Organic Chemistry Introduction to Hydrocarbons Alkanes What is

Organic Chemistry Introduction to Hydrocarbons Alkanes
What is Organic Chemistry • This is the branch of chemistry describing the structure and behaviors of compounds composed primarily of the elements Carbon and Hydrogen. • Just like general chemistry, there a number of classes of compounds within this branch of the field. • Hydrocarbons are simply one of those groups of classes.
Hydrocarbons • This group of compounds within the field of Organic Chemistry consists of compounds containing ONLY carbon and hydrogen. • There are three separate classes of hydrocarbons – the bonding between the carbon atoms differentiates the classes.
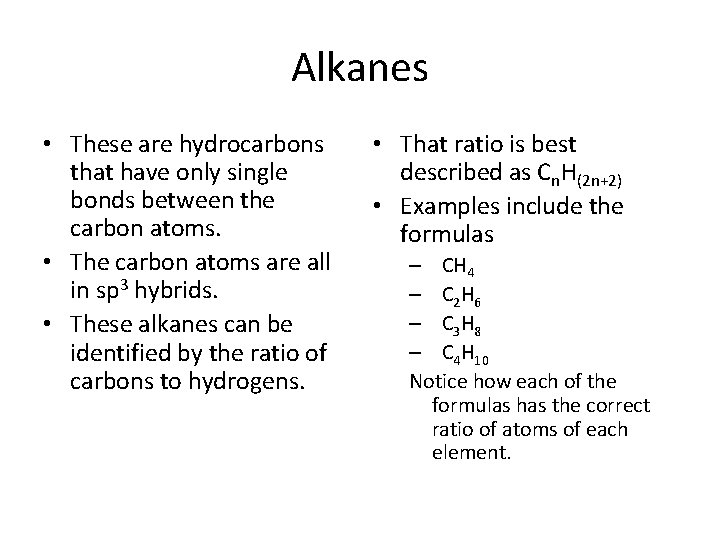
Alkanes • These are hydrocarbons that have only single bonds between the carbon atoms. • The carbon atoms are all in sp 3 hybrids. • These alkanes can be identified by the ratio of carbons to hydrogens. • That ratio is best described as Cn. H(2 n+2) • Examples include the formulas – CH 4 – C 2 H 6 – C 3 H 8 – C 4 H 10 Notice how each of the formulas has the correct ratio of atoms of each element.

Drawing Alkanes • Remember that the Carbon atoms will be in sp 3 hybrids and therefore, each carbon atom will form 4 bonds. • If there is more than one carbon atom, start by connecting them in a chain. • Then add the Hydrogen atoms on the outside of that carbon “backbone”.
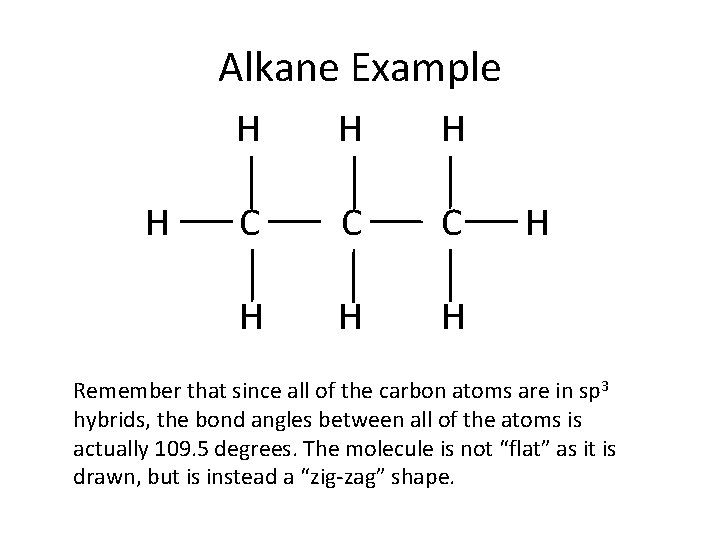
Alkane Example H H C C C H H Remember that since all of the carbon atoms are in sp 3 hybrids, the bond angles between all of the atoms is actually 109. 5 degrees. The molecule is not “flat” as it is drawn, but is instead a “zig-zag” shape.

Naming Alkanes • The key to naming these compounds is to learn the prefixes that correspond to the number of carbon atoms in the formula. • All that has to be added is the suffix “-ane” because the compound is an alkane. • • • C C 2 C 3 C 4 C 5 C 6 C 7 C 8 C 9 C 10 = = = = = meth prop but pent hex hept oct non dec
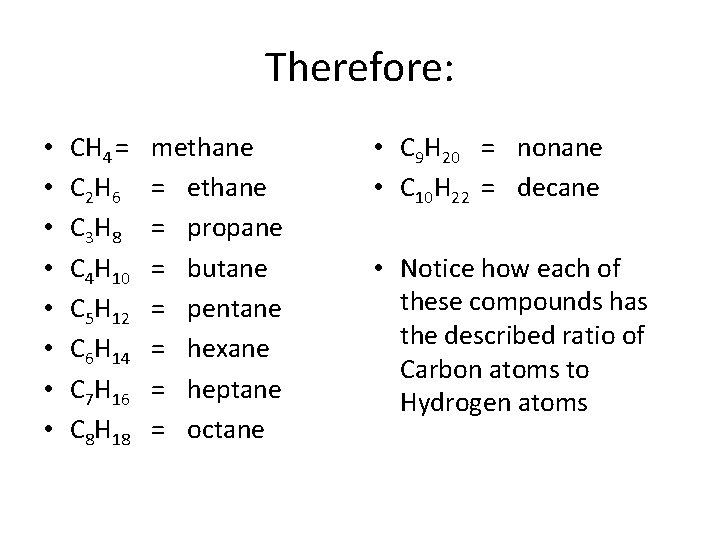
Therefore: • • CH 4 = C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 methane = propane = butane = pentane = hexane = heptane = octane • C 9 H 20 = nonane • C 10 H 22 = decane • Notice how each of these compounds has the described ratio of Carbon atoms to Hydrogen atoms
- Slides: 8