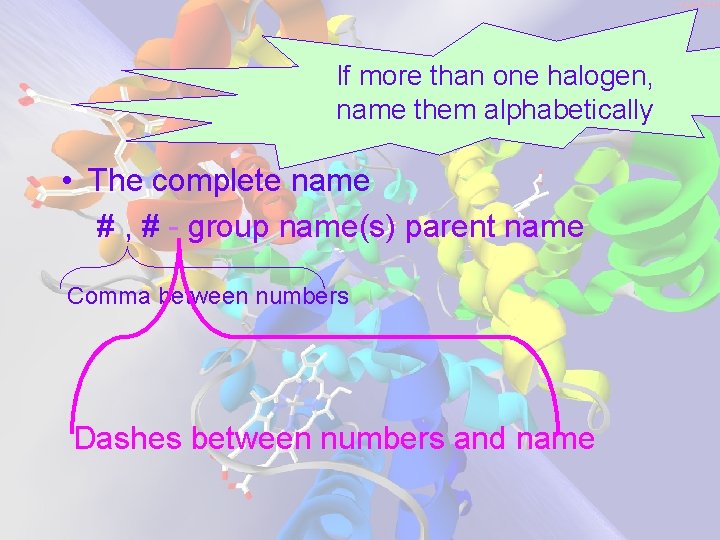
If more than one halogen, name them alphabetically • The complete name # , # - group name(s) parent name Comma between numbers Dashes between numbers and name
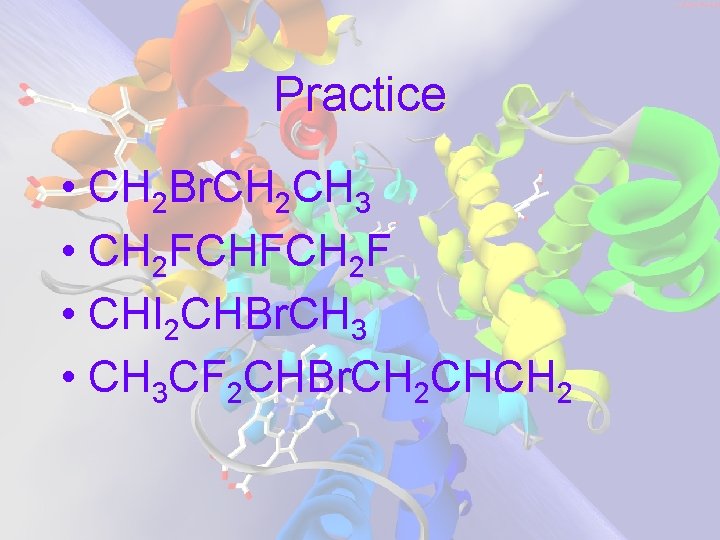
Practice • CH 2 Br. CH 2 CH 3 • CH 2 FCHFCH 2 F • CHI 2 CHBr. CH 3 • CH 3 CF 2 CHBr. CH 2 CHCH 2
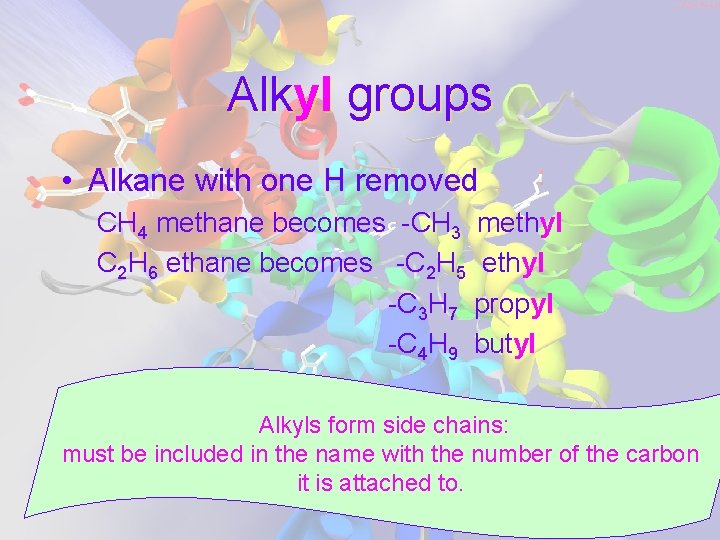
Alkyl groups • Alkane with one H removed CH 4 methane becomes -CH 3 methyl C 2 H 6 ethane becomes -C 2 H 5 ethyl -C 3 H 7 propyl -C 4 H 9 butyl Alkyls form side chains: must be included in the name with the number of the carbon it is attached to.

• If a particular alkyl group appears more than once use prefixes – di – tri - • Which carbon is it on? Lowest number C

If more than one. . . ABC’s • The complete name # , # - alkyl name(s) parent name Comma between numbers Dashes between numbers and name
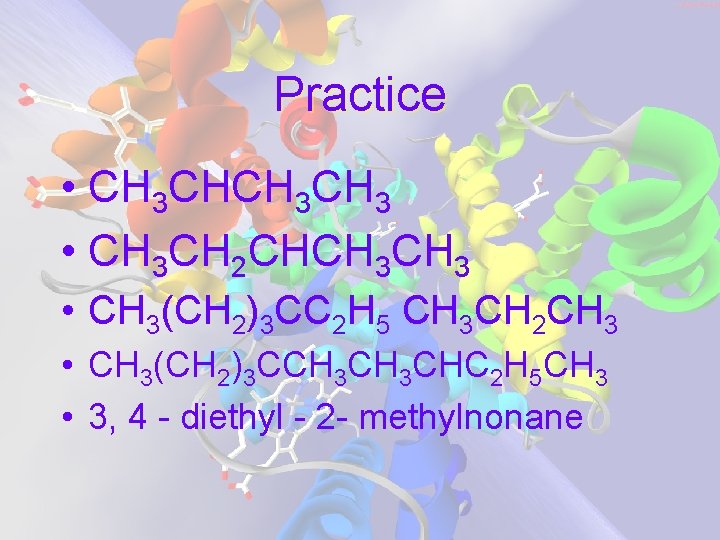
Practice • CH 3 CH 3 • CH 3 CH 2 CHCH 3 • CH 3(CH 2)3 CC 2 H 5 CH 3 CH 2 CH 3 • CH 3(CH 2)3 CCH 3 CHC 2 H 5 CH 3 • 3, 4 - diethyl - 2 - methylnonane

What if you have halogens and alkyl groups? ?
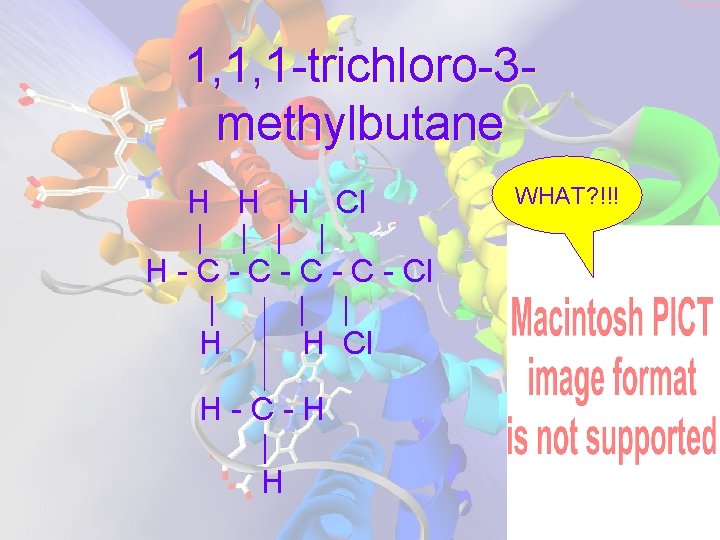
1, 1, 1 -trichloro-3 methylbutane H H H Cl | | H - C - C - Cl | | | H H Cl H-C-H | H WHAT? !!!
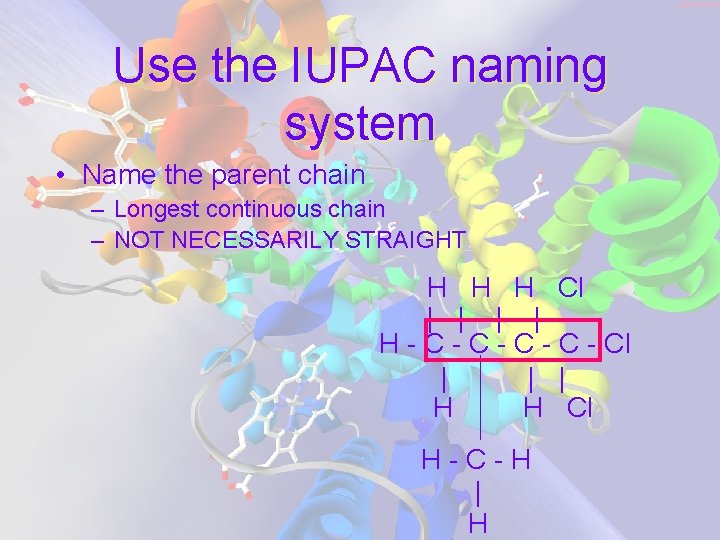
Use the IUPAC naming system • Name the parent chain – Longest continuous chain – NOT NECESSARILY STRAIGHT H H H Cl | | H - C - C - Cl | | | H H Cl H-C-H | H
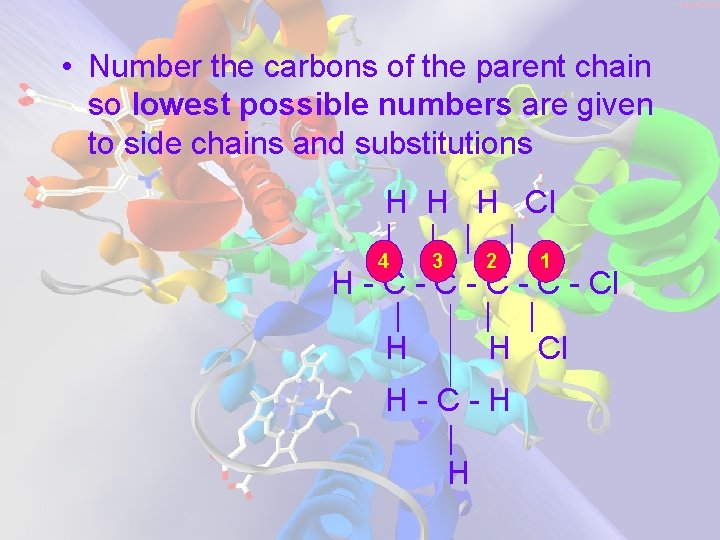
• Number the carbons of the parent chain so lowest possible numbers are given to side chains and substitutions H H H Cl | | 4 3 2 1 H - C - C - Cl | | | H H Cl H-C-H | H
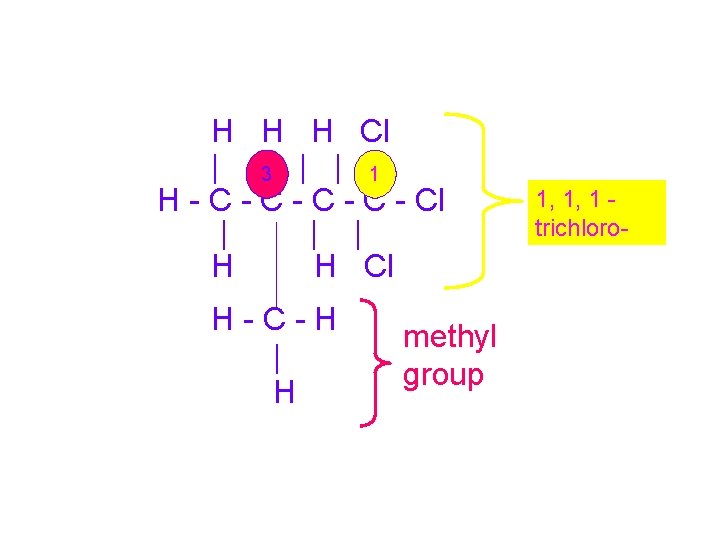
H H H Cl | |3 | | 1 H - C - C - Cl | | | H H Cl H-C-H | H methyl group 1, 1, 1 trichloro-
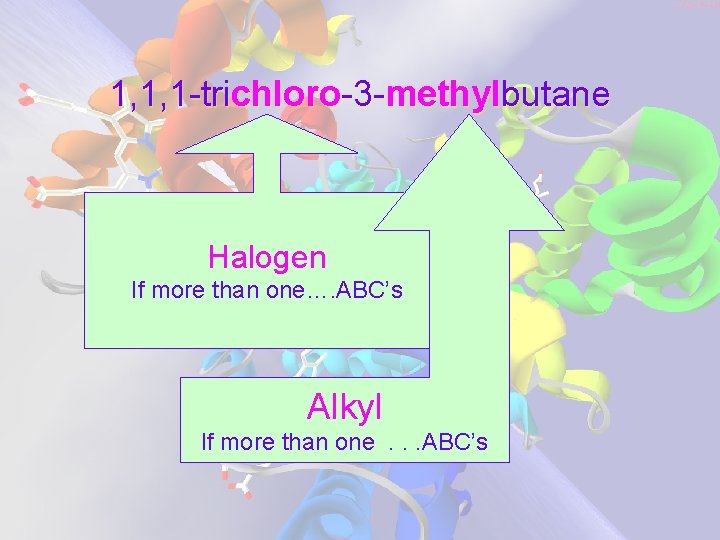
1, 1, 1 -trichloro-3 -methylbutane Halogen If more than one…. ABC’s Alkyl If more than one. . . ABC’s
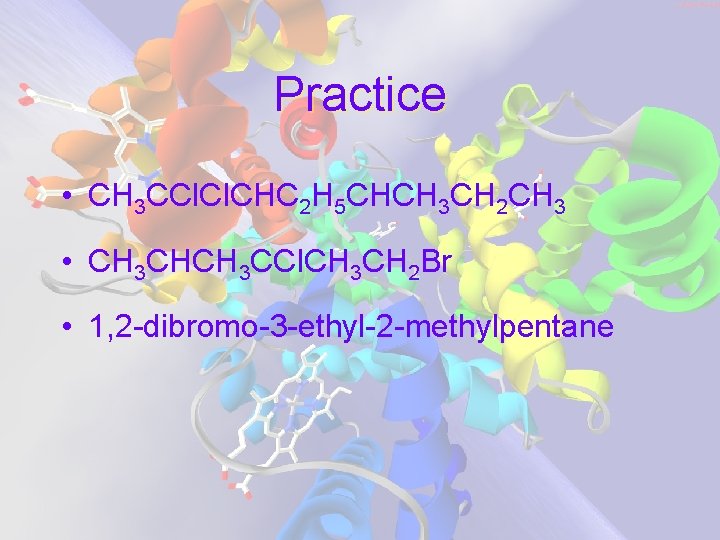
Practice • CH 3 CCl. CHC 2 H 5 CHCH 3 CH 2 CH 3 • CH 3 CHCH 3 CCl. CH 3 CH 2 Br • 1, 2 -dibromo-3 -ethyl-2 -methylpentane
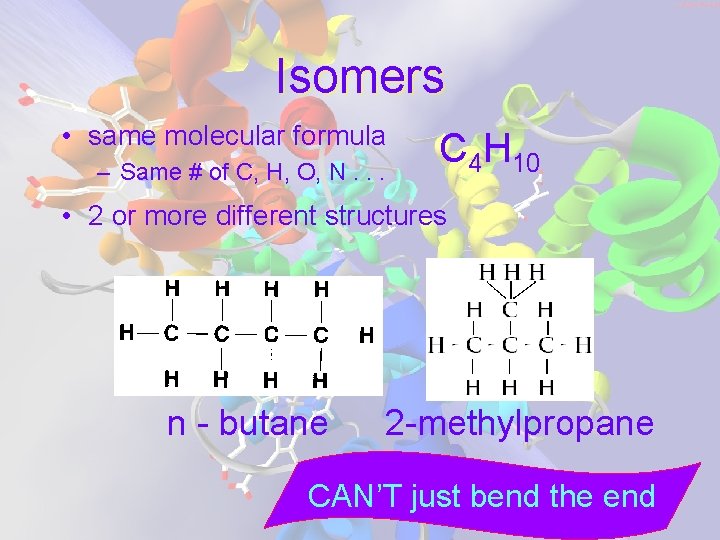
Isomers • same molecular formula – Same # of C, H, O, N. . . C 4 H 10 • 2 or more different structures n - butane 2 -methylpropane CAN’T just bend the end
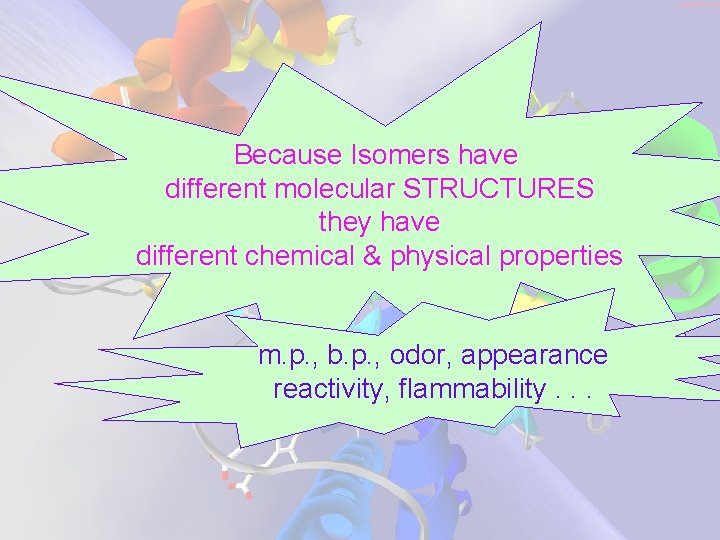
Because Isomers have different molecular STRUCTURES they have different chemical & physical properties m. p. , b. p. , odor, appearance reactivity, flammability. . .
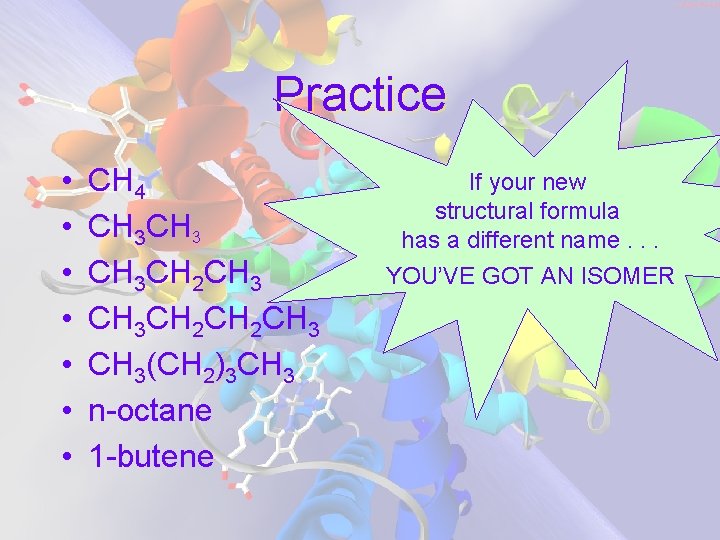
Practice • • CH 4 CH 3 CH 2 CH 3 CH 2 CH 3(CH 2)3 CH 3 n-octane 1 -butene If your new structural formula has a different name. . . YOU’VE GOT AN ISOMER
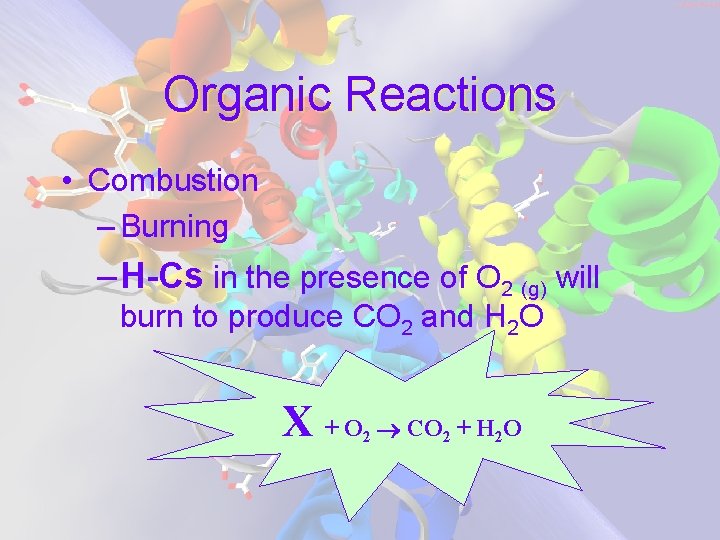
Organic Reactions • Combustion – Burning – H-Cs in the presence of O 2 (g) will burn to produce CO 2 and H 2 O X +O 2 CO 2 + H 2 O
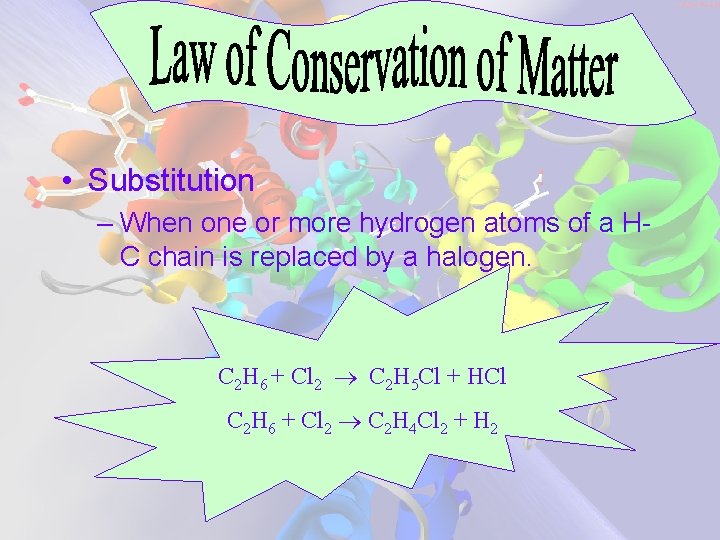
• Substitution – When one or more hydrogen atoms of a HC chain is replaced by a halogen. C 2 H 6 + Cl 2 C 2 H 5 Cl + HCl C 2 H 6 + Cl 2 C 2 H 4 Cl 2 + H 2

Addition • Involves breaking a multiple bond • Unsaturated compounds become more saturated – Alkyne becomes an alkene – Alkene becomes an alkane C 2 H 2 + Br 2 C 2 H 2 Br 2 C 2 H 4 + Br 2 C 2 H 4 Br 2
Polymerization • The process of bonding many small molecules (monomers) together to make a very long chain (polymer) • Water must be a product • Examples of polymers – starches – Proteins – nylon

To be continued. . .
- Slides: 43