Organic Chemistry Hydrocarbons Chemistry 100 Organic Compounds Study

Organic Chemistry: Hydrocarbons Chemistry 100

Organic Compounds • • • Study of Carbon Compounds Carbon atom containing compounds has one or more C atoms has many H atoms may also contain O, S, N, and halogens usually has carbon written first Carbon is special because of its ability to form covalent bonds, especially with hydrogen and other carbon atoms.

Properties of Organic Compound Typical Organic Compounds • • • have covalent bonds have low melting points have low boiling points are flammable are soluble in nonpolar solvents are not soluble in water
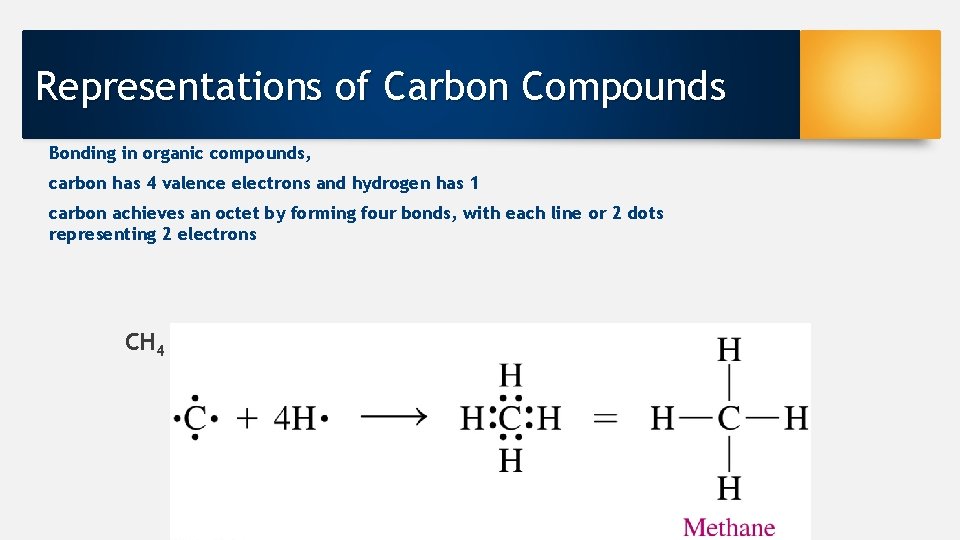
Representations of Carbon Compounds Bonding in organic compounds, carbon has 4 valence electrons and hydrogen has 1 carbon achieves an octet by forming four bonds, with each line or 2 dots representing 2 electrons CH 4
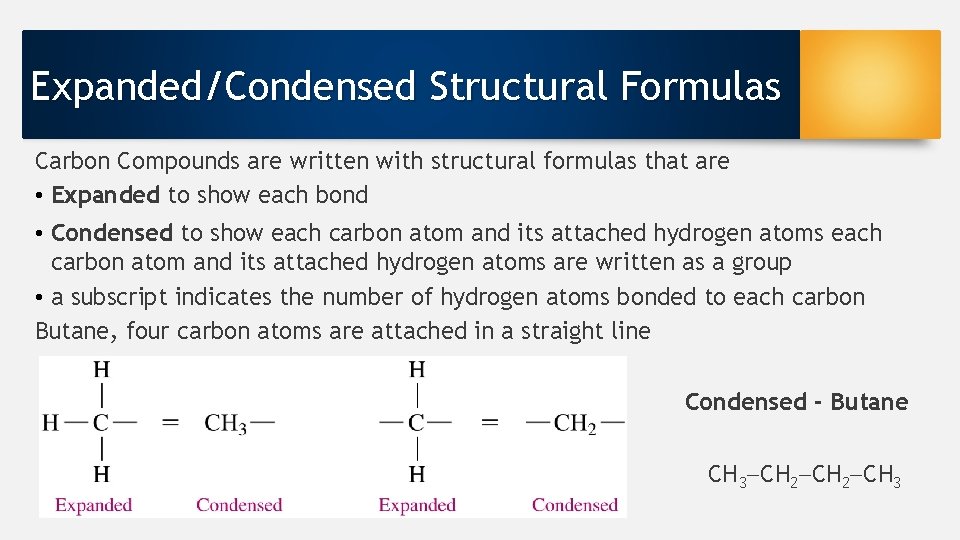
Expanded/Condensed Structural Formulas Carbon Compounds are written with structural formulas that are • Expanded to show each bond • Condensed to show each carbon atom and its attached hydrogen atoms are written as a group • a subscript indicates the number of hydrogen atoms bonded to each carbon Butane, four carbon atoms are attached in a straight line Condensed - Butane CH 3 CH 2 CH 3
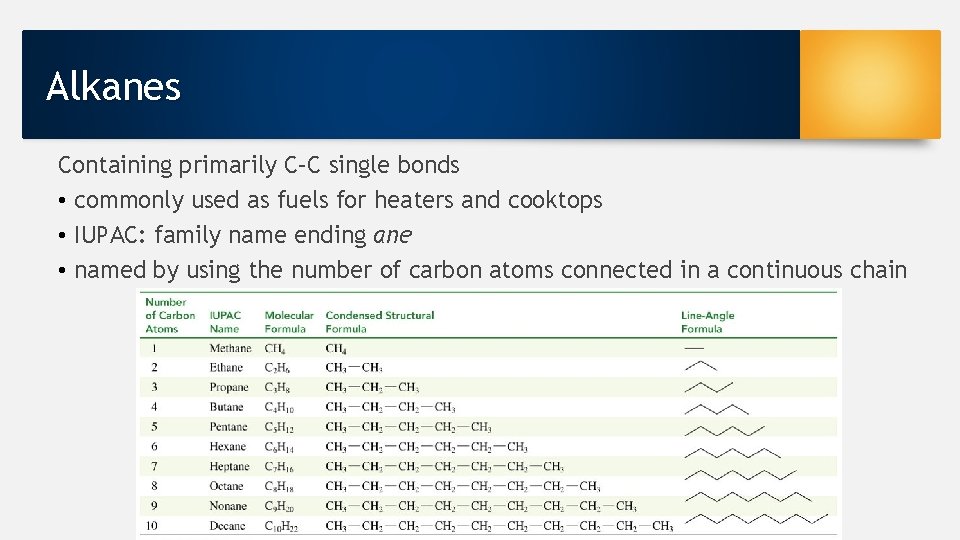
Alkanes Containing primarily C–C single bonds • commonly used as fuels for heaters and cooktops • IUPAC: family name ending ane • named by using the number of carbon atoms connected in a continuous chain
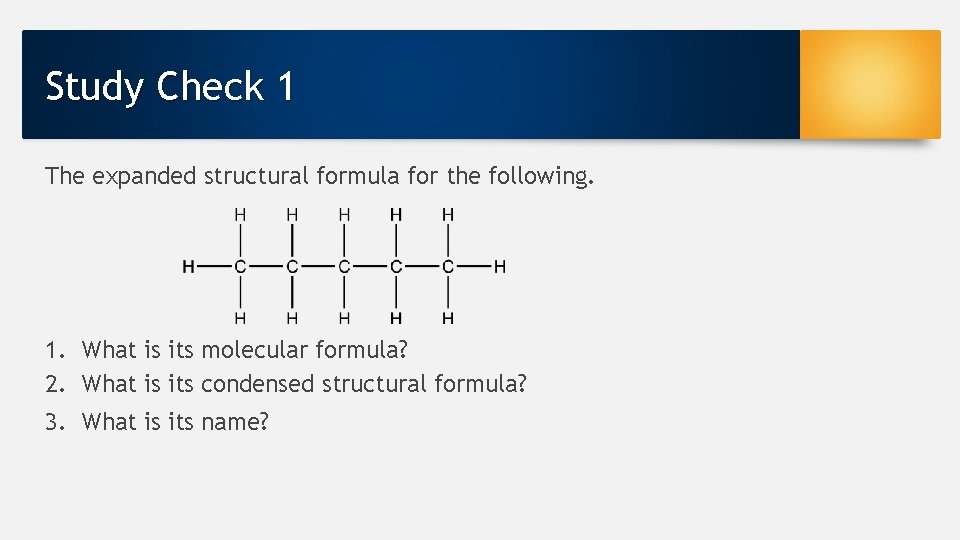
Study Check 1 The expanded structural formula for the following. 1. What is its molecular formula? 2. What is its condensed structural formula? 3. What is its name?
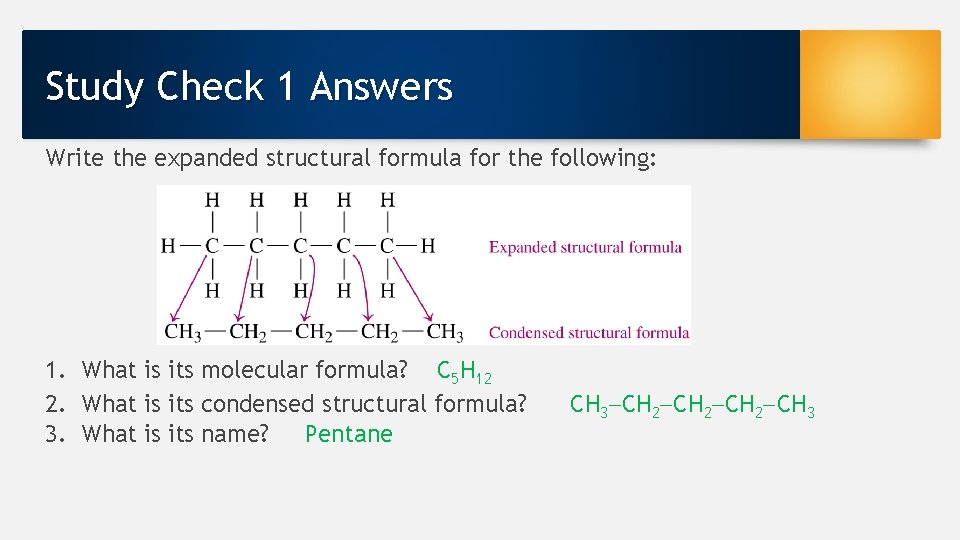
Study Check 1 Answers Write the expanded structural formula for the following: 1. What is its molecular formula? C 5 H 12 2. What is its condensed structural formula? 3. What is its name? Pentane CH 3 CH 2 CH 3

Study Check 2 Draw the condensed structural formula for: A. Ethane B. Heptane

Study Check 2 Answers Draw the condensed structural formula for: A. Ethane: CH 3 B. Heptane: CH 3 CH 2 CH 2 CH 3
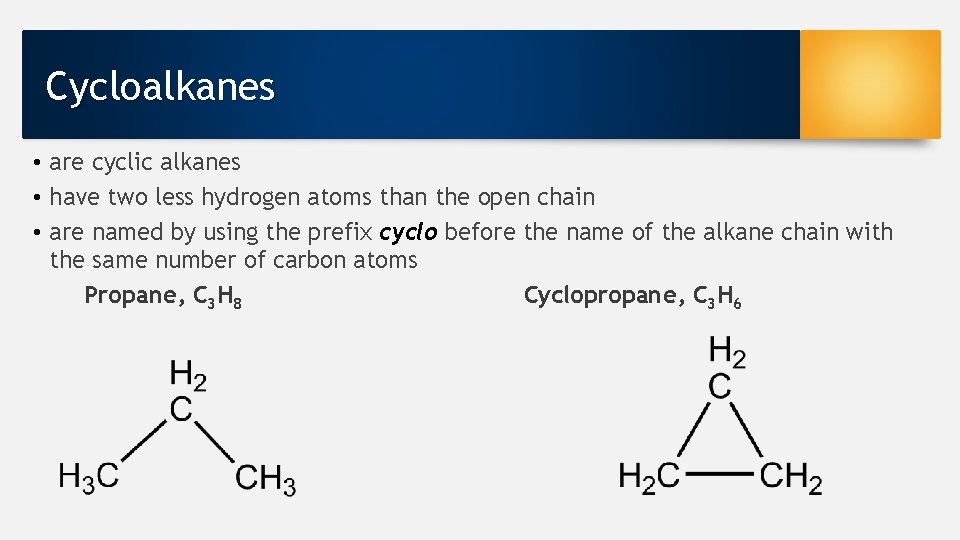
Cycloalkanes • are cyclic alkanes • have two less hydrogen atoms than the open chain • are named by using the prefix cyclo before the name of the alkane chain with the same number of carbon atoms Propane, C 3 H 8 Cyclopropane, C 3 H 6
Alkanes with Substituents (branch) are atoms or groups of atoms attached to the carbon chain and include alkyl and halo groups. Alkyl groups are • carbon branches attached to carbon chains • named with a yl ending Halo substituents are • halogens attached to the carbon chain • named as fluoro, chloro, bromo, or iodo
IUPAC System of Naming Compounds Step 1 Find the longest chain of carbon atoms that contains the functional group. Step 2 Number the carbon atoms starting from the end nearer to the functional group. Step 3 Location and name of each substituent (in alphabetical order) as a prefix to the name of the main chain.
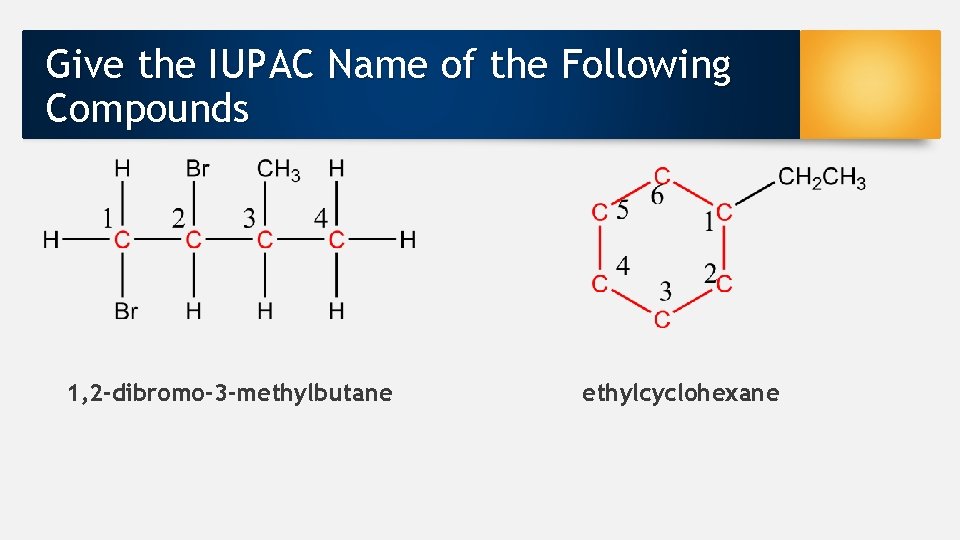
Give the IUPAC Name of the Following Compounds 1, 2 -dibromo-3 -methylbutane ethylcyclohexane

Drawing Formulas for Alkanes Draw the structure for 1, 2 -dichloro, 2 -methylheptane – 7 -carbon chain methyl group on carbon 2 chlorine on carbon 1 and 1 Cl CH CH CH 2 CH 3 Cl CH 3

Study Check 3 Draw the condensed structural formula for 3 -bromo-1 -chlorobutane.
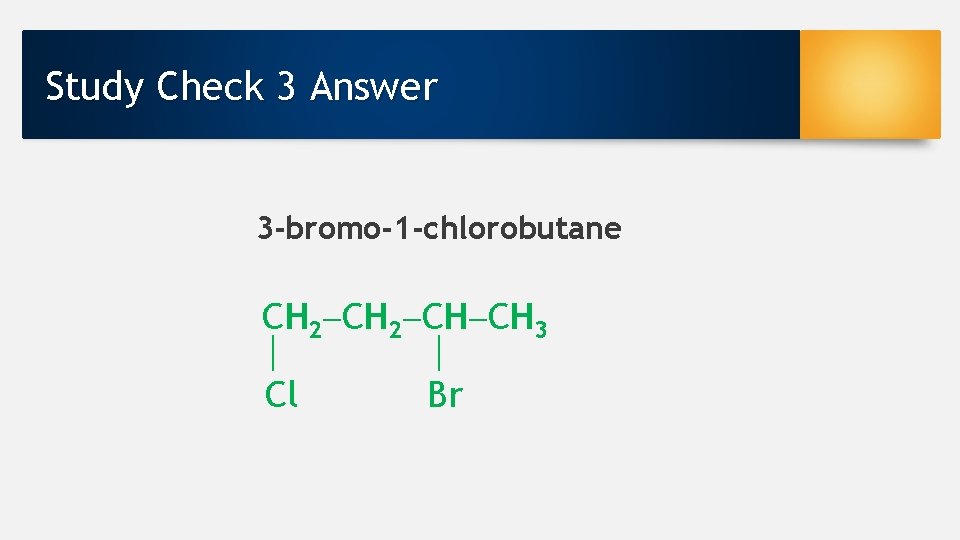
Study Check 3 Answer 3 -bromo-1 -chlorobutane CH 2 CH CH 3 Cl Br

Properties of Alkanes Some Uses of Alkanes One to four carbons are gases at room temperature and are widely used as heating fuels; methane, propane, butane. Five to eight carbons are highly volatile liquids at room temperature, making them useful in fuels such as gasoline. 9− 17 carbons are liquids with higher boiling points and are found in motor oils, mineral oil, kerosene, diesel, and jet fuels. 18 or more carbon atoms are waxy solids at room temperature, are waxy coatings of fruits and vegetables.

Solubility and Density • • nonpolar insoluble in water less dense than water flammable in air The crude oil in oil spills floats on top of the water, forming a thin layer on the surface because it is less dense than water.

Combustion of Alkanes have strong C C bonds Least reactive family Combustion Reaction • Burn readily- react with oxygen gas to make carbon dioxide and water • Release energy when C C bonds are broken in CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g) + energy

Functional Groups A characteristic feature of organic molecules that behave in a predictable way • Composed of an atom or group of atoms that replace a hydrogen atom in the corresponding alkane • A way to classify families of organic compounds with similar physical and chemical properties Identify the Functional Group Name compound predict physical properties predict chemical properties and their products
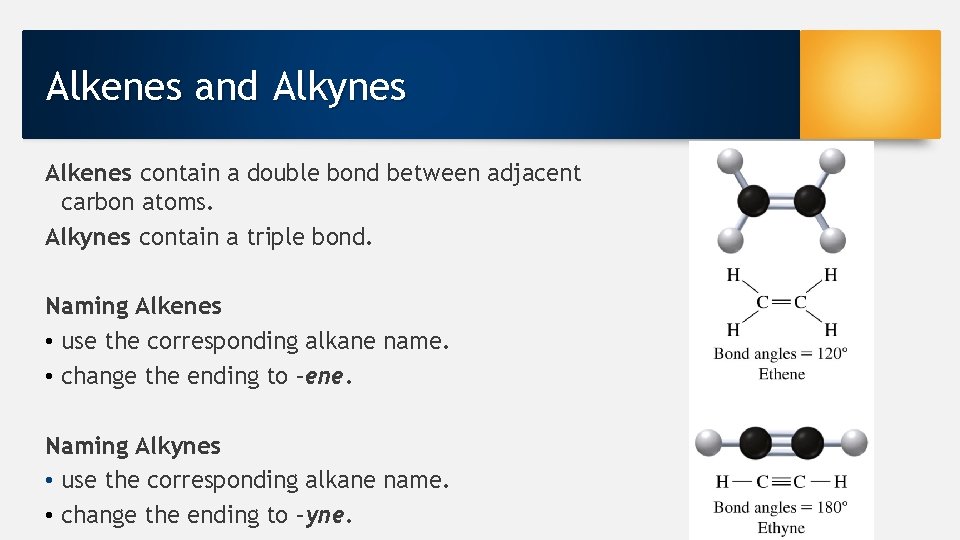
Alkenes and Alkynes Alkenes contain a double bond between adjacent carbon atoms. Alkynes contain a triple bond. Naming Alkenes • use the corresponding alkane name. • change the ending to –ene. Naming Alkynes • use the corresponding alkane name. • change the ending to –yne.
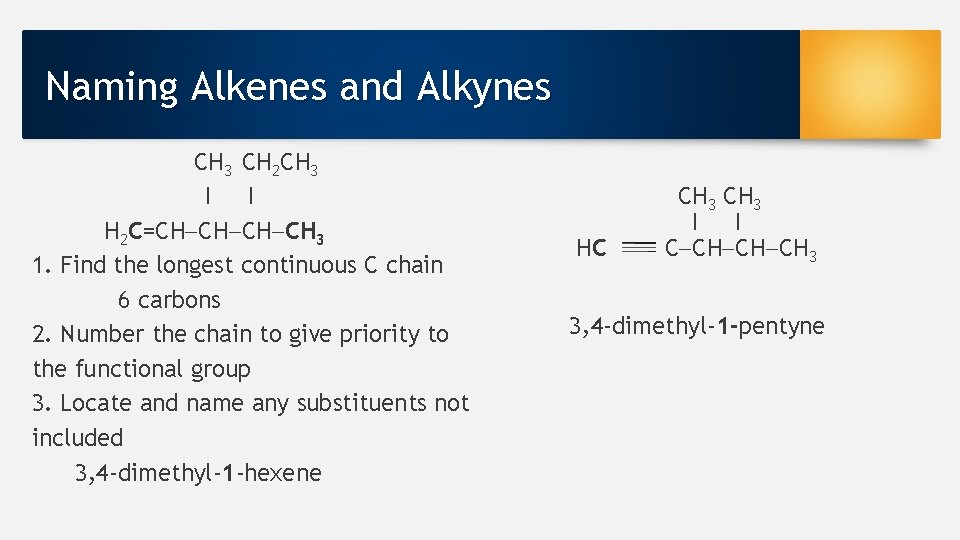
Naming Alkenes and Alkynes CH 3 CH 2 CH 3 I I H 2 C=CH CH 3 1. Find the longest continuous C chain 6 carbons 2. Number the chain to give priority to the functional group 3. Locate and name any substituents not included 3, 4 -dimethyl-1 -hexene HC CH 3 I I C CH CH CH 3 3, 4 -dimethyl-1 -pentyne
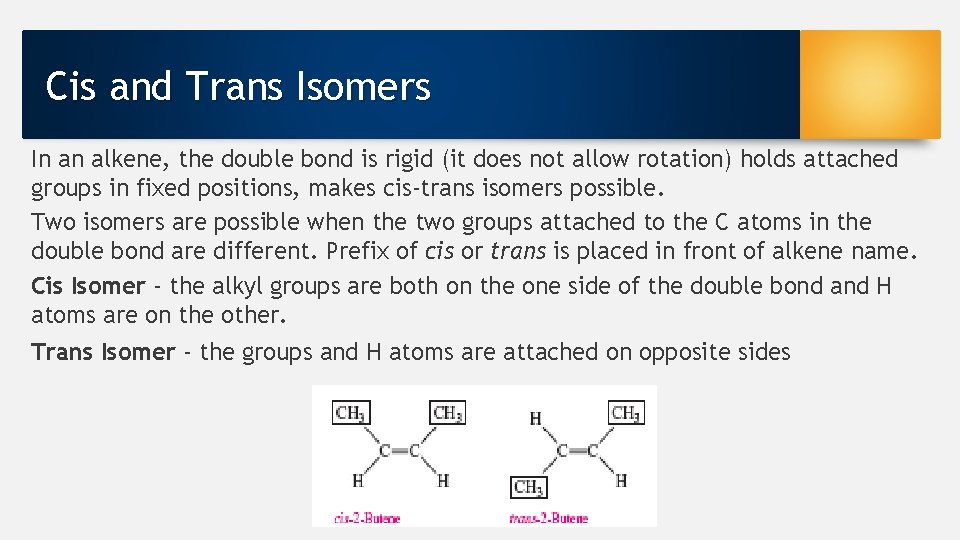
Cis and Trans Isomers In an alkene, the double bond is rigid (it does not allow rotation) holds attached groups in fixed positions, makes cis-trans isomers possible. Two isomers are possible when the two groups attached to the C atoms in the double bond are different. Prefix of cis or trans is placed in front of alkene name. Cis Isomer - the alkyl groups are both on the one side of the double bond and H atoms are on the other. Trans Isomer - the groups and H atoms are attached on opposite sides
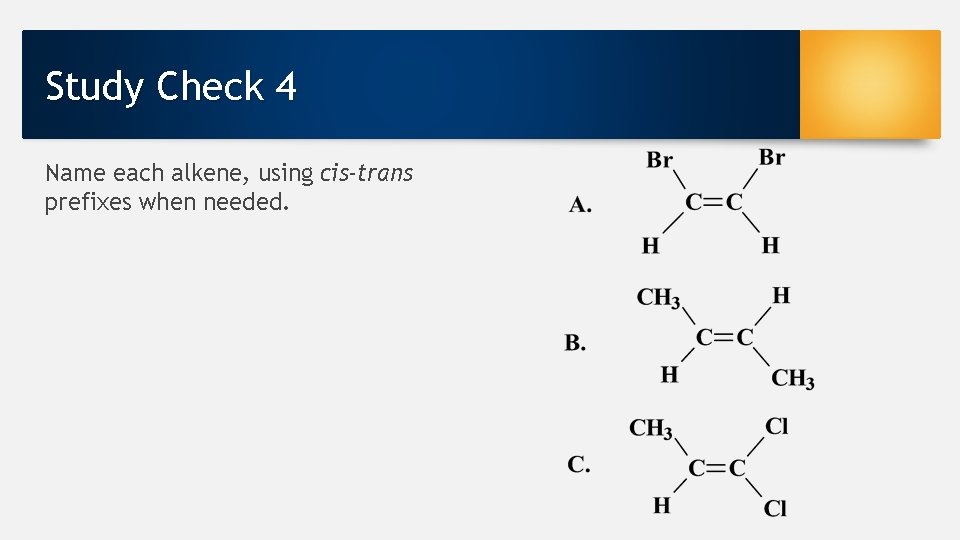
Study Check 4 Name each alkene, using cis-trans prefixes when needed.
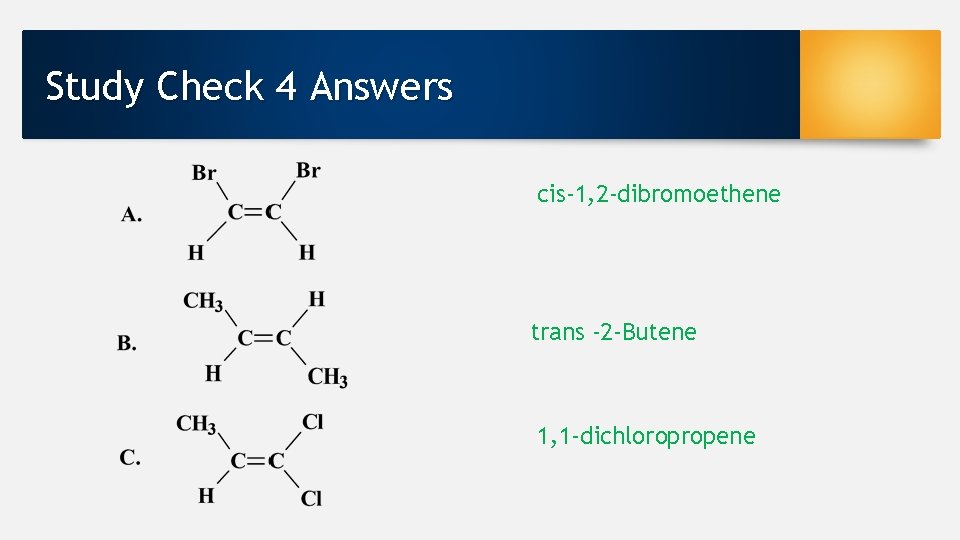
Study Check 4 Answers cis-1, 2 -dibromoethene trans -2 -Butene 1, 1 -dichloropropene
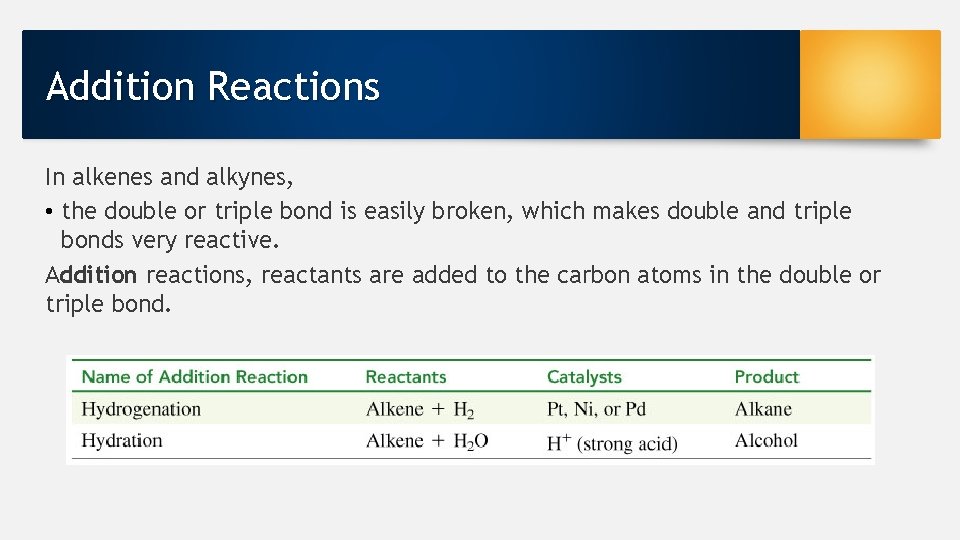
Addition Reactions In alkenes and alkynes, • the double or triple bond is easily broken, which makes double and triple bonds very reactive. Addition reactions, reactants are added to the carbon atoms in the double or triple bond.
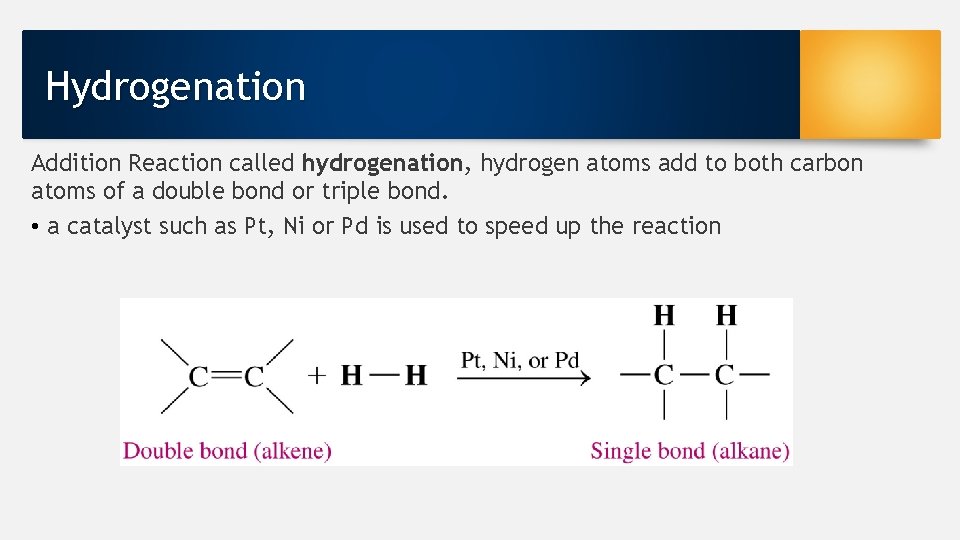
Hydrogenation Addition Reaction called hydrogenation, hydrogen atoms add to both carbon atoms of a double bond or triple bond. • a catalyst such as Pt, Ni or Pd is used to speed up the reaction
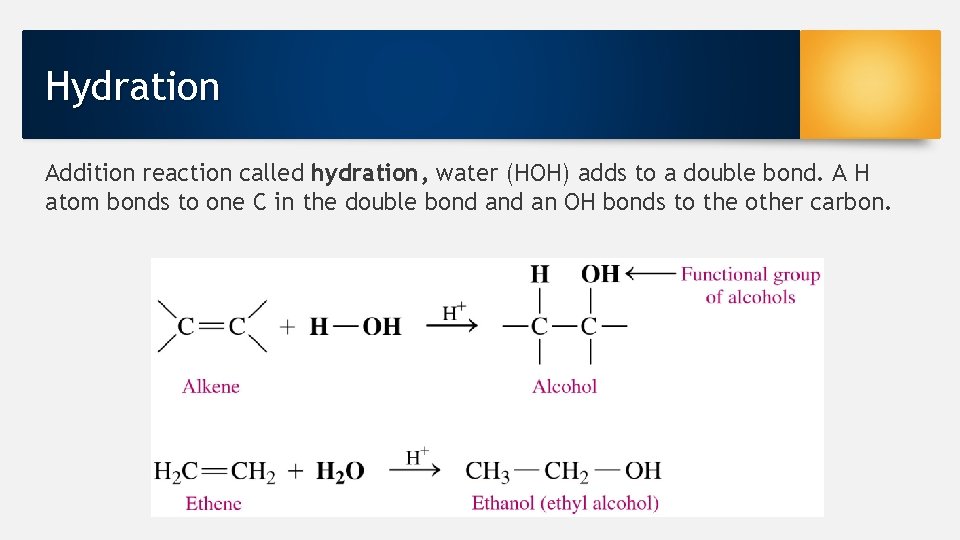
Hydration Addition reaction called hydration, water (HOH) adds to a double bond. A H atom bonds to one C in the double bond an OH bonds to the other carbon.
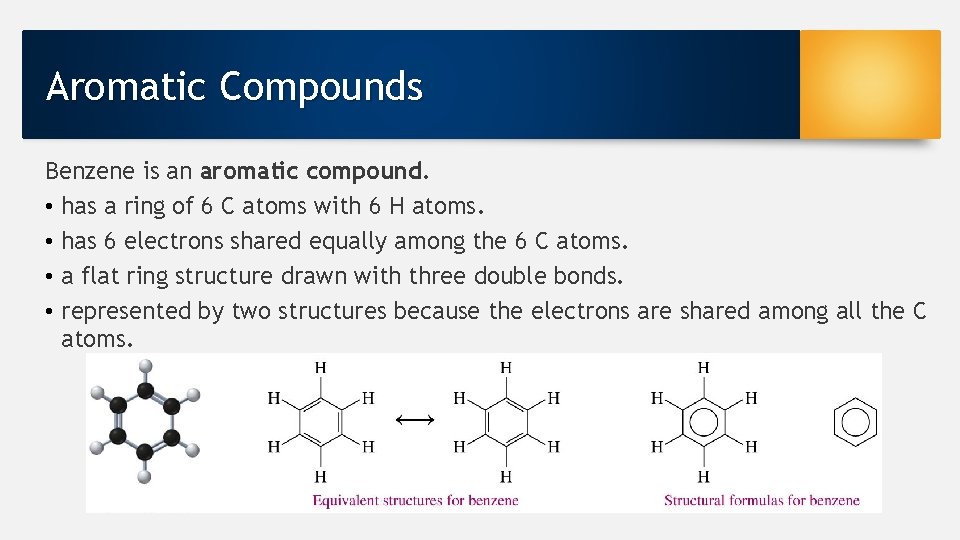
Aromatic Compounds Benzene is an aromatic compound. • has a ring of 6 C atoms with 6 H atoms. • has 6 electrons shared equally among the 6 C atoms. • a flat ring structure drawn with three double bonds. • represented by two structures because the electrons are shared among all the C atoms.
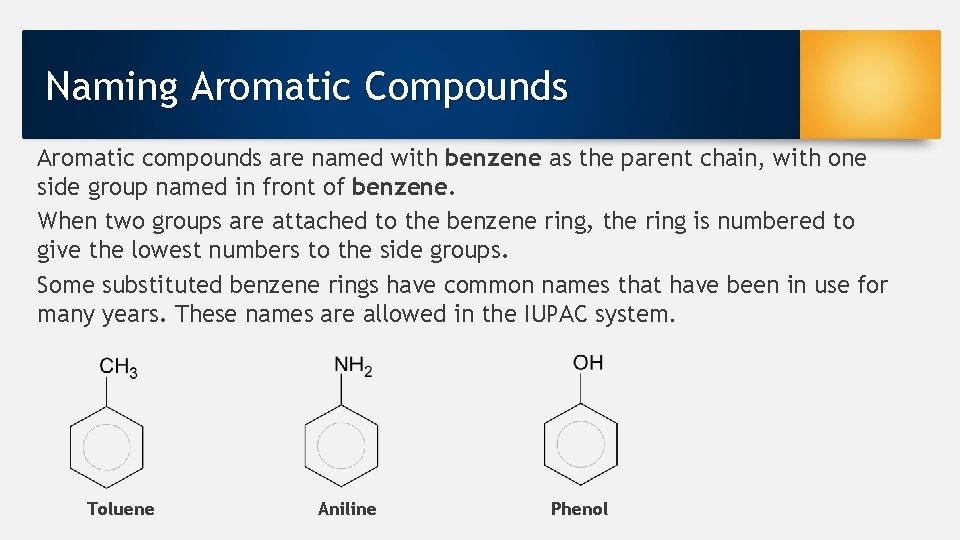
Naming Aromatic Compounds Aromatic compounds are named with benzene as the parent chain, with one side group named in front of benzene. When two groups are attached to the benzene ring, the ring is numbered to give the lowest numbers to the side groups. Some substituted benzene rings have common names that have been in use for many years. These names are allowed in the IUPAC system. Toluene Aniline Phenol
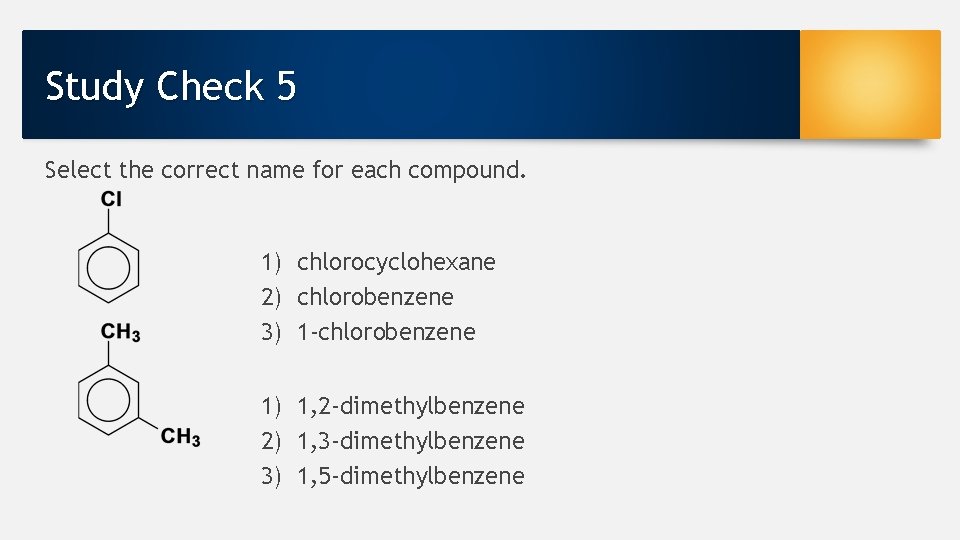
Study Check 5 Select the correct name for each compound. 1) chlorocyclohexane 2) chlorobenzene 3) 1 -chlorobenzene 1) 1, 2 -dimethylbenzene 2) 1, 3 -dimethylbenzene 3) 1, 5 -dimethylbenzene
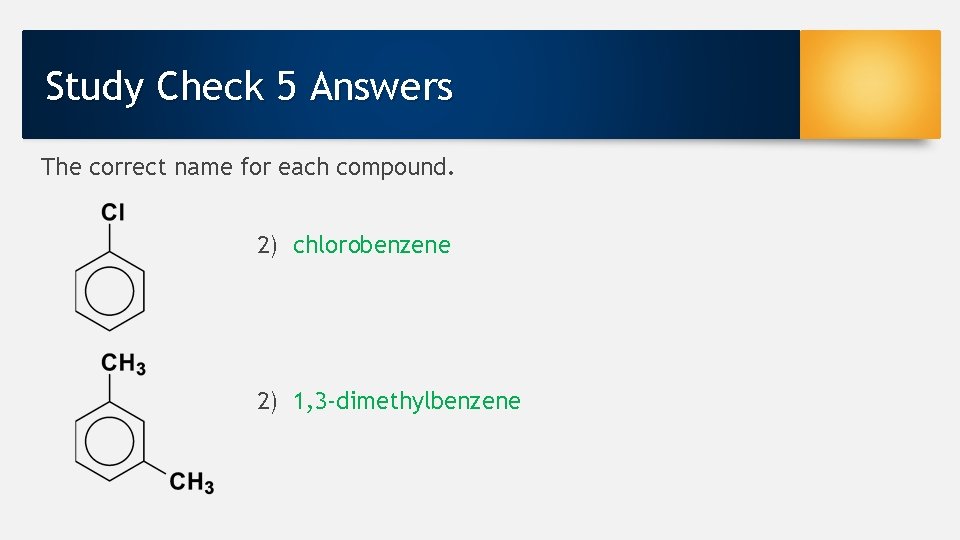
Study Check 5 Answers The correct name for each compound. 2) chlorobenzene 2) 1, 3 -dimethylbenzene
- Slides: 33