ORGANIC CHEMISTRY Organic compounds are synthesized by cells
ORGANIC CHEMISTRY Organic compounds are synthesized by cells and contain Carbon – made of carbon skeleton. BUILDING macromolecules (AKA. organic compounds): Macromolecules are large molecules called polymers. These polymers are composed of monomer subunits.
REACTIONS • Condensation (dehydration synthesis) – monomers are connected to produce polymers; releases H 2 O during the reaction (building up) • Hydrolysis – polymers are broken down into their monomers; H 2 O is needed for the reaction to occur (breaking down) • ENZYMES assist in both reactions!
4 TYPES OF MACROMOLECULES • Carbohydrates • Lipids • Nucleic Acids • Proteins

1. CARBOHYDRATES consist of C, H, O in a 1: 2: 1 ratio FUNCTION – Main source of energy – breakdown of sugar supplies quick energy to cells; excess is stored as complex sugars in cells
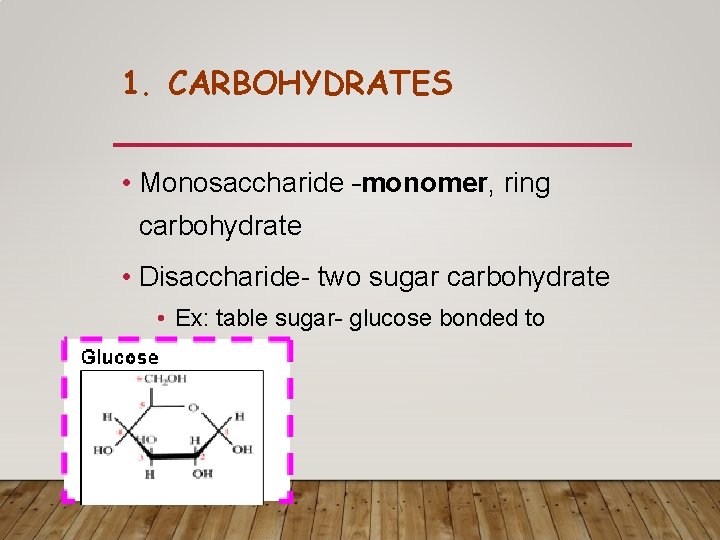
1. CARBOHYDRATES • Monosaccharide –monomer, ring carbohydrate • Disaccharide- two sugar carbohydrate • Ex: table sugar- glucose bonded to fructose

1. CARBOHYDRATES • Polysaccharide- polymer carbohydrate. Made up of long chains of glucose • Starch- a spiral chain of glucose. Made by plants as a storage molecule • Glycogen- a chain of glucose made by animals to store excess glucose • Cellulose- chain of glucose hooked together like a chained link fence • Made by plants for cell wall support • Humans cannot digest
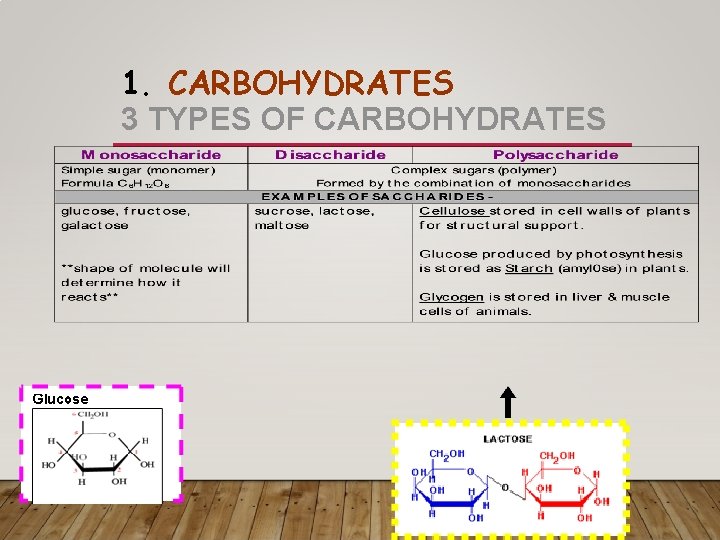
1. CARBOHYDRATES 3 TYPES OF CARBOHYDRATES
2. LIPIDS consist of C, H, O • hydrophobic – insoluble in water • FUNCTIONS: • Energy Storage – breakdown of lipids provides long-term energy supply; excess is stored in fat cells; yields twice as much energy as carbohydrates • Component of cell membrane (phospholipids) • provides cushions, insulates and waterproofing (wax)
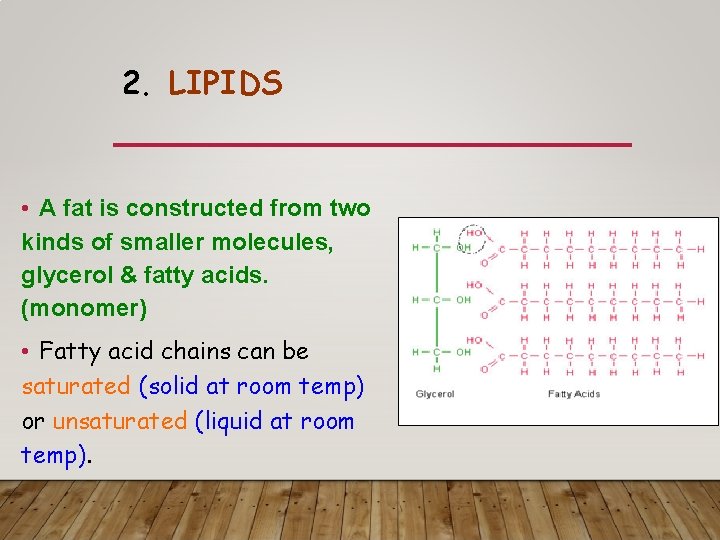
2. LIPIDS • A fat is constructed from two kinds of smaller molecules, glycerol & fatty acids. (monomer) • Fatty acid chains can be saturated (solid at room temp) or unsaturated (liquid at room temp).

2. LIPIDS • Fats- solid at room temperature, Saturated Fatty Acid • Oils- liquid at room temperature, Unsaturated Fatty Acid • Waxes- waterproofing (skin, fur, feathers) protective coating on plants • Steroids- maintain and control functions throughout the body
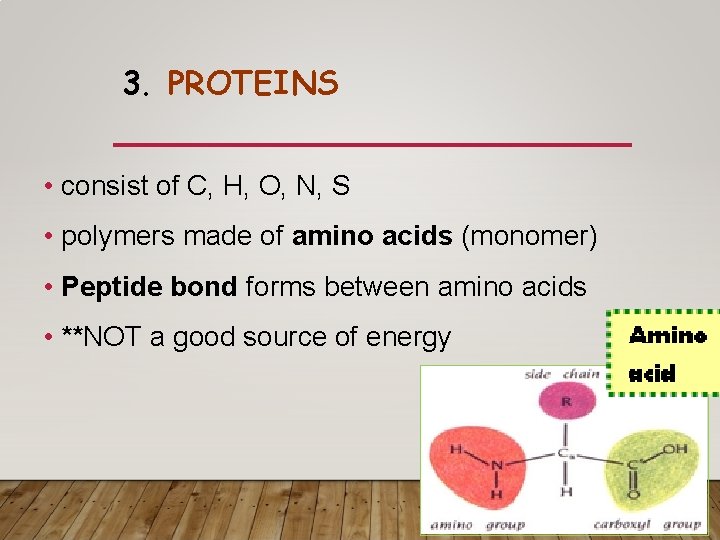
3. PROTEINS • consist of C, H, O, N, S • polymers made of amino acids (monomer) • Peptide bond forms between amino acids • **NOT a good source of energy
3. PROTEINS FUNCTIONS: Structural element of hair/nails (keratin) & bone/cartilage (collagen) Increase rate of reaction as an enzyme (biological catalyst) Transport and storage of molecules Control of metabolism Receptor proteins - signaling from cell to cell Tissue defense (antibodies)
4. NUCLEIC ACIDS consist of C, H, O, N, P polymer of nucleotides (monomer) stores and transmits genetic information • Two types of nucleic acids – 1. DNA (deoxyribonucleic acid) – double strand of genetic information 2. RNA (ribonucleic acid) – single strand copy of DNA used to build proteins
ENZYMES • Enzymes are a type of protein • They speed up a chemical reaction (serve as a catalyst) by lowering the activation rate • Almost all metabolic reactions are helped along by enzymes • Enzymes function at a specific temperature, p. H, and concentration (amount of enzyme) • Ex: pepsin enzyme in the stomach works at a p. H of 2
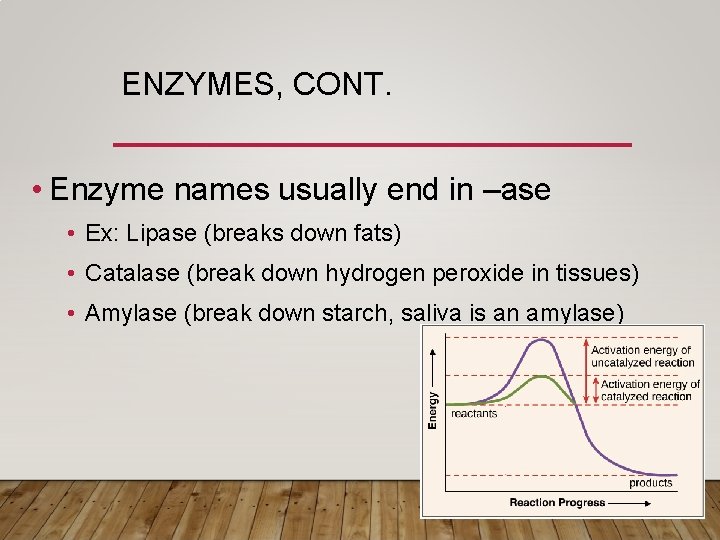
ENZYMES, CONT. • Enzyme names usually end in –ase • Ex: Lipase (breaks down fats) • Catalase (break down hydrogen peroxide in tissues) • Amylase (break down starch, saliva is an amylase)
- Slides: 15