Organic Chemistry In a nutshell Important Elements CARBON

Organic Chemistry In a nutshell. .

Important Elements • CARBON is the most important element in organic chemistry • Also important… – Hydrogen – Oxygen – Nitrogen – Sulphur – Phosphorus – Chlorine
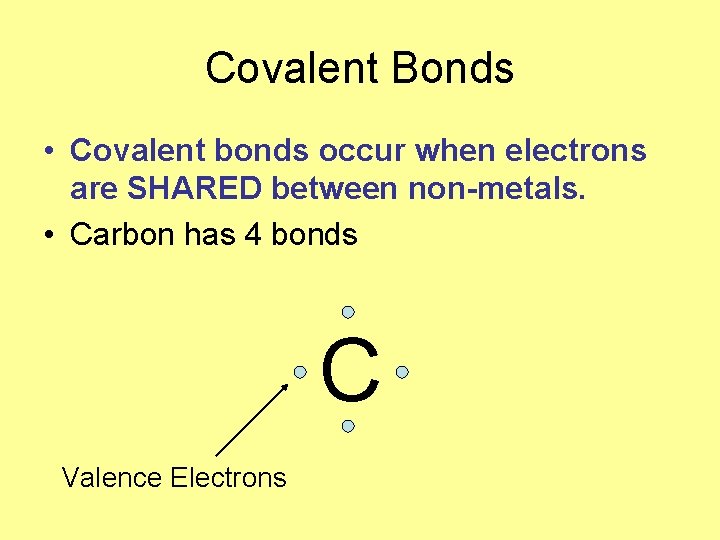
Covalent Bonds • Covalent bonds occur when electrons are SHARED between non-metals. • Carbon has 4 bonds C Valence Electrons

Other Bonds • • Hydrogen one bond Oxygen two bonds Nitrogen three bonds Sulphur two bonds (varies) Phosphorus three bonds (varies) Chlorine one bond Bonds = # valence electrons needed to be Happy
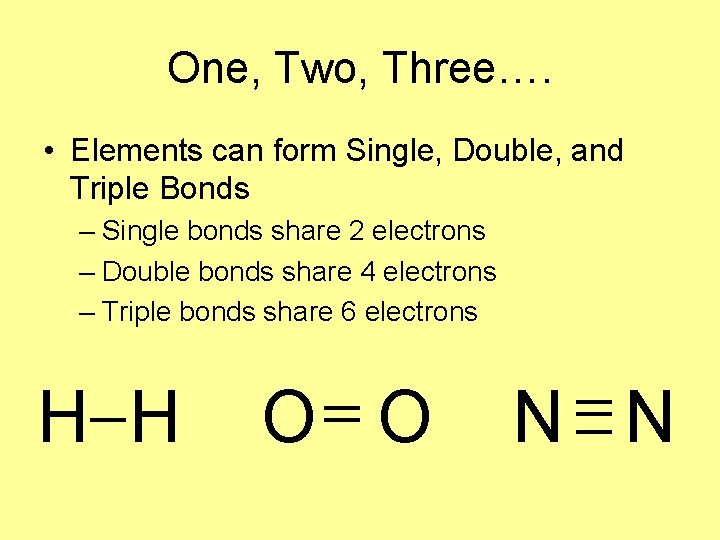
One, Two, Three…. • Elements can form Single, Double, and Triple Bonds – Single bonds share 2 electrons – Double bonds share 4 electrons – Triple bonds share 6 electrons H H O O N N

Hydrocarbons • Hydrocarbons are made up of hydrogen and carbon only. • They are divided up based on the number of bonds. – alkanes have Single Bonds – alkenes have Double Bonds – alkynes have Triple Bonds
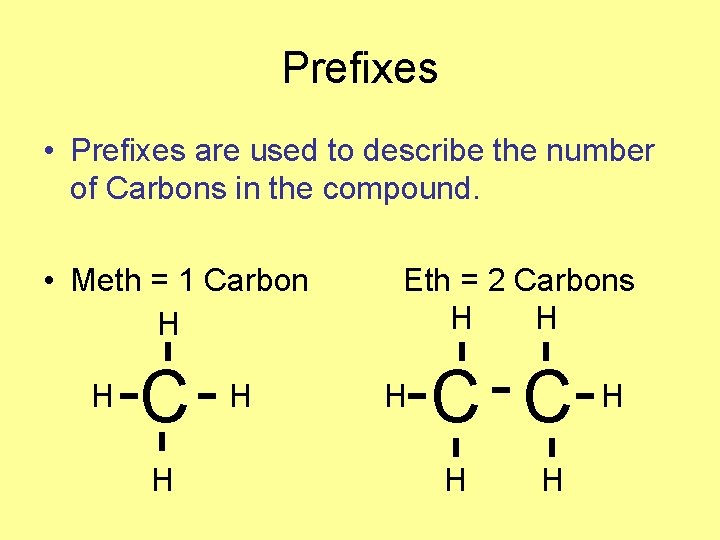
Prefixes • Prefixes are used to describe the number of Carbons in the compound. • Meth = 1 Carbon H H C H H Eth = 2 Carbons H H H C C H H H

Prefixes • Meth 1 carbon Hex • Eth 2 carbons Hept 7 carbons • Prop 3 carbons Oct 8 carbons • But 4 carbons Non 9 carbons • Pent 5 carbons Dec 10 carbons 6 carbons
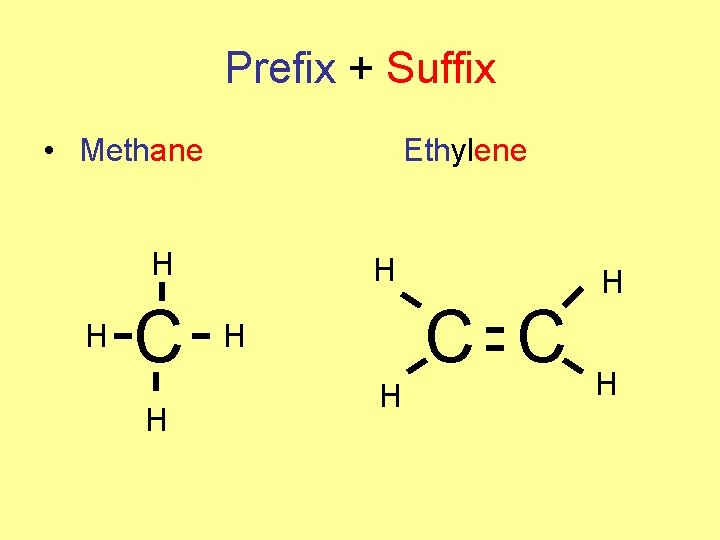
Prefix + Suffix • Methane Ethylene H H C C H H
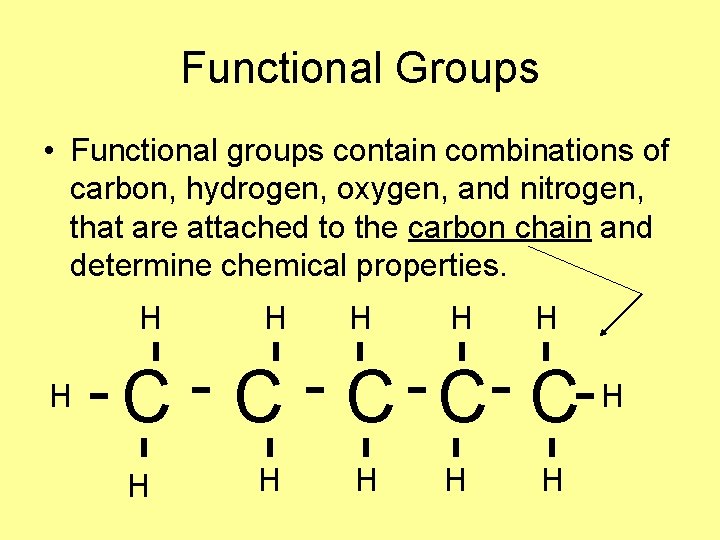
Functional Groups • Functional groups contain combinations of carbon, hydrogen, oxygen, and nitrogen, that are attached to the carbon chain and determine chemical properties. H H H C C C H H H
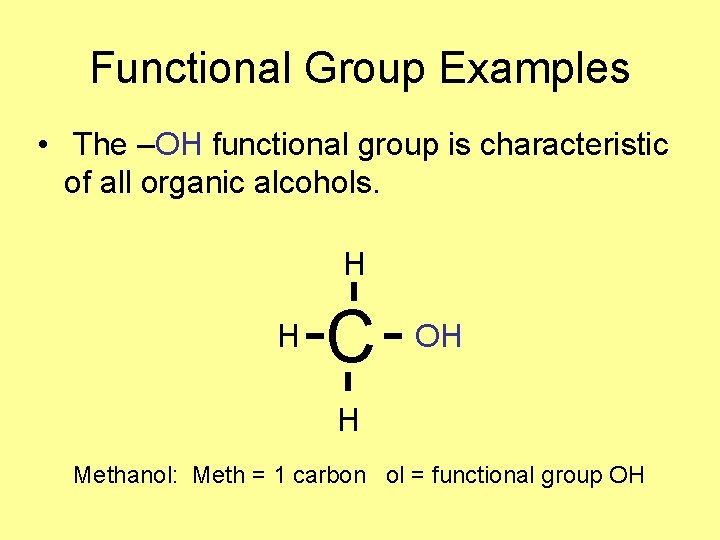
Functional Group Examples • The –OH functional group is characteristic of all organic alcohols. H H C OH H Methanol: Meth = 1 carbon ol = functional group OH
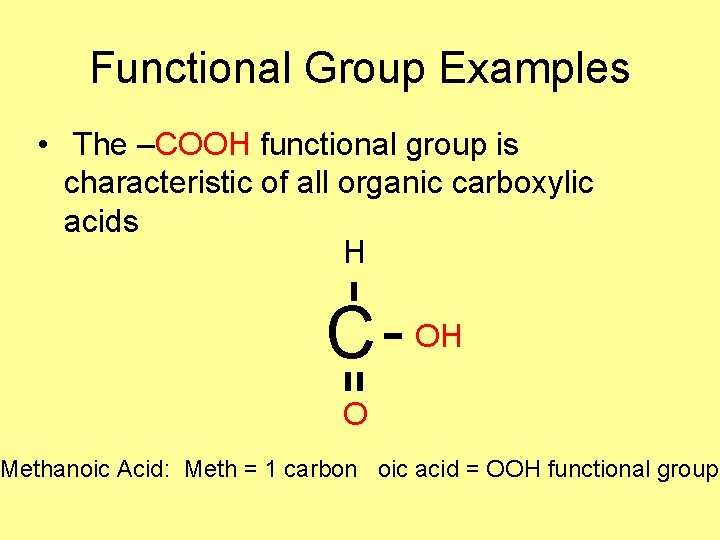
Functional Group Examples • The –COOH functional group is characteristic of all organic carboxylic acids H C OH O Methanoic Acid: Meth = 1 carbon oic acid = OOH functional group
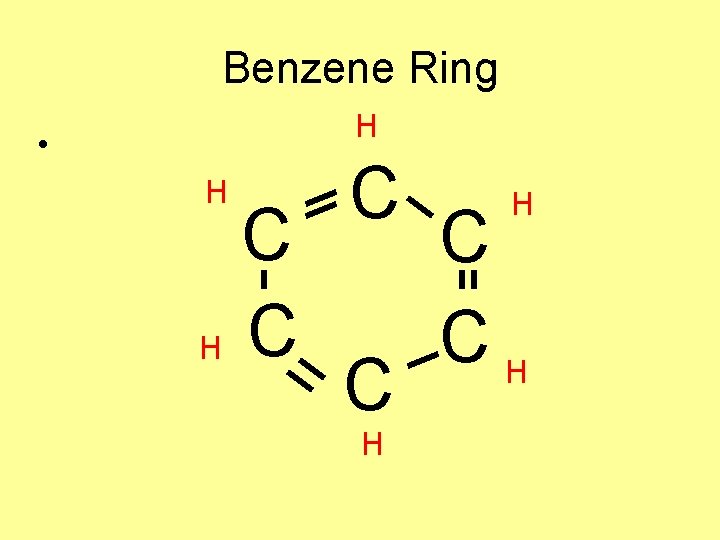
Benzene Ring H • H C C C H H C CH
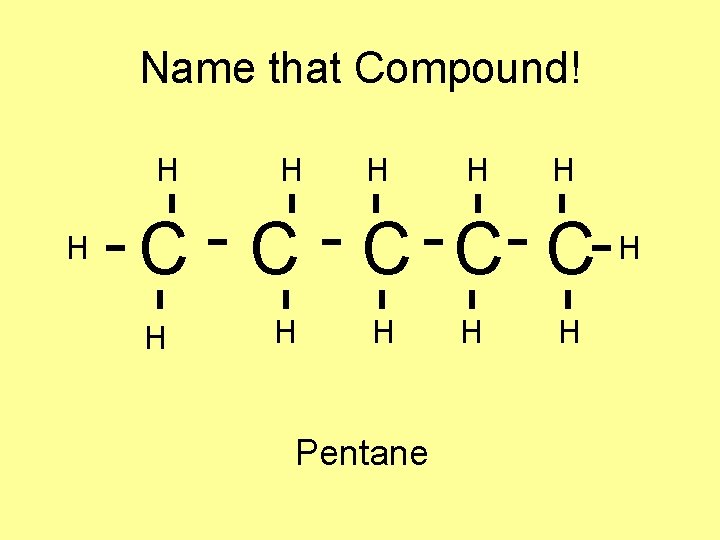
Name that Compound! H H H C C C H H H Pentane H H H
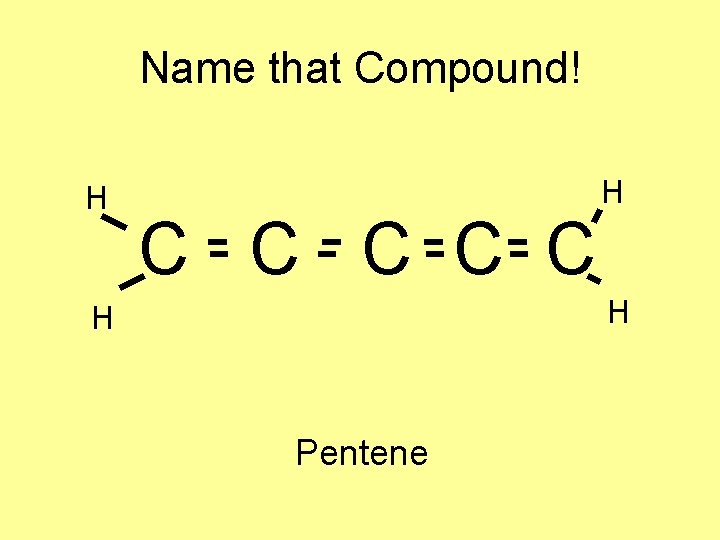
Name that Compound! H C C C H H H Pentene
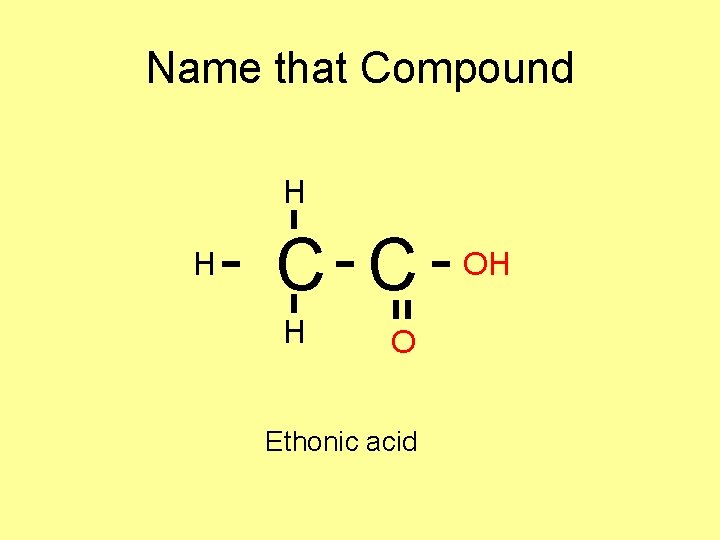
Name that Compound H H C C H O Ethonic acid OH
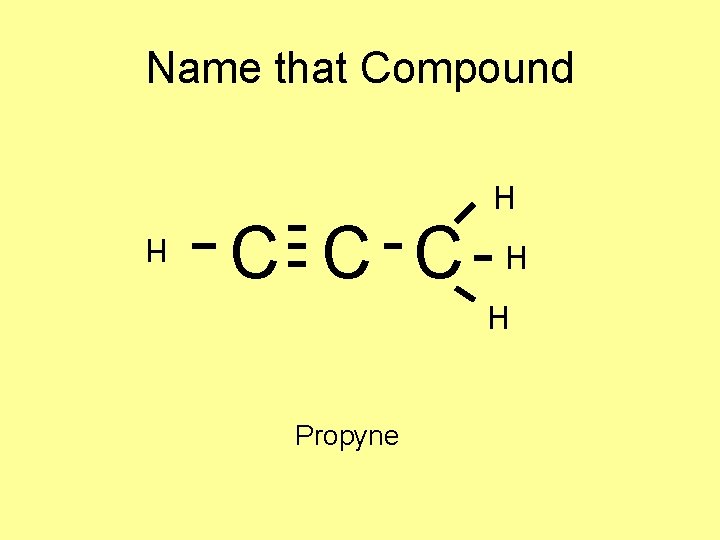
Name that Compound C C C H H H Propyne H
Crystal Meth N-methyl-1 -phenylpropan-2 -amine
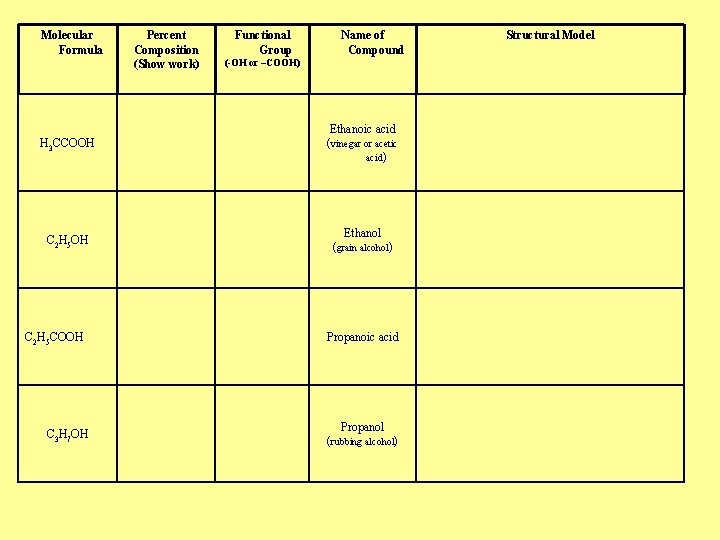
Molecular Formula Percent Composition (Show work) Functional Group Name of Compound (-OH or –COOH) H 3 CCOOH Ethanoic acid (vinegar or acetic acid) C 2 H 5 OH Ethanol (grain alcohol) C 2 H 5 COOH C 3 H 7 OH Propanoic acid Propanol (rubbing alcohol) Structural Model
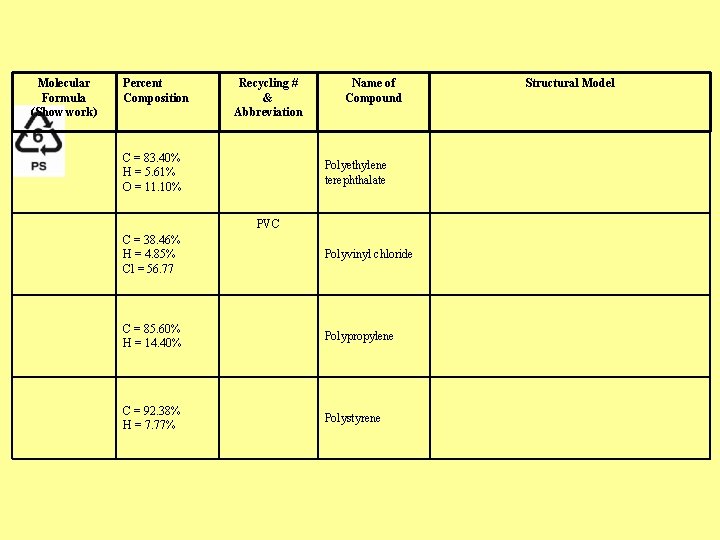
Molecular Formula (Show work) Percent Composition Recycling # & Abbreviation C = 83. 40% H = 5. 61% O = 11. 10% Name of Compound Polyethylene terephthalate PVC C = 38. 46% H = 4. 85% Cl = 56. 77 Polyvinyl chloride C = 85. 60% H = 14. 40% Polypropylene C = 92. 38% H = 7. 77% Polystyrene Structural Model
- Slides: 20