Organic Chemistry Objectives Define the characteristics of organic
Organic Chemistry Objectives • Define the characteristics of organic substances • Explain the relationship among different types of organic compounds • Define hydrocarbons • Define the hydrocarbon prefixes • Know the general formulas for alkanes, alkenes, and alkynes
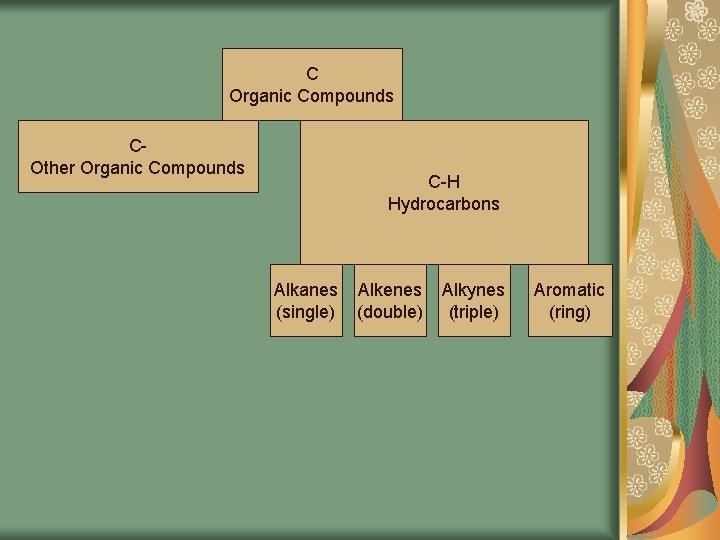
C Organic Compounds COther Organic Compounds C-H Hydrocarbons Alkanes (single) Alkenes (double) Alkynes (triple) Aromatic (ring)

Characteristics Contain carbon Most linked together by covalent bonds (pairs of shared electrons) Generally nonpolar (equal attracting power) so usually insoluble in water (exception: acetic acid) Tend to dissolve in nonpolar solvents

Molecules are not composed of ions (exception: some organic acids) so not electrolytes Low melting points Reactions involving organic compounds are slow Decomposed by heat at relatively low temperatures

Activity Draw the map of the relationships among different types of organic compounds Saturated (single bonds) – contains the maximum number of hydrogens Unsaturated – organic compounds that contain double or triple carbon bonds

Hydrocarbons Compounds composed ONLY of carbon and hydrogen atoms bonded to each other covalently They are alkanes, alkenes, alkynes, and arenes Used as fuels or as building blocks

Prefixes Meth 1 Eth 2 Prop 3 But 4 Pent 5 Hex 6 Hept 7 Oct 8 Non 9 Dec 10

General Formulas Alkanes Cn. H 2 n+2 Alkenes Cn. H 2 n Alkynes Cn. H 2 n-2

Objectives Define the –yl groups Draw the structural formulas of the various alkane derived compounds Define isomers Draw the structural formulas of various isomers Define the functional groups
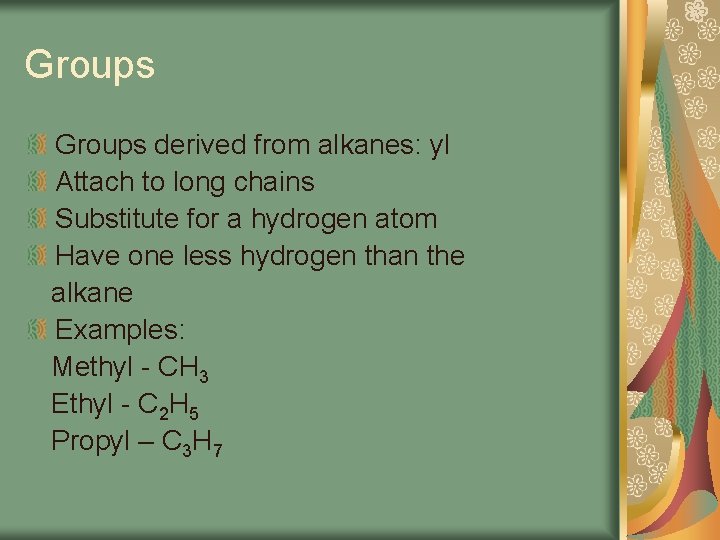
Groups derived from alkanes: yl Attach to long chains Substitute for a hydrogen atom Have one less hydrogen than the alkane Examples: Methyl - CH 3 Ethyl - C 2 H 5 Propyl – C 3 H 7

Rules for Attaching Groups Refer to the IUPAC rules for naming branched-chain alkanes 1. 2. 3. 4. Activity: Draw 2 -ethylpentane Draw 3 -ethylpentane Draw 4 -ethyl-2, 3, 4 -trimethyloctane Complete the Practice Problems
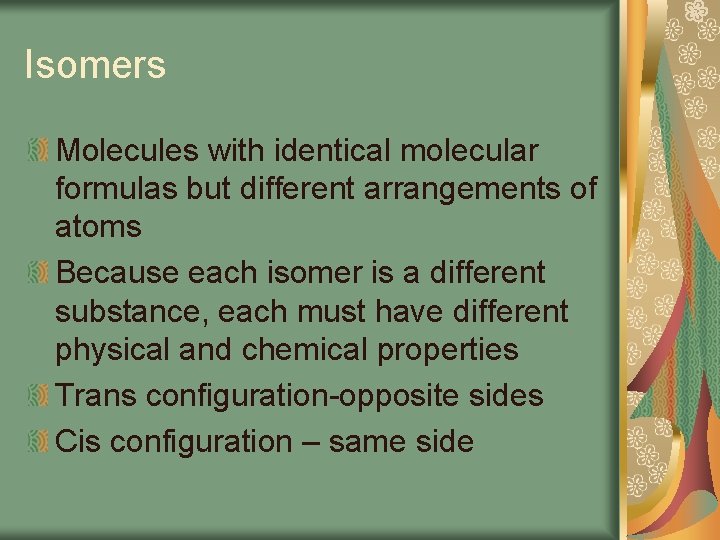
Isomers Molecules with identical molecular formulas but different arrangements of atoms Because each isomer is a different substance, each must have different physical and chemical properties Trans configuration-opposite sides Cis configuration – same side
- Slides: 12