Organic chemistry Organic Chemistry Organic means living Hydrocarbons

Organic chemistry
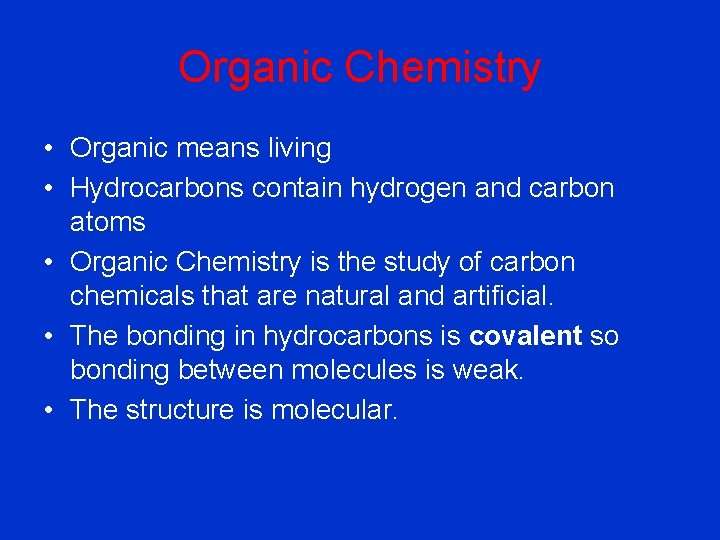
Organic Chemistry • Organic means living • Hydrocarbons contain hydrogen and carbon atoms • Organic Chemistry is the study of carbon chemicals that are natural and artificial. • The bonding in hydrocarbons is covalent so bonding between molecules is weak. • The structure is molecular.
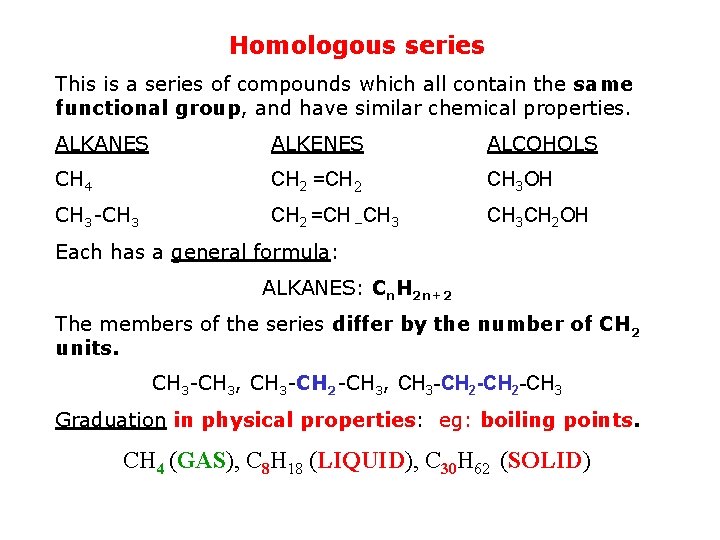
Homologous series This is a series of compounds which all contain the same functional group, and have similar chemical properties. ALKANES ALKENES ALCOHOLS CH 4 CH 2 =CH 2 CH 3 OH CH 3 -CH 3 CH 2 =CH –CH 3 CH 2 OH Each has a general formula: ALKANES: Cn. H 2 n+2 The members of the series differ by the number of CH 2 units. CH 3 -CH 3, CH 3 -CH 2 -CH 3 Graduation in physical properties: eg: boiling points. CH 4 (GAS), C 8 H 18 (LIQUID), C 30 H 62 (SOLID)

IUPAC The International Union of Pure and Applied Chemistry Why? Historical names (e. g. acetic acid, formic acid) meant nothing to other scientists in different countries

IUPAC Rules for Alkane Nomenclature 1. Find and name the longest continuous carbon chain. AKA parent chain 2. Identify and name groups attached to this chain. 3. Number the chain consecutively, starting at the end nearest a substituent group. 4. Designate the location of each substituent group by an appropriate number and name. 5. Assemble the name, listing groups in alphabetical order. The prefixes di, tri, tetra etc. , used to designate several groups of the same kind, are not considered when alphabetizing.
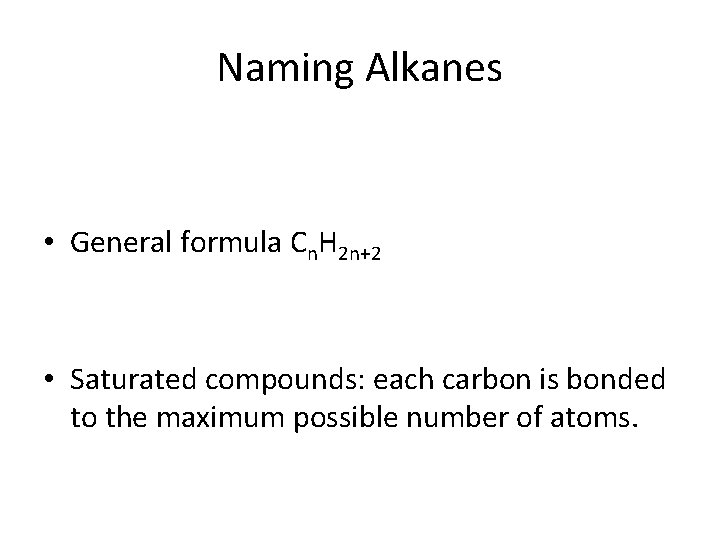
Naming Alkanes • General formula Cn. H 2 n+2 • Saturated compounds: each carbon is bonded to the maximum possible number of atoms.
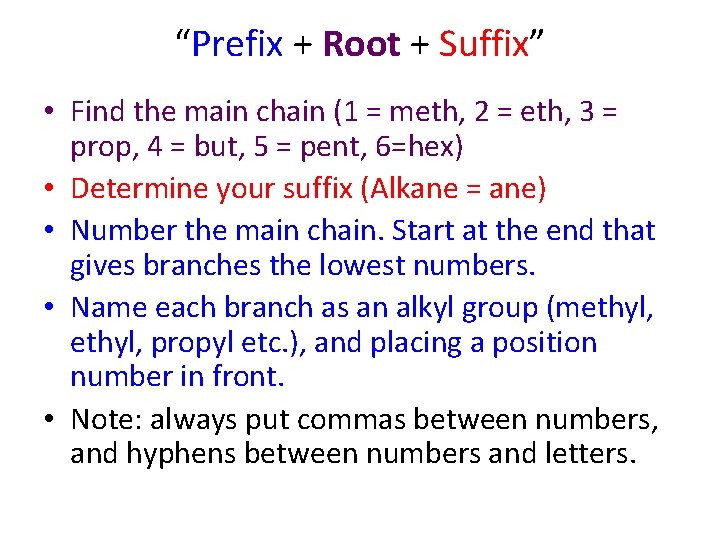
“Prefix + Root + Suffix” • Find the main chain (1 = meth, 2 = eth, 3 = prop, 4 = but, 5 = pent, 6=hex) • Determine your suffix (Alkane = ane) • Number the main chain. Start at the end that gives branches the lowest numbers. • Name each branch as an alkyl group (methyl, propyl etc. ), and placing a position number in front. • Note: always put commas between numbers, and hyphens between numbers and letters.
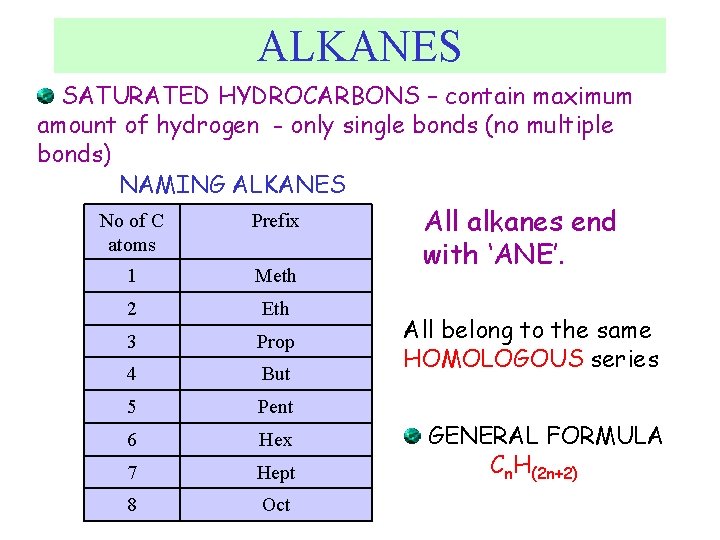
ALKANES SATURATED HYDROCARBONS – contain maximum amount of hydrogen - only single bonds (no multiple bonds) NAMING ALKANES No of C atoms Prefix 1 Meth 2 Eth 3 Prop 4 But 5 Pent 6 Hex 7 Hept 8 Oct All alkanes end with ‘ANE’. All belong to the same HOMOLOGOUS series GENERAL FORMULA Cn. H(2 n+2)
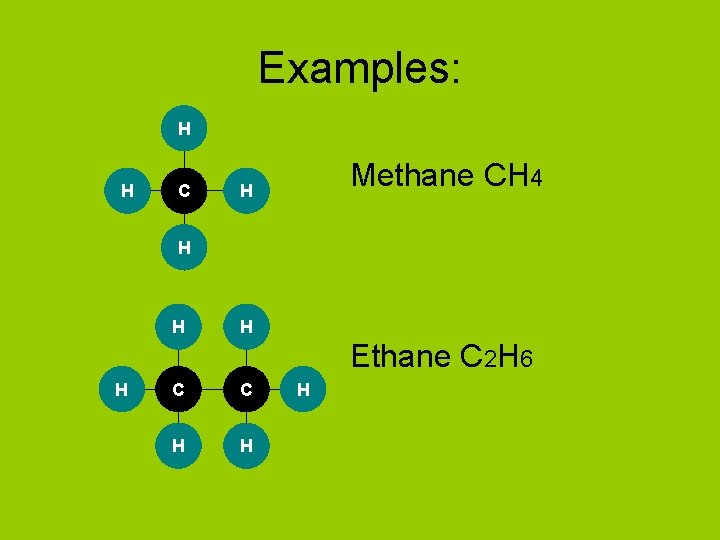
Examples: H H C Methane CH 4 H H Ethane C 2 H 6 H C C H H H
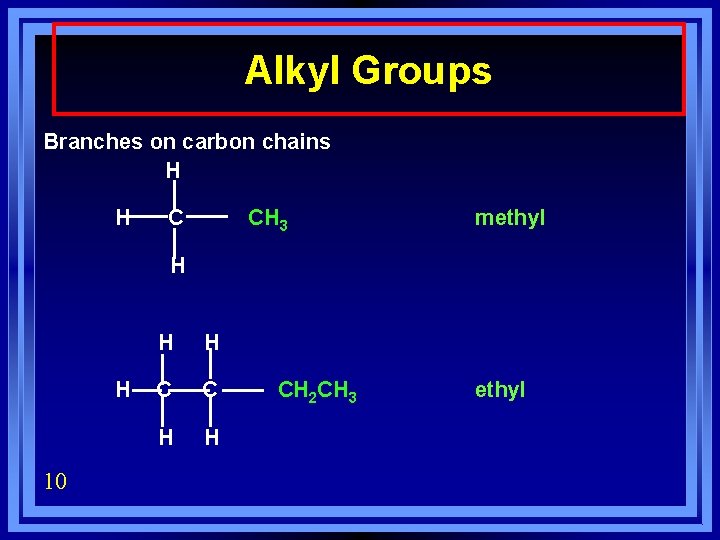
Alkyl Groups Branches on carbon chains H H C CH 3 methyl H H 10 H H C C H H CH 2 CH 3 ethyl
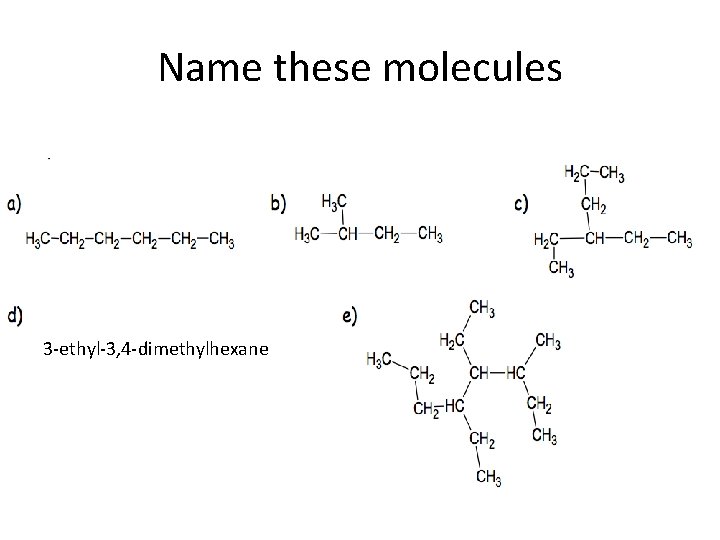
Name these molecules 3 -ethyl-3, 4 -dimethylhexane
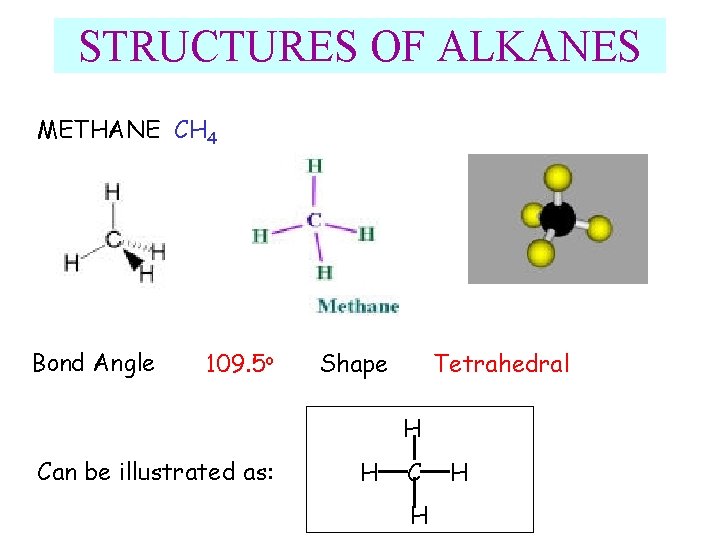
STRUCTURES OF ALKANES METHANE CH 4 Bond Angle 109. 5 o Shape Tetrahedral H Can be illustrated as: H C H H
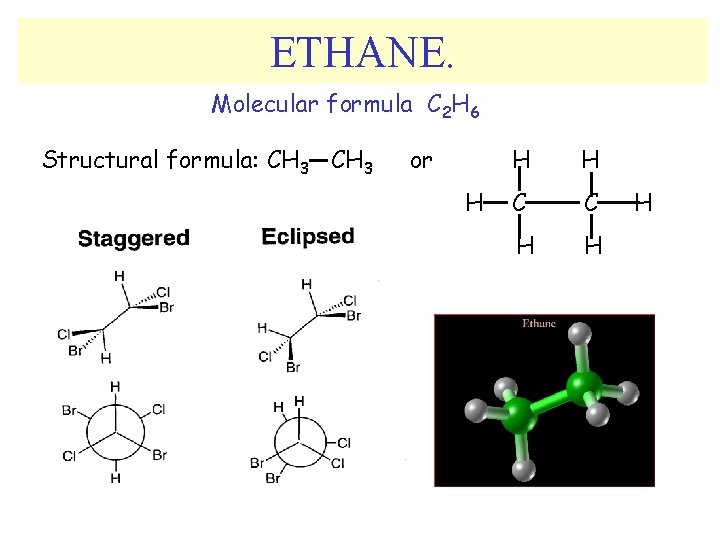
ETHANE. Molecular formula C 2 H 6 Structural formula: CH 3 or H H H C C H H H
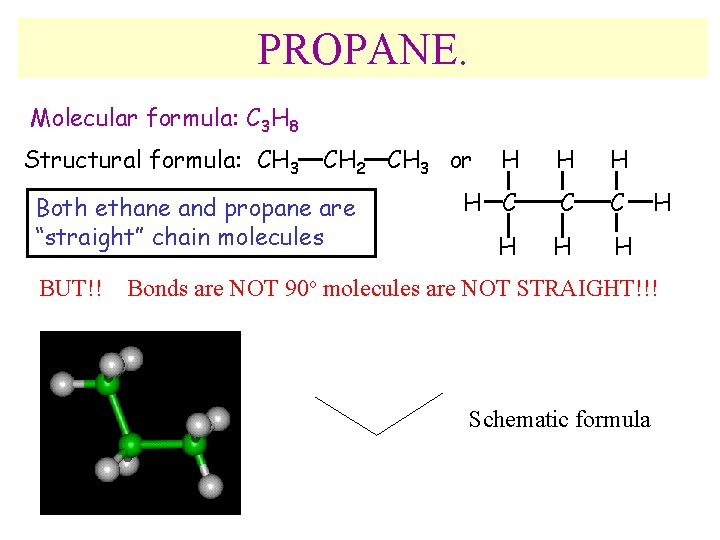
PROPANE. Molecular formula: C 3 H 8 Structural formula: CH 3 CH 2 CH 3 or Both ethane and propane are “straight” chain molecules BUT!! H H C C C H H Bonds are NOT 90 o molecules are NOT STRAIGHT!!! Schematic formula
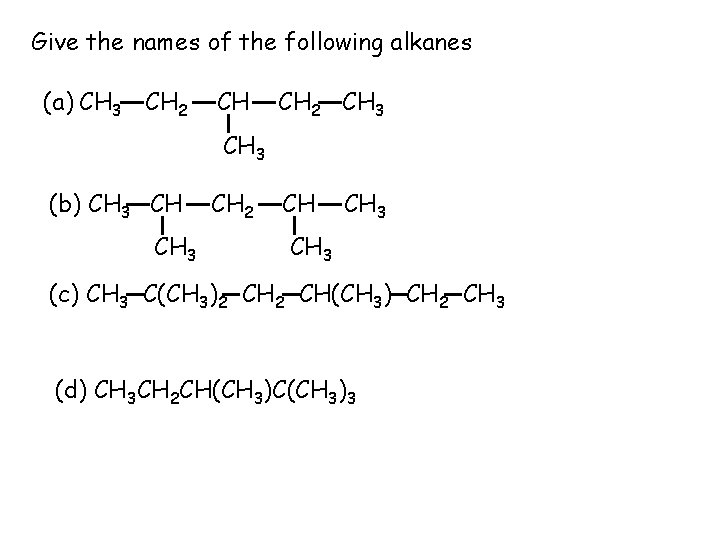
Give the names of the following alkanes (a) CH 3 CH 2 CH 3 (b) CH 3 CH 2 CH CH 3 (c) CH 3 C(CH 3)2 CH(CH 3) CH 2 CH 3 (d) CH 3 CH 2 CH(CH 3)C(CH 3)3
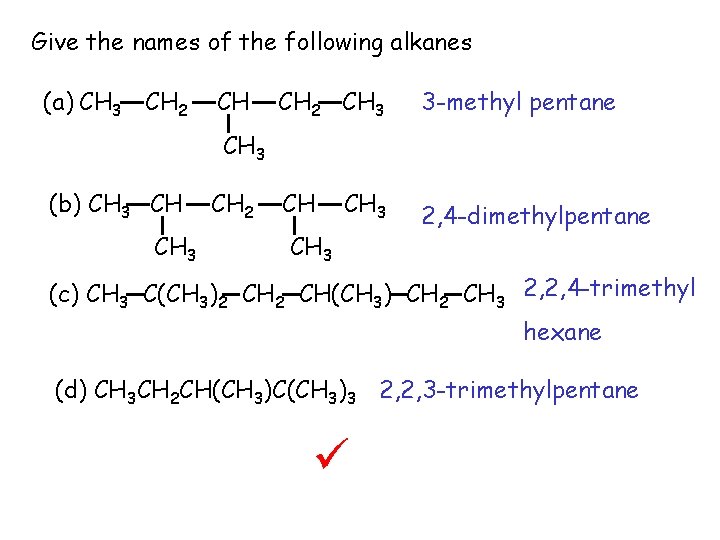
Give the names of the following alkanes (a) CH 3 CH 2 CH 3 3 -methyl pentane CH 3 (b) CH 3 CH 2 CH CH 3 2, 4 -dimethylpentane (c) CH 3 C(CH 3)2 CH(CH 3) CH 2 CH 3 2, 2, 4 -trimethyl hexane (d) CH 3 CH 2 CH(CH 3)C(CH 3)3 2, 2, 3 -trimethylpentane ü
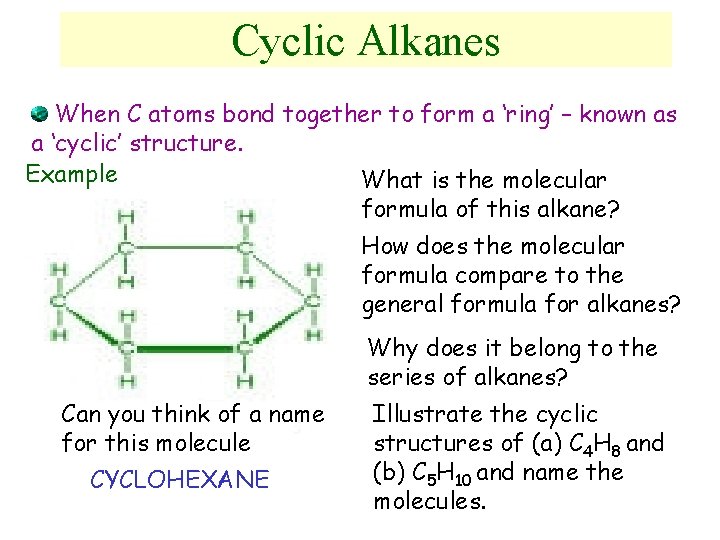
Cyclic Alkanes When C atoms bond together to form a ‘ring’ – known as a ‘cyclic’ structure. Example What is the molecular formula of this alkane? How does the molecular formula compare to the general formula for alkanes? Why does it belong to the series of alkanes? Can you think of a name for this molecule CYCLOHEXANE Illustrate the cyclic structures of (a) C 4 H 8 and (b) C 5 H 10 and name the molecules.

Commas and dashes • Use commas between numbers • Use dashes between numbers and letters • And one other thing; when arranging your substituent groups in alphabetical order the di, tri, tetra, etc do not count

Correct the mistakes You have 1 minute to discuss with a partner all the mistakes in the naming of the following compounds
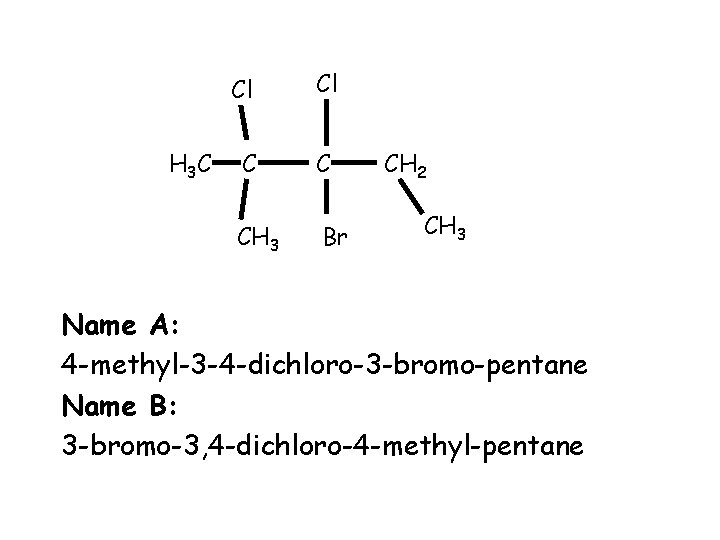
H 3 C Cl Cl C C CH 3 Br CH 2 CH 3 Name A: 4 -methyl-3 -4 -dichloro-3 -bromo-pentane Name B: 3 -bromo-3, 4 -dichloro-4 -methyl-pentane
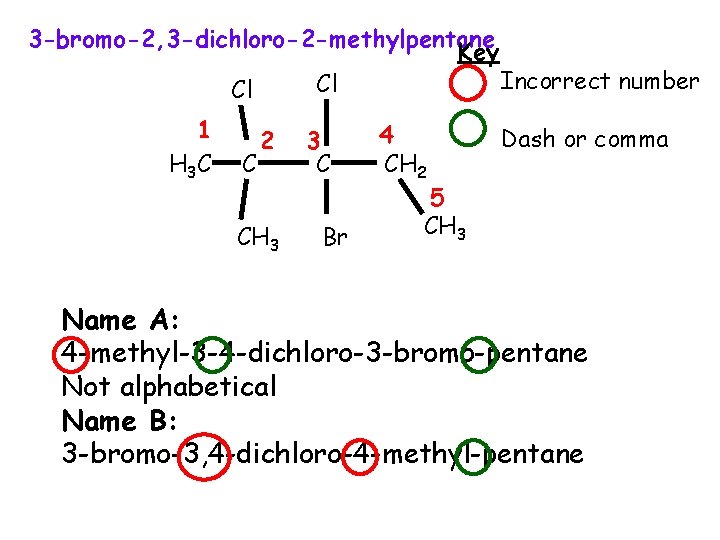
3 -bromo-2, 3 -dichloro-2 -methylpentane Key Incorrect number Cl Cl 1 H 3 C C 2 CH 3 3 C Br 4 CH 2 Dash or comma 5 CH 3 Name A: 4 -methyl-3 -4 -dichloro-3 -bromo-pentane Not alphabetical Name B: 3 -bromo-3, 4 -dichloro-4 -methyl-pentane
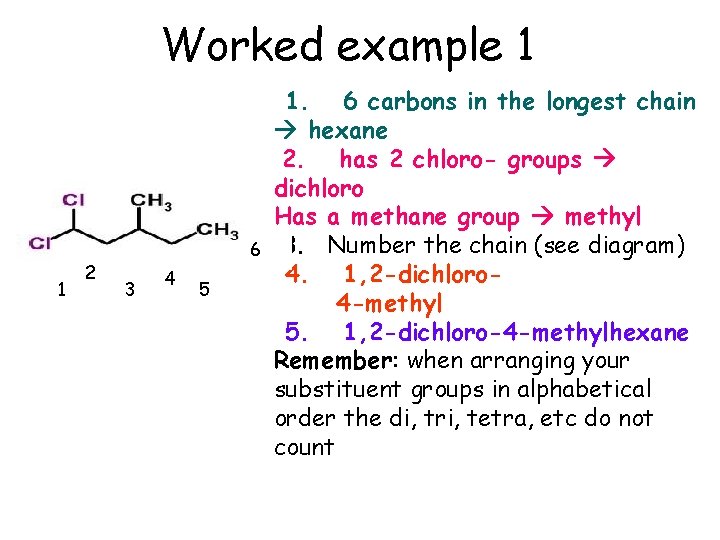
Worked example 1 1 2 2 1 3 3 6 4 5 1. 6 carbons in the longest chain hexane 2. has 2 chloro- groups dichloro Has a methane group methyl 3. Number the chain (see diagram) 4. 1, 2 -dichloro 4 -methyl 5. 1, 2 -dichloro-4 -methylhexane Remember: when arranging your substituent groups in alphabetical order the di, tri, tetra, etc do not count
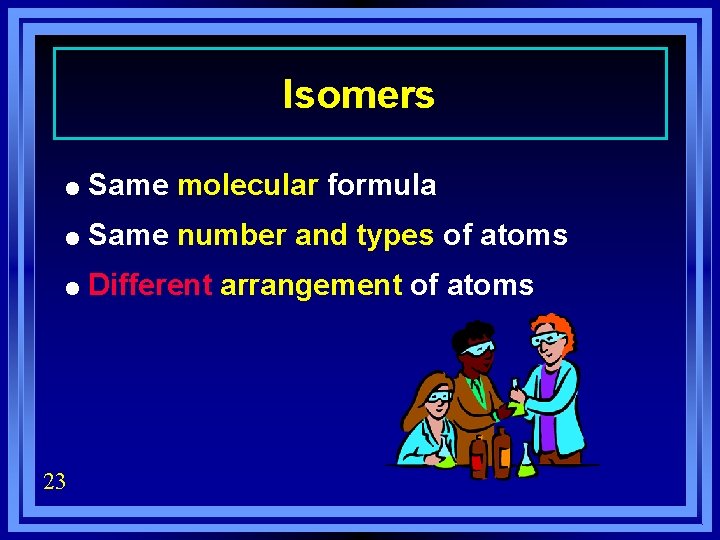
Isomers = Same molecular formula = Same number and types of atoms = Different 23 arrangement of atoms
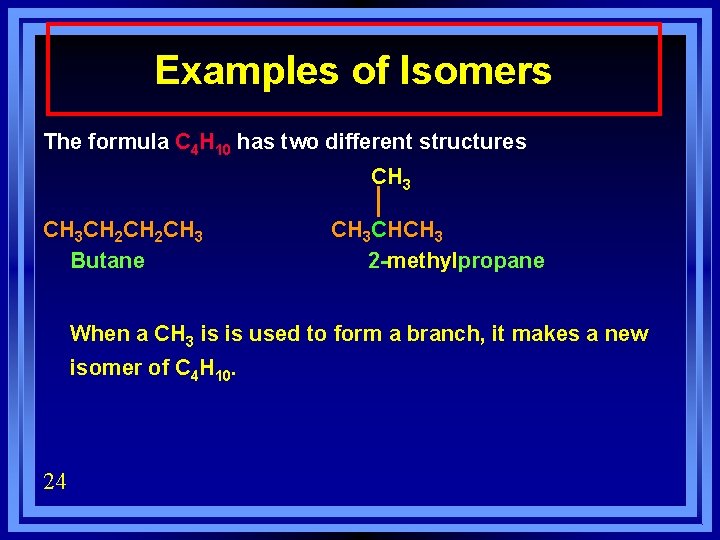
Examples of Isomers The formula C 4 H 10 has two different structures CH 3 CH 2 CH 3 Butane CH 3 CHCH 3 2 -methylpropane When a CH 3 is is used to form a branch, it makes a new isomer of C 4 H 10. 24
Structural Isomers l Compounds pp that have the same molecular formula, but different molecular structures, are called structural isomers l Butane and 2 -methylpropane on previous slide. l Also have different properties, such as b. p. , m. p. , and reactivity 25
Geometric Isomers pp l There is a lack of rotation around a carbon to carbon multiple bond – has an important structural implication – Two possible methyl arrangements: 1. trans configuration - substituted groups on opposite sides of double bond 2. cis configuration - same side 26
Geometric Isomers l Differ pp only in the geometry of their substituted groups l Like other structural isomers, have different physical and chemical properties 27
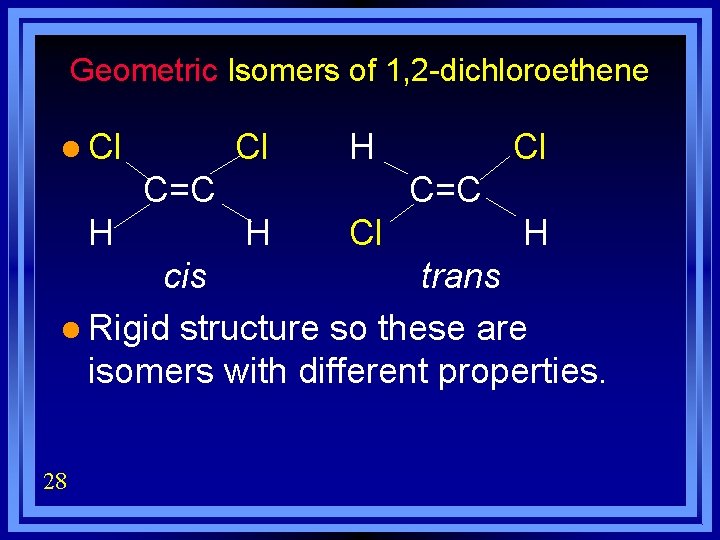
Geometric Isomers of 1, 2 -dichloroethene l Cl Cl H C=C H Cl H cis trans l Rigid structure so these are isomers with different properties. 28
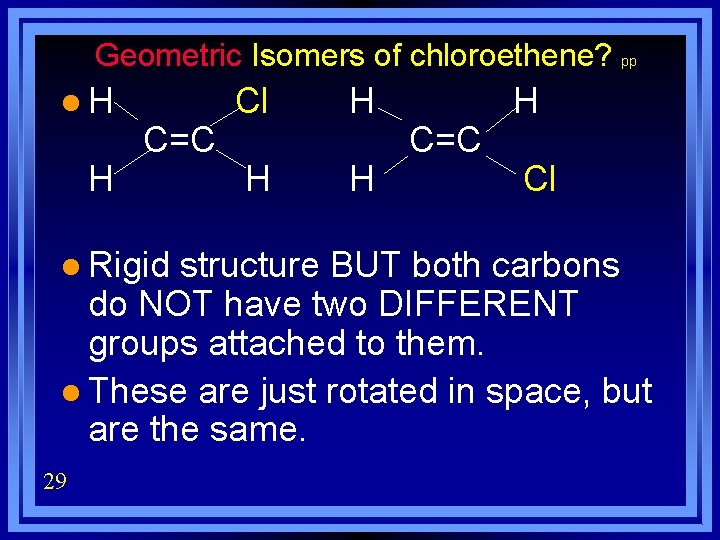
Geometric Isomers of chloroethene? pp l. H H C=C l Rigid Cl H H H C=C H Cl structure BUT both carbons do NOT have two DIFFERENT groups attached to them. l These are just rotated in space, but are the same. 29
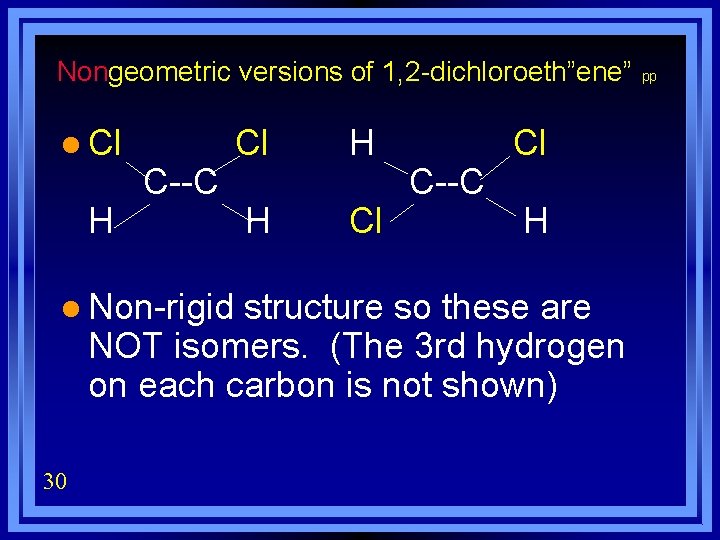
Nongeometric versions of 1, 2 -dichloroeth”ene” l Cl H C--C l Non-rigid Cl H H Cl C--C Cl H structure so these are NOT isomers. (The 3 rd hydrogen on each carbon is not shown) 30 pp
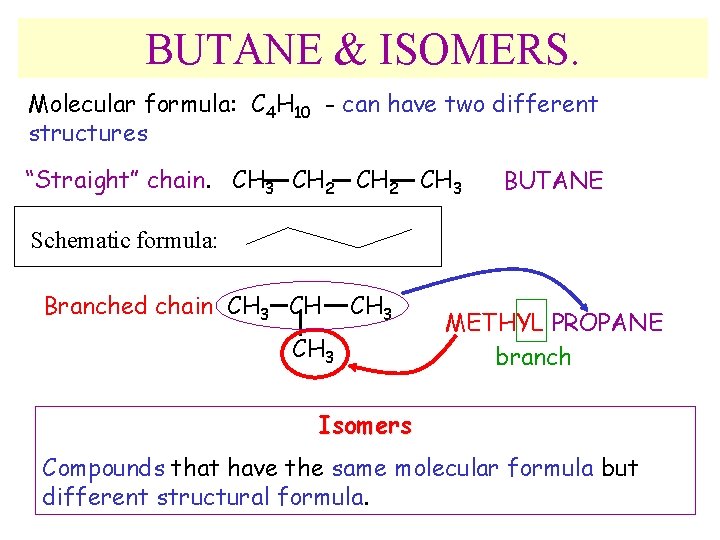
BUTANE & ISOMERS. Molecular formula: C 4 H 10 - can have two different structures “Straight” chain. CH 3 CH 2 CH 3 BUTANE Schematic formula: Branched chain CH 3 METHYL PROPANE branch Isomers Compounds that have the same molecular formula but different structural formula.
TASK: Illustrate the structures of the three different isomers of C 5 H 12.
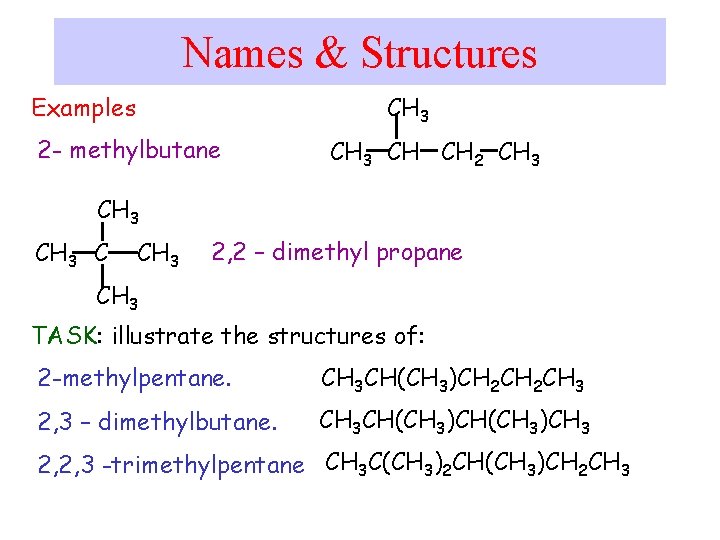
Names & Structures CH 3 Examples 2 - methylbutane CH 3 CH CH 2 CH 3 C CH 3 2, 2 – dimethyl propane CH 3 TASK: illustrate the structures of: 2 -methylpentane. CH 3 CH(CH 3)CH 2 CH 3 2, 3 – dimethylbutane. CH 3 CH(CH 3)CH 3 2, 2, 3 -trimethylpentane CH 3 C(CH 3)2 CH(CH 3)CH 2 CH 3
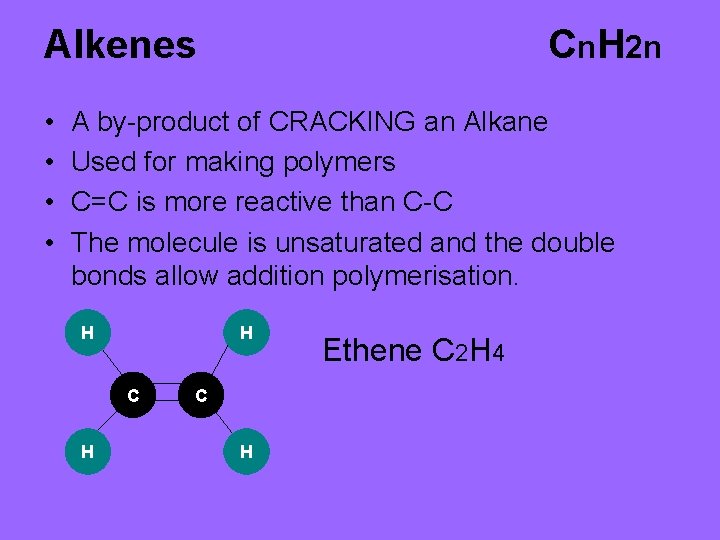
Alkenes • • Cn. H 2 n A by-product of CRACKING an Alkane Used for making polymers C=C is more reactive than C-C The molecule is unsaturated and the double bonds allow addition polymerisation. H H C H Ethene C 2 H 4
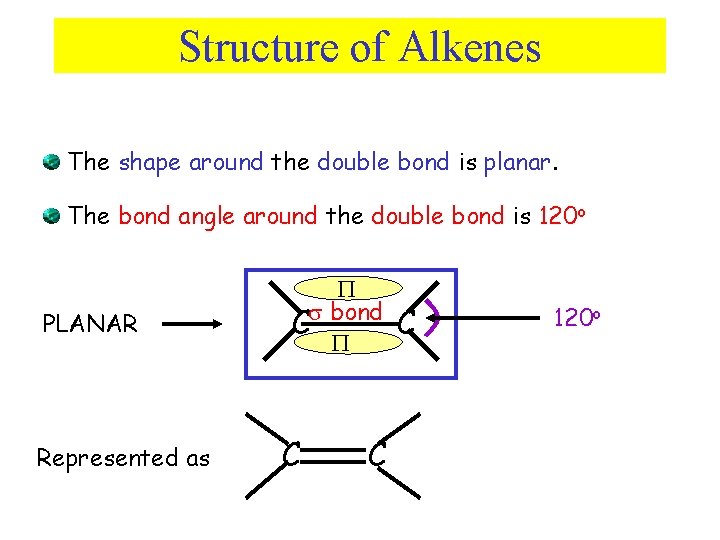
Structure of Alkenes The shape around the double bond is planar. The bond angle around the double bond is 120 o PLANAR Represented as C bond C C C 120 o
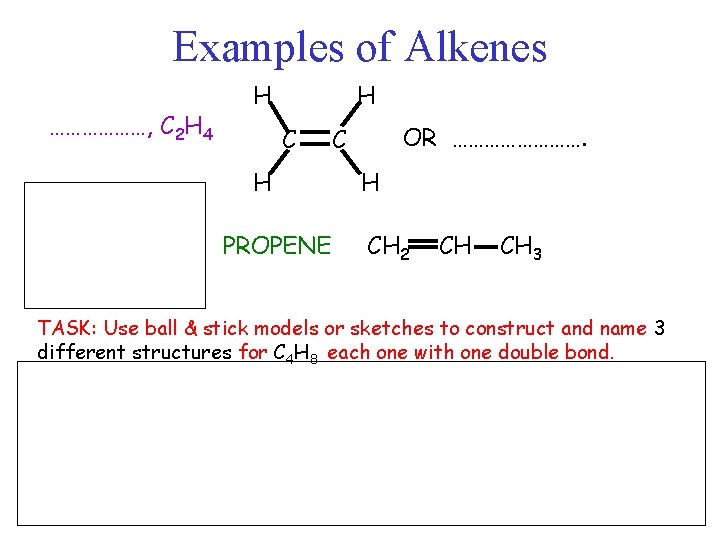
Examples of Alkenes ………………, C 2 H 4 H H C H PROPENE OR …………. C H CH 2 CH CH 3 TASK: Use ball & stick models or sketches to construct and name 3 different structures for C 4 H 8 each one with one double bond.
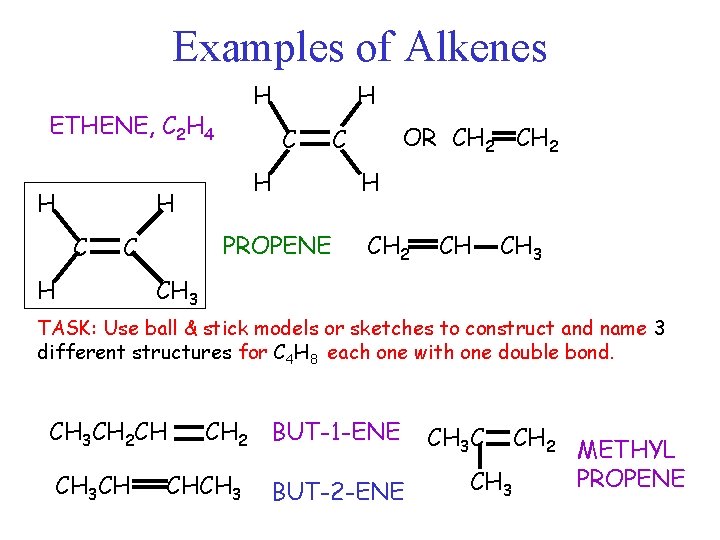
Examples of Alkenes H ETHENE, C 2 H 4 H C H H C PROPENE C H H OR CH 2 C H CH 2 CH CH 3 TASK: Use ball & stick models or sketches to construct and name 3 different structures for C 4 H 8 each one with one double bond. CH 3 CH 2 CH CH 3 CH CH 2 BUT-1 -ENE CHCH 3 BUT-2 -ENE CH 3 C CH 3 CH 2 METHYL PROPENE
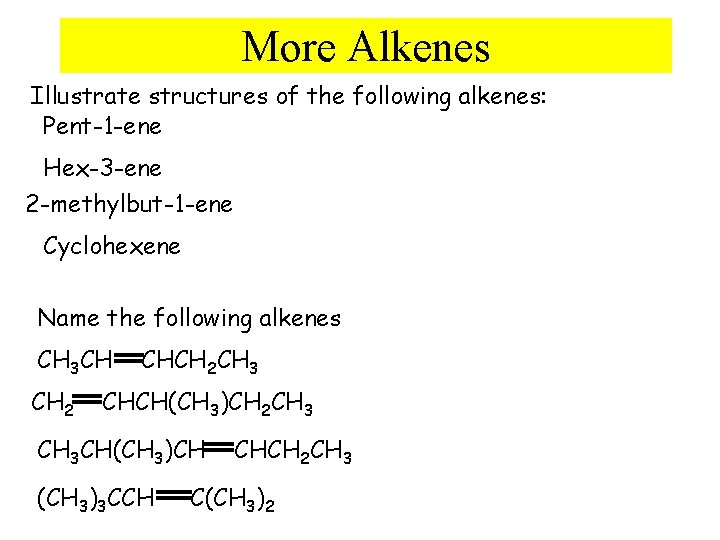
More Alkenes Illustrate structures of the following alkenes: Pent-1 -ene Hex-3 -ene 2 -methylbut-1 -ene Cyclohexene Name the following alkenes CH 3 CH CH 2 CHCH 2 CH 3 CHCH(CH 3)CH 2 CH 3 CH(CH 3)CH (CH 3)3 CCH CHCH 2 CH 3 C(CH 3)2
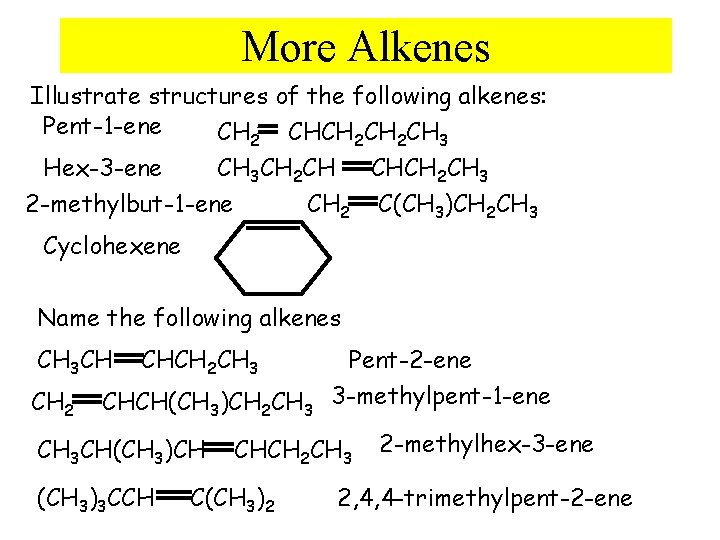
More Alkenes Illustrate structures of the following alkenes: Pent-1 -ene CH 2 CHCH 2 CH 3 CH 2 CH CHCH 2 CH 3 Hex-3 -ene CH 2 C(CH 3)CH 2 CH 3 2 -methylbut-1 -ene Cyclohexene Name the following alkenes CH 3 CH CH 2 CHCH 2 CH 3 CHCH(CH 3)CH 2 CH 3 CH(CH 3)CH (CH 3)3 CCH Pent-2 -ene 3 -methylpent-1 -ene CHCH 2 CH 3 C(CH 3)2 2 -methylhex-3 -ene 2, 4, 4 -trimethylpent-2 -ene
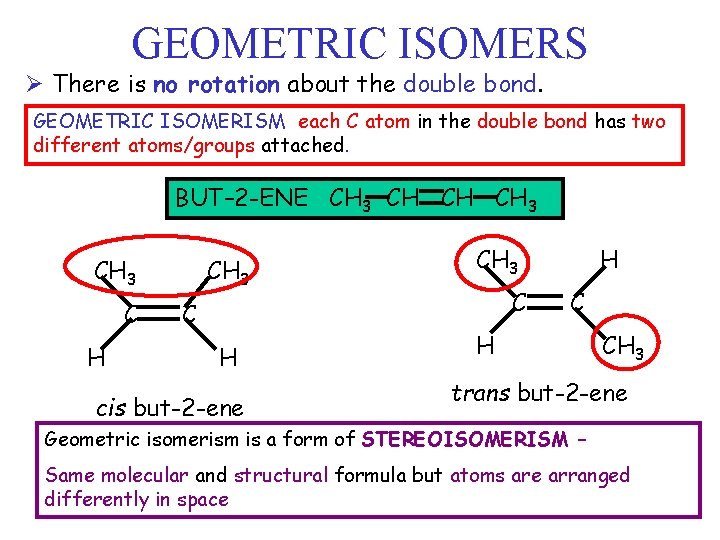
GEOMETRIC ISOMERS Ø There is no rotation about the double bond. GEOMETRIC ISOMERISM each C atom in the double bond has two different atoms/groups attached. BUT– 2 -ENE CH 3 CH CH CH 3 C H cis but-2 -ene CH 3 C H CH 3 trans but-2 -ene Geometric isomerism is a form of STEREOISOMERISM – Same molecular and structural formula but atoms are arranged differently in space
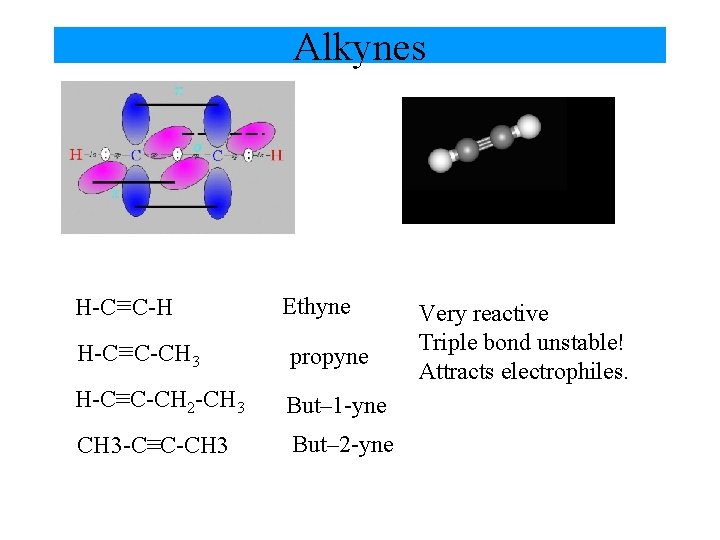
Alkynes H-C≡C-H Ethyne H-C≡C-CH 3 propyne H-C≡C-CH 2 -CH 3 But– 1 -yne CH 3 -C≡C-CH 3 But– 2 -yne Very reactive Triple bond unstable! Attracts electrophiles.
- Slides: 41