Organic Chemistry ELEVENTH EDITION Solomons Fryhle Snyder Chapter




- Slides: 98

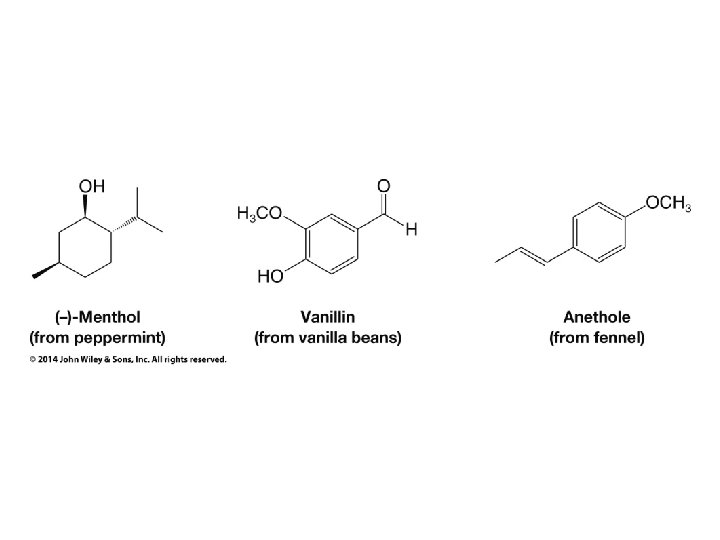
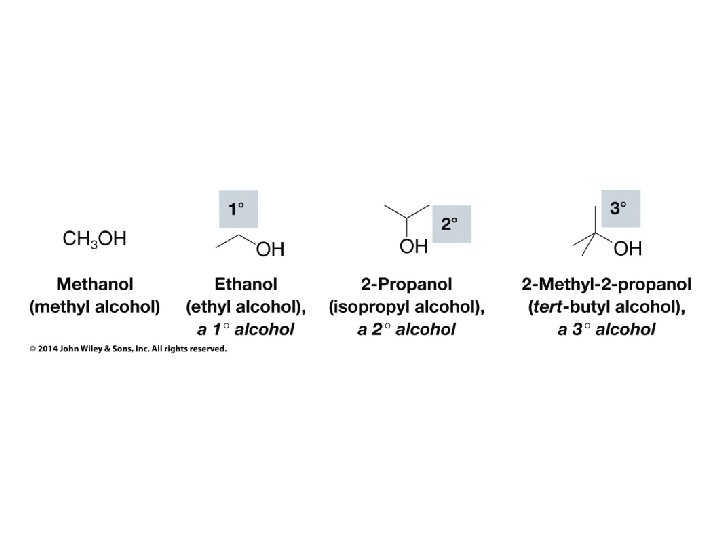
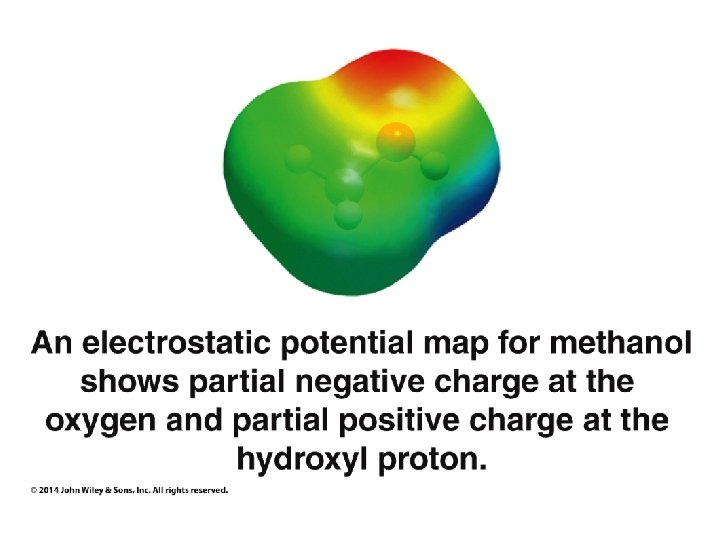
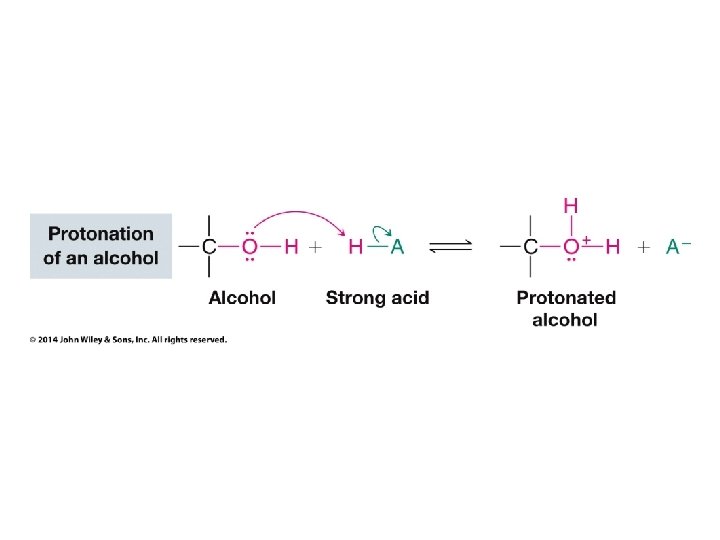
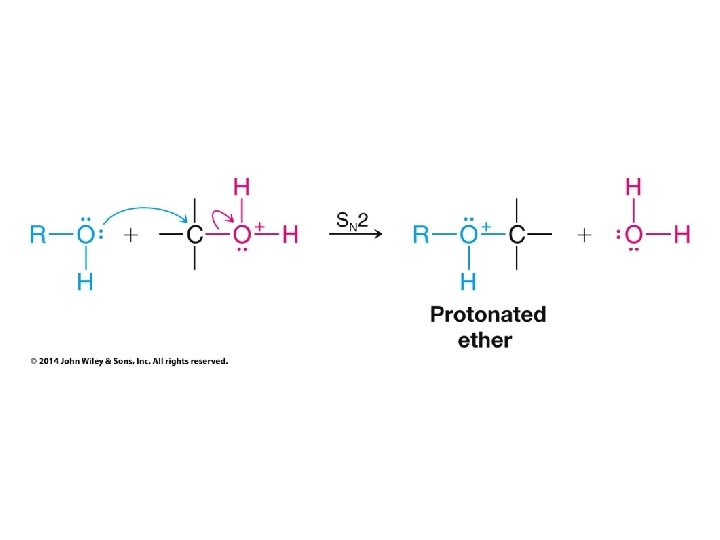
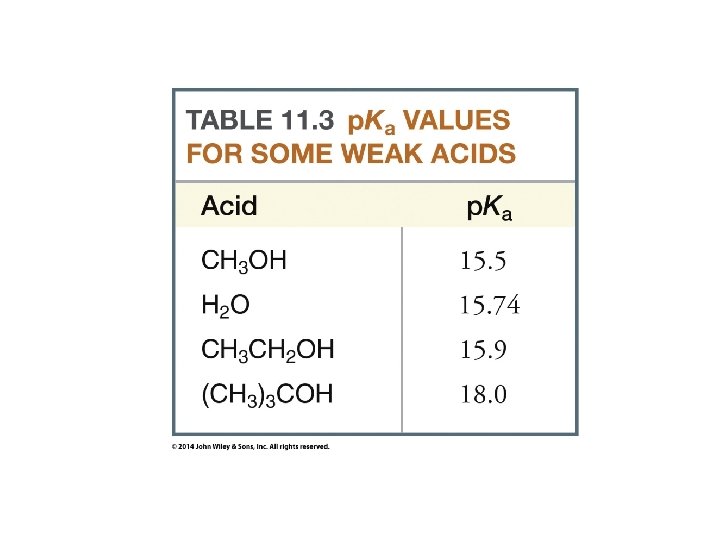
Organic Chemistry ELEVENTH EDITION Solomons • Fryhle • Snyder Chapter 11 Alcohols and Ethers Copyright © 2014 by John Wiley & Sons, Inc. All rights reserved.



















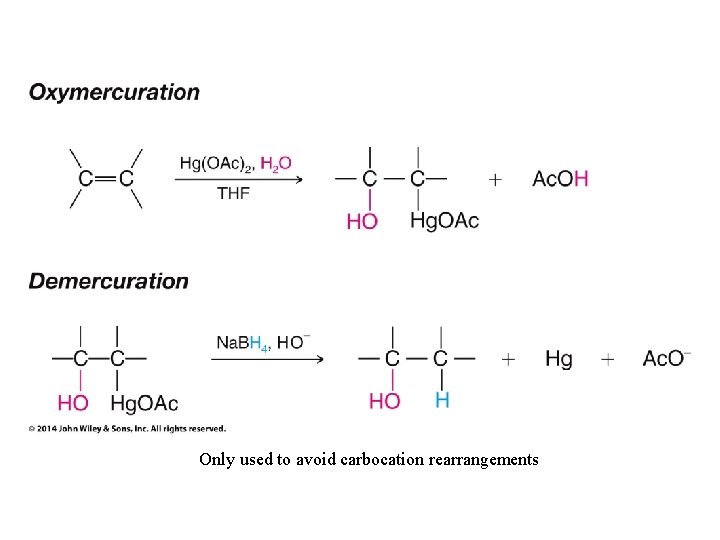
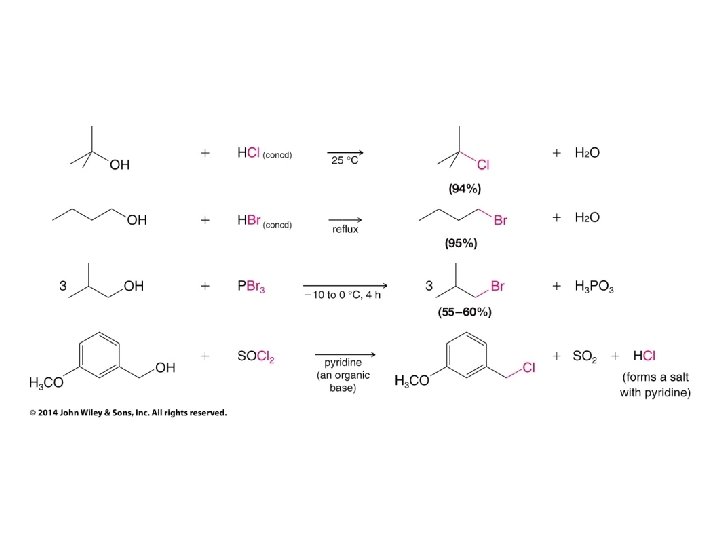
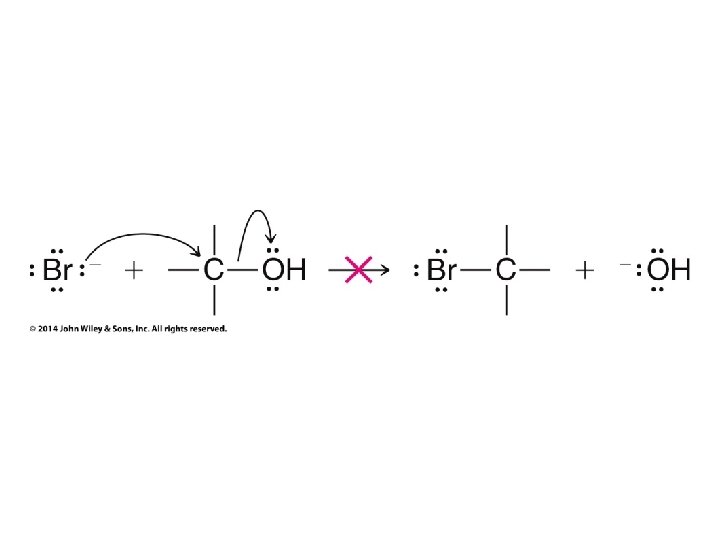
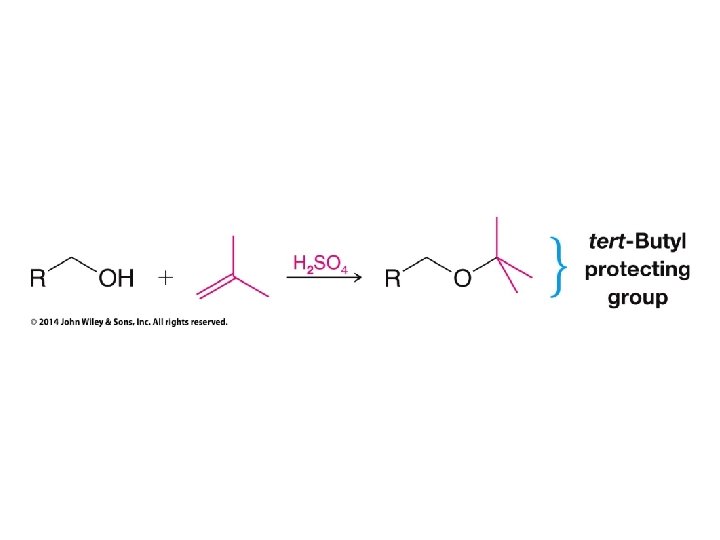
Only used to avoid carbocation rearrangements




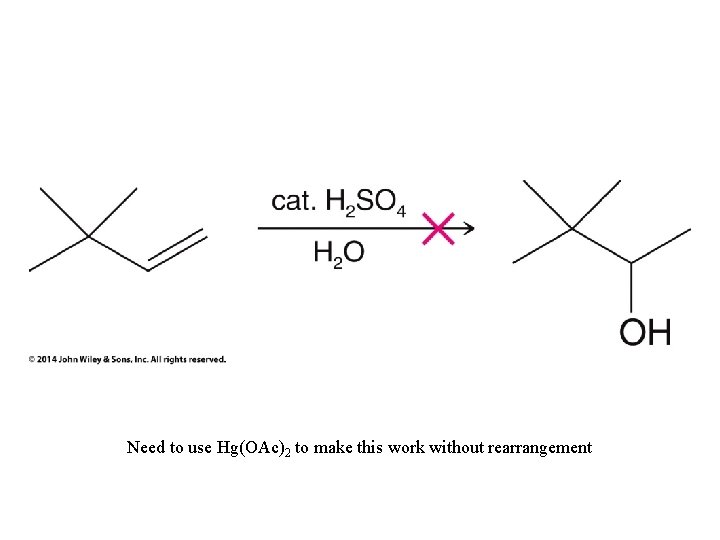
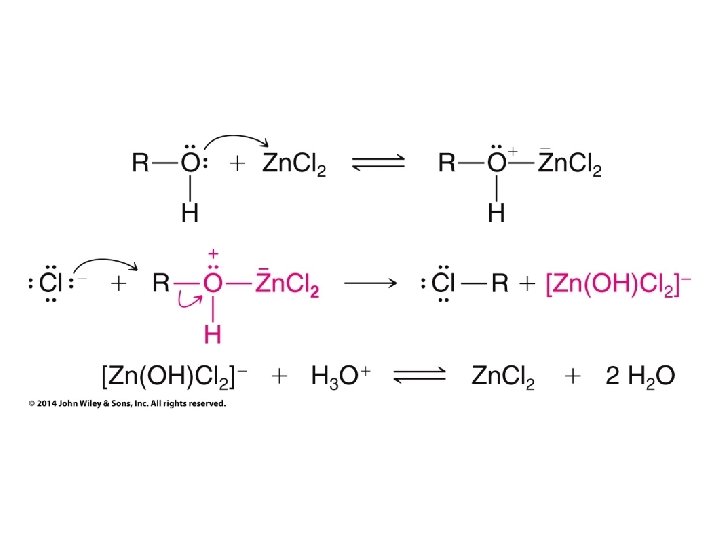
Need to use Hg(OAc)2 to make this work without rearrangement








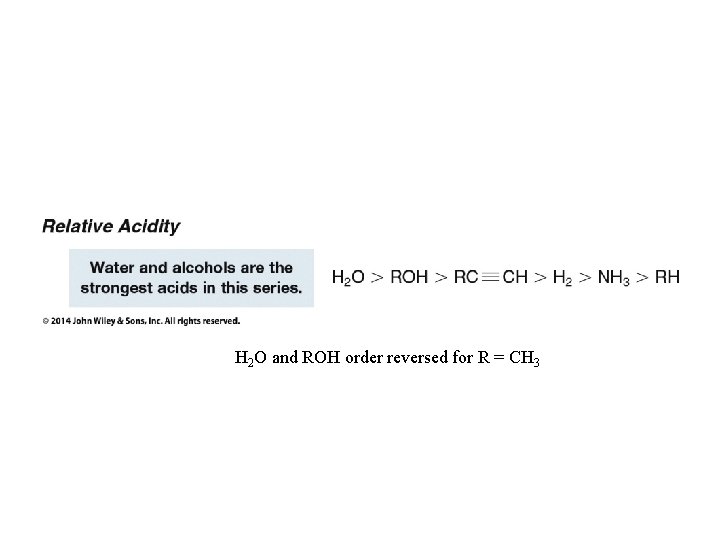
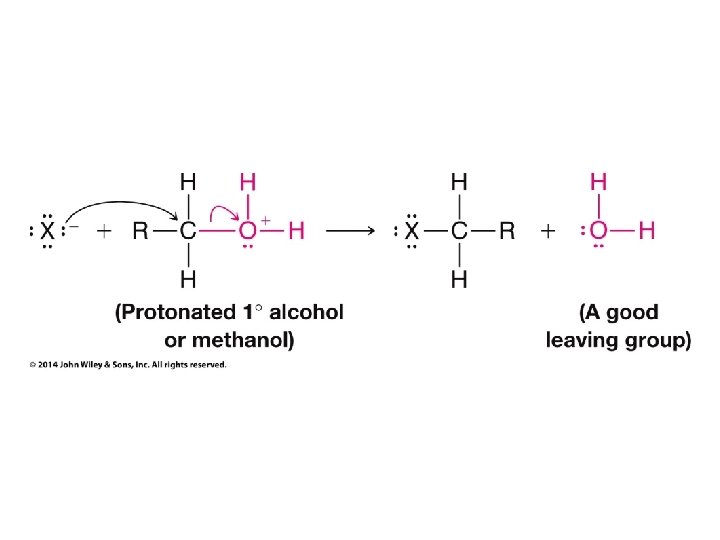
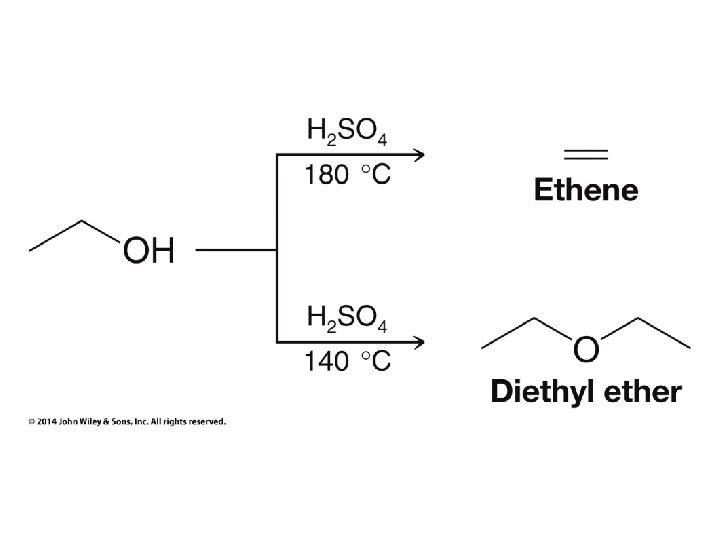
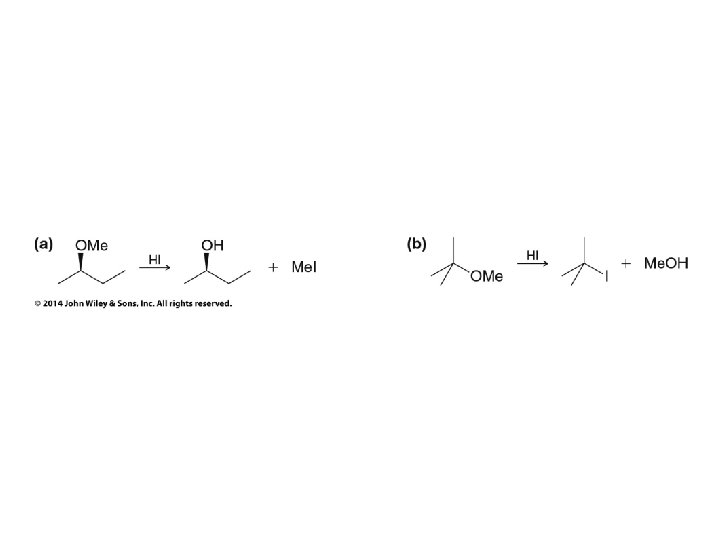
H 2 O and ROH order reversed for R = CH 3

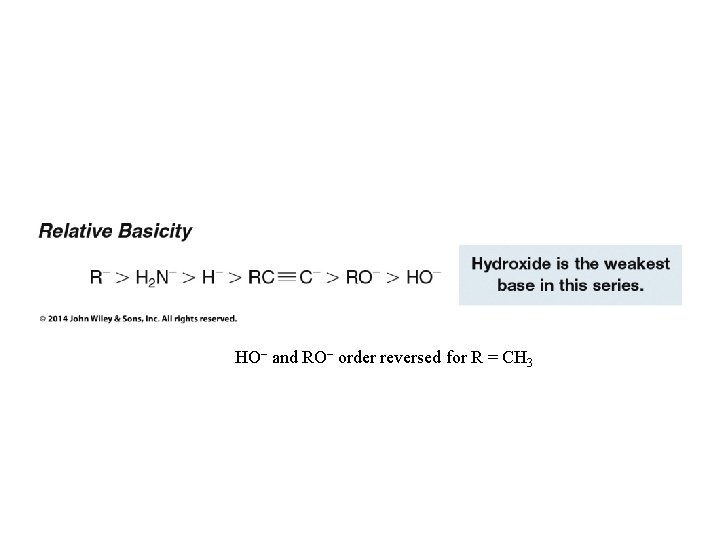
HO– and RO– order reversed for R = CH 3

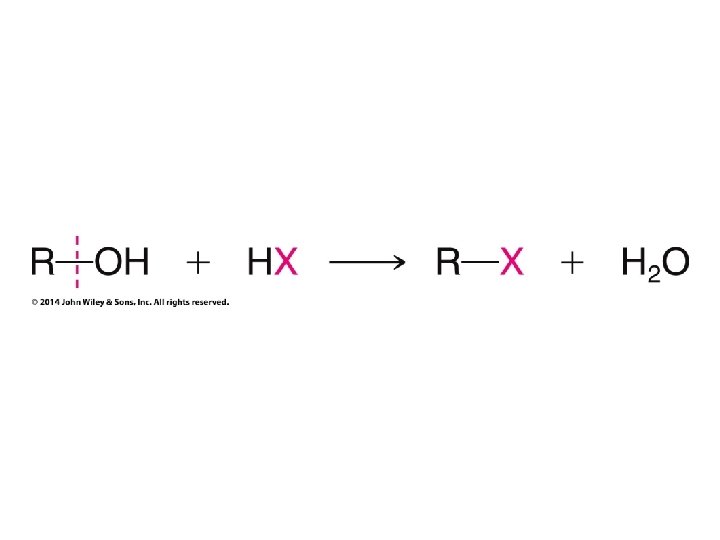







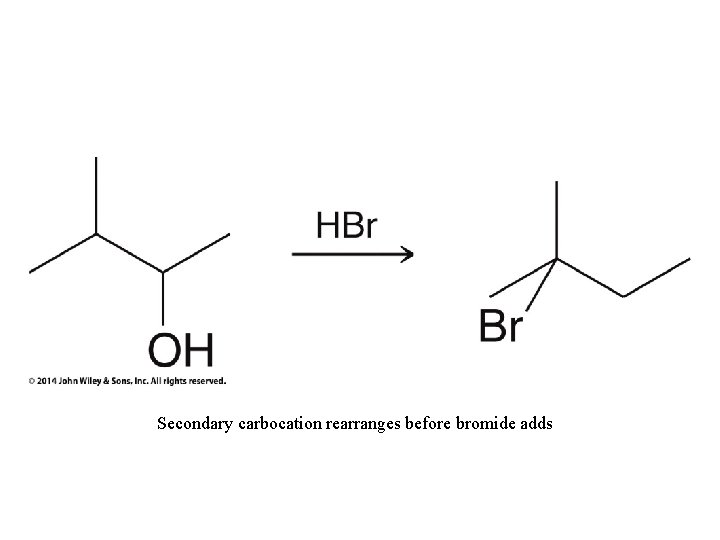
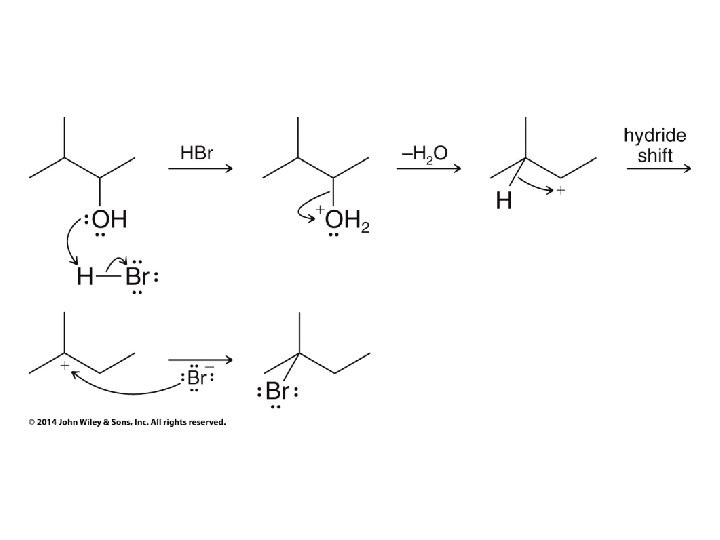
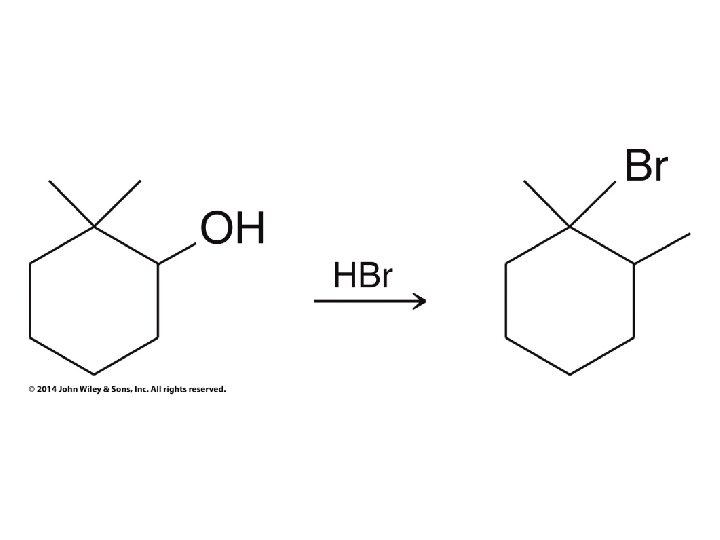
Secondary carbocation rearranges before bromide adds



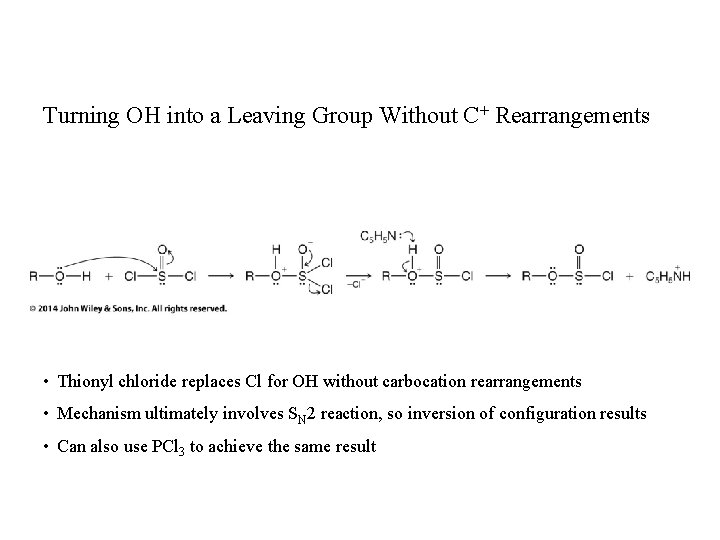
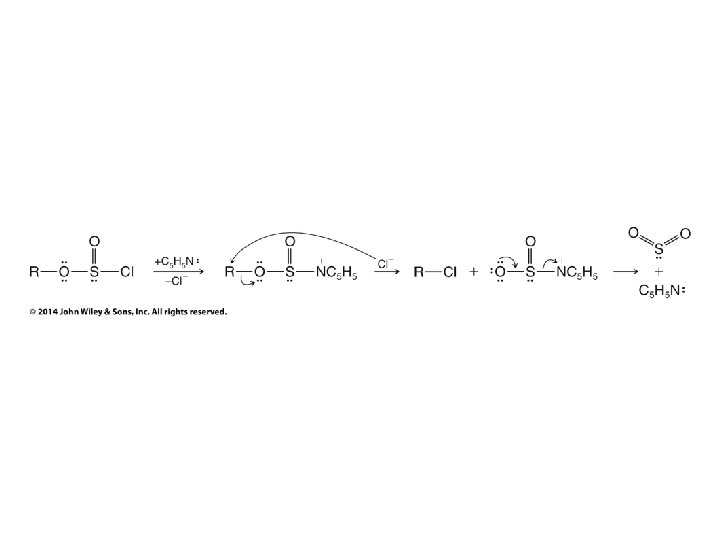
Turning OH into a Leaving Group Without C+ Rearrangements • Thionyl chloride replaces Cl for OH without carbocation rearrangements • Mechanism ultimately involves SN 2 reaction, so inversion of configuration results • Can also use PCl 3 to achieve the same result


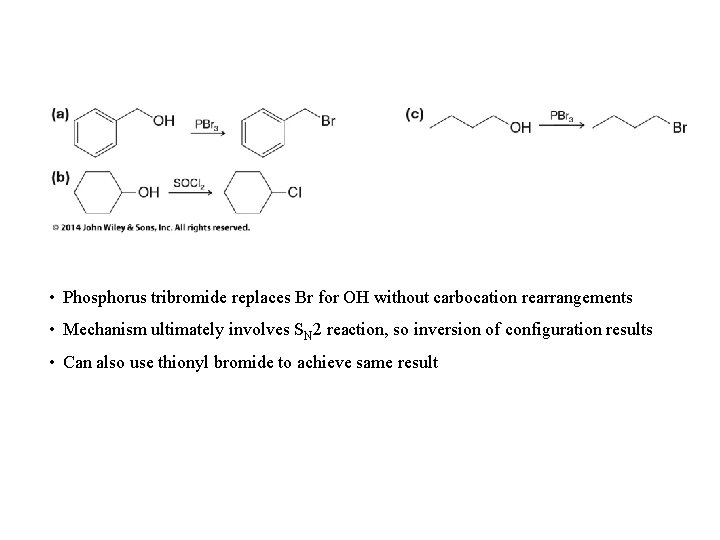
• Phosphorus tribromide replaces Br for OH without carbocation rearrangements • Mechanism ultimately involves SN 2 reaction, so inversion of configuration results • Can also use thionyl bromide to achieve same result



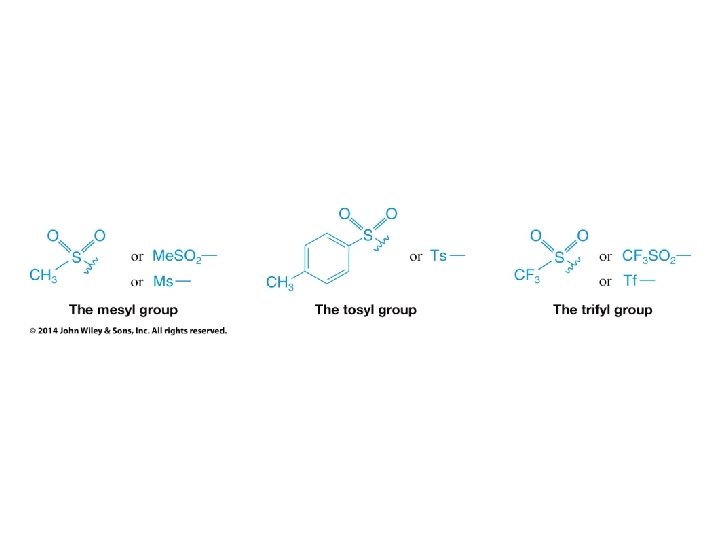
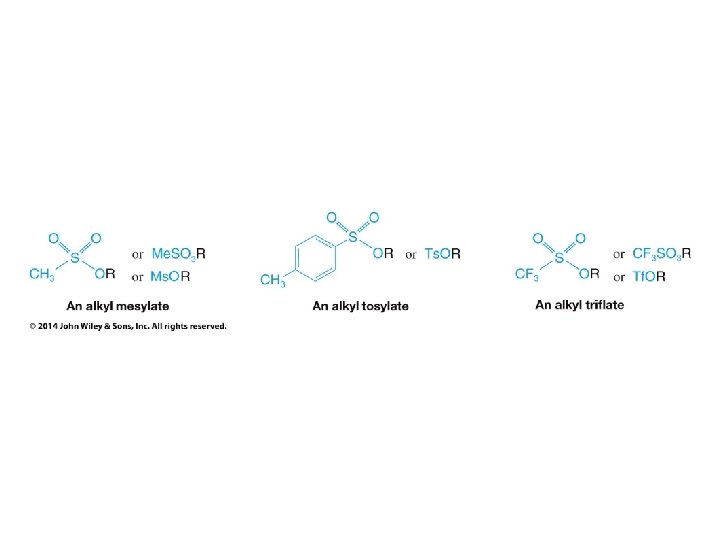
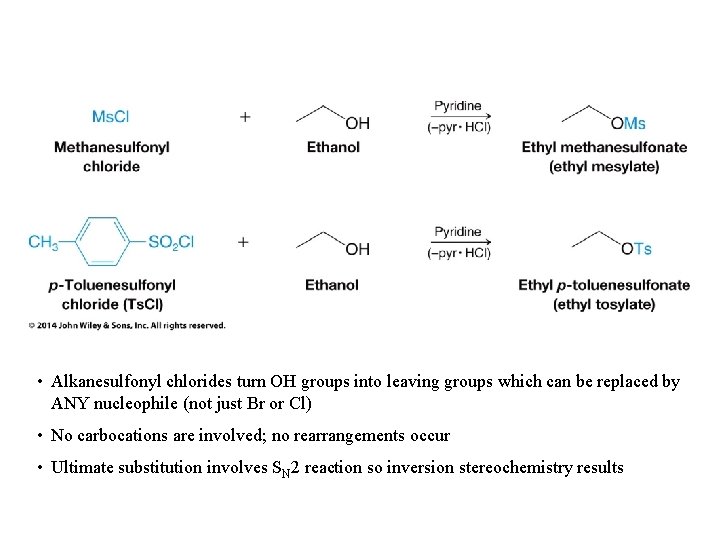
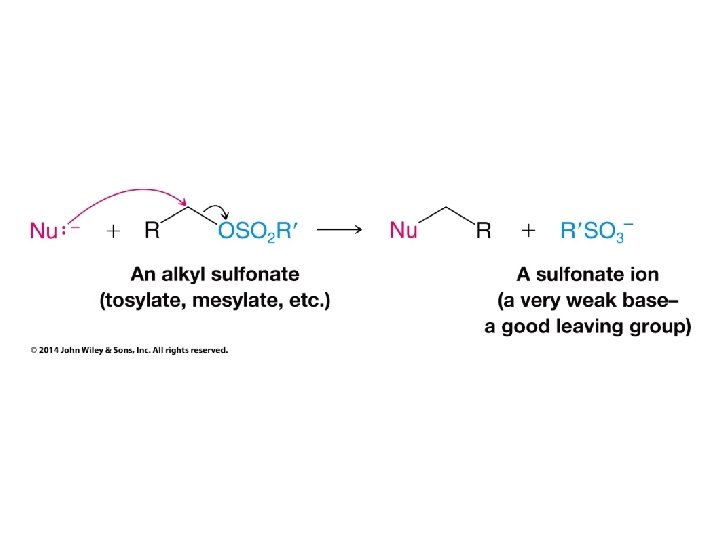
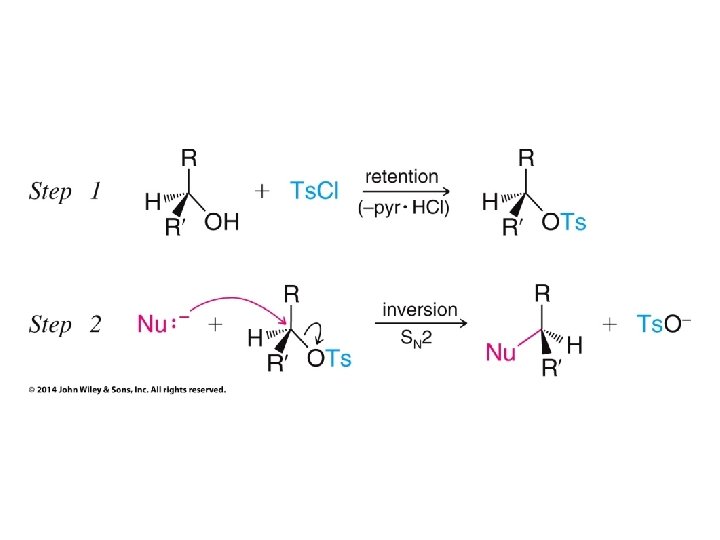
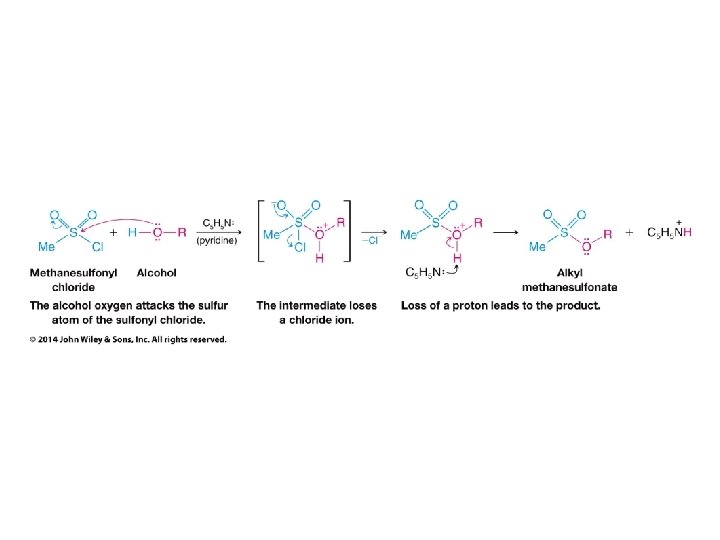
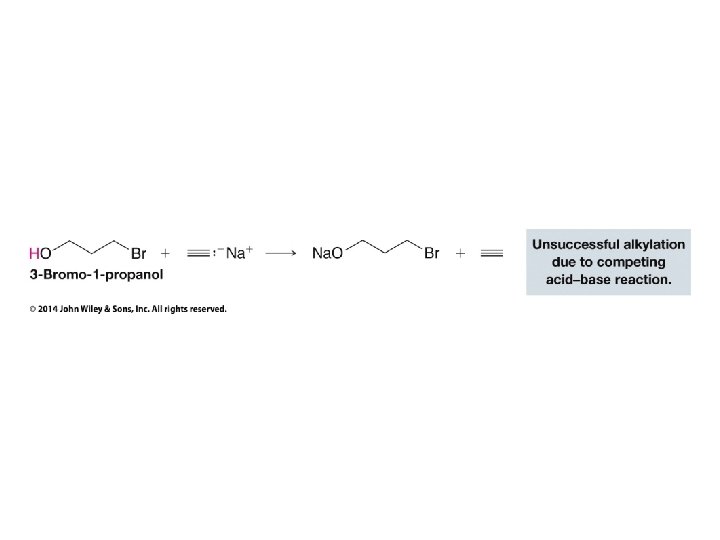
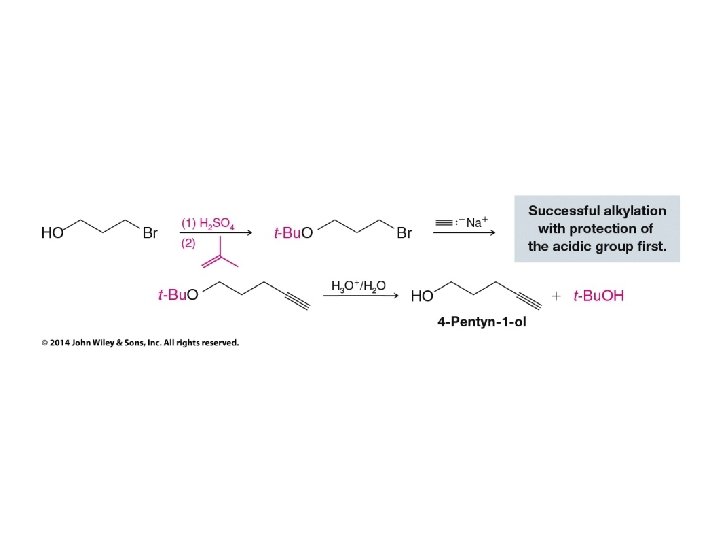
• Alkanesulfonyl chlorides turn OH groups into leaving groups which can be replaced by ANY nucleophile (not just Br or Cl) • No carbocations are involved; no rearrangements occur • Ultimate substitution involves SN 2 reaction so inversion stereochemistry results













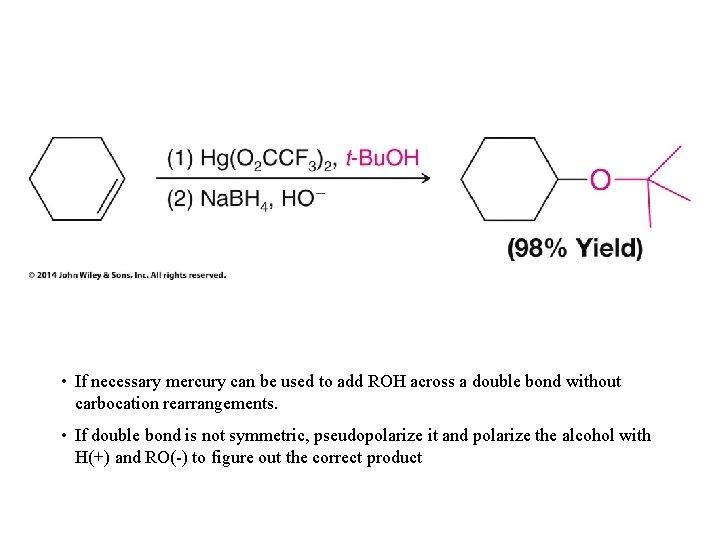
• If necessary mercury can be used to add ROH across a double bond without carbocation rearrangements. • If double bond is not symmetric, pseudopolarize it and polarize the alcohol with H(+) and RO(-) to figure out the correct product




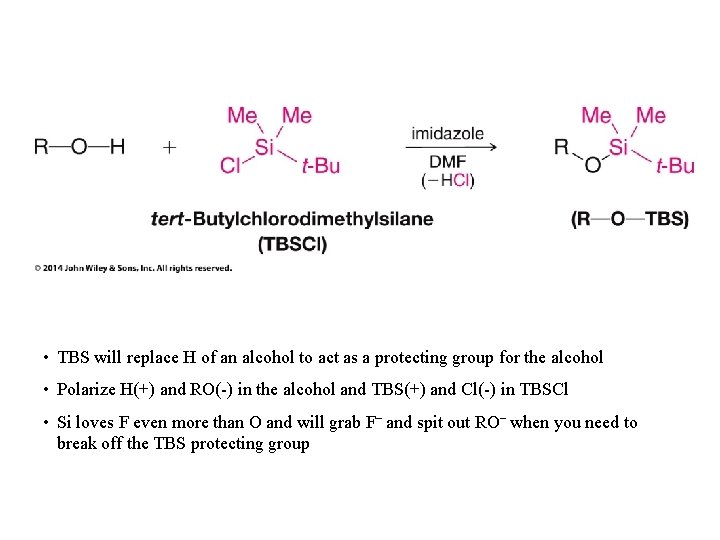
• TBS will replace H of an alcohol to act as a protecting group for the alcohol • Polarize H(+) and RO(-) in the alcohol and TBS(+) and Cl(-) in TBSCl • Si loves F even more than O and will grab F– and spit out RO– when you need to break off the TBS protecting group


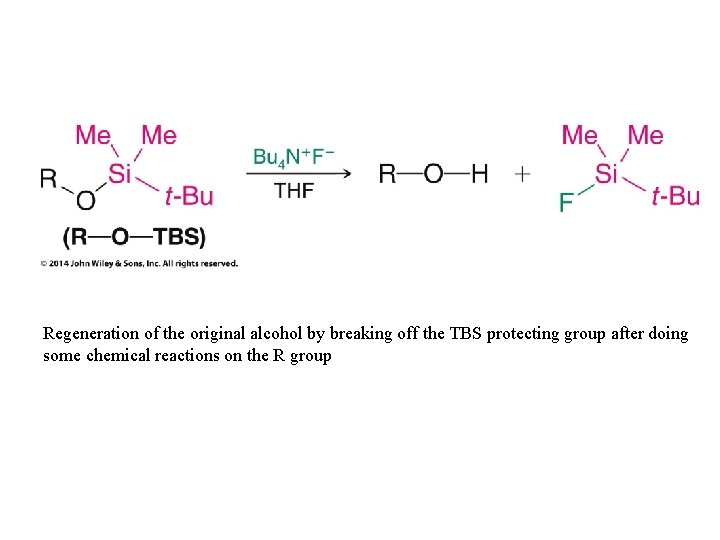
Regeneration of the original alcohol by breaking off the TBS protecting group after doing some chemical reactions on the R group








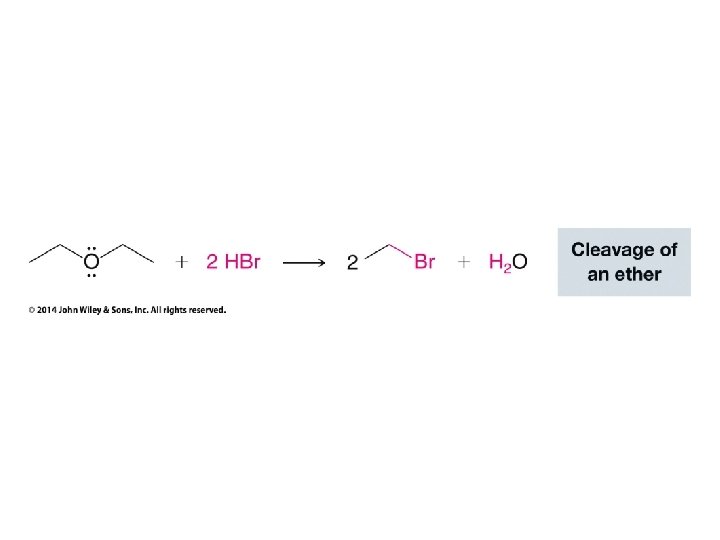
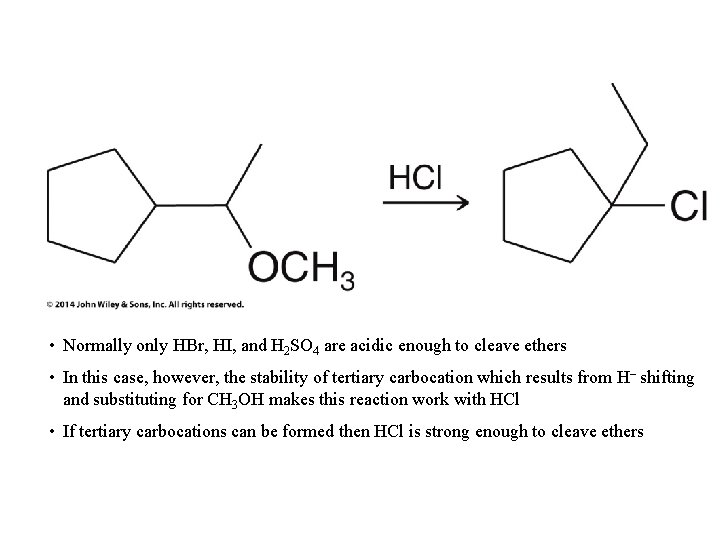
• Normally only HBr, HI, and H 2 SO 4 are acidic enough to cleave ethers • In this case, however, the stability of tertiary carbocation which results from H– shifting and substituting for CH 3 OH makes this reaction work with HCl • If tertiary carbocations can be formed then HCl is strong enough to cleave ethers







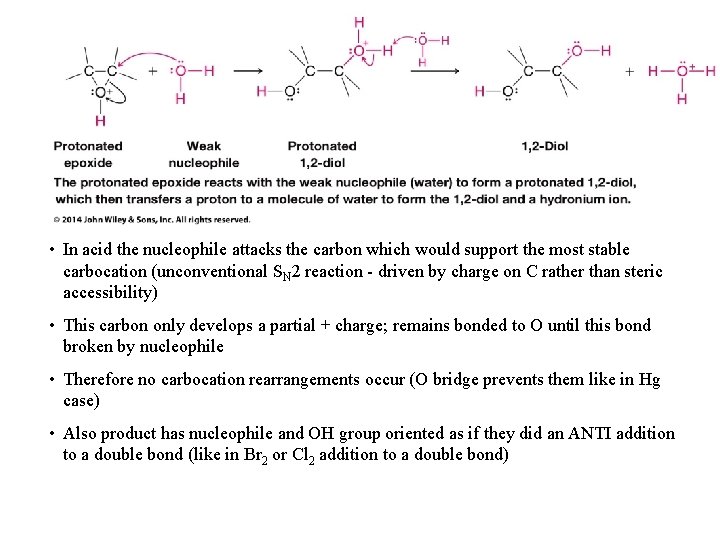
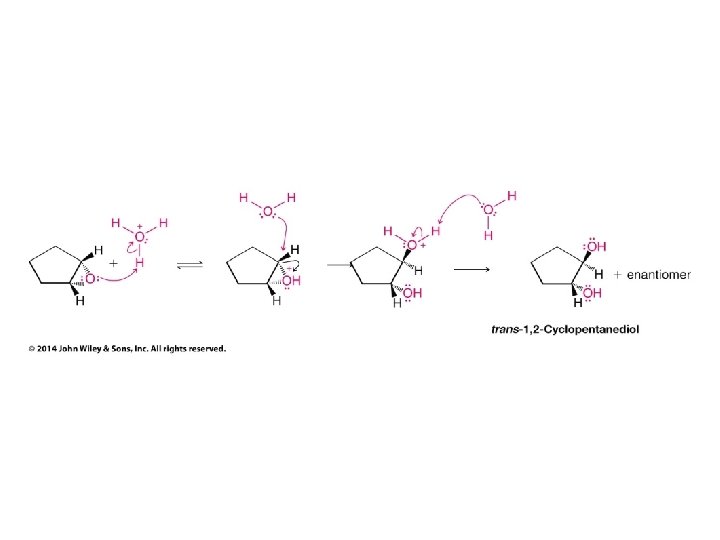
• In acid the nucleophile attacks the carbon which would support the most stable carbocation (unconventional SN 2 reaction - driven by charge on C rather than steric accessibility) • This carbon only develops a partial + charge; remains bonded to O until this bond broken by nucleophile • Therefore no carbocation rearrangements occur (O bridge prevents them like in Hg case) • Also product has nucleophile and OH group oriented as if they did an ANTI addition to a double bond (like in Br 2 or Cl 2 addition to a double bond)

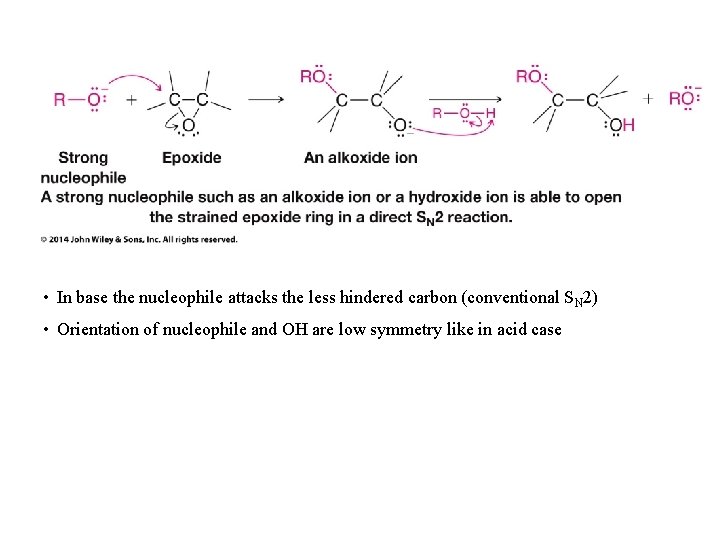
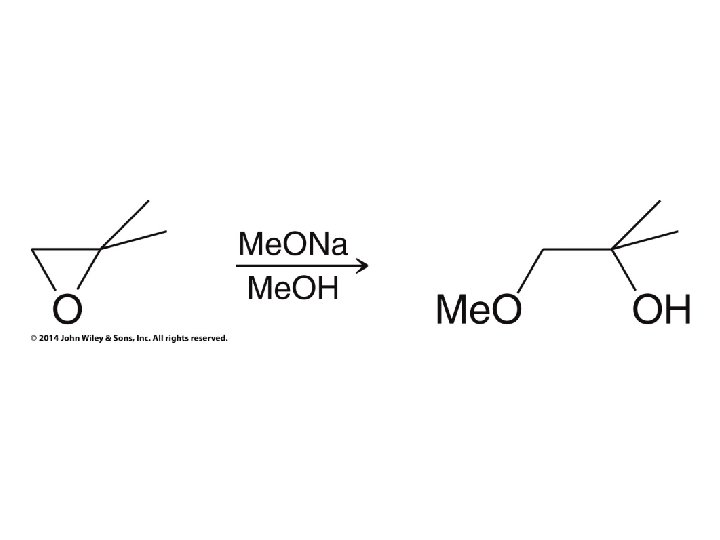
• In base the nucleophile attacks the less hindered carbon (conventional SN 2) • Orientation of nucleophile and OH are low symmetry like in acid case

• For product analysis polarize the less hindered C(+) and the epoxide O(–) • Polarize the nucleophile in the normal manner, break the correct C-O epoxide bond, and add the + fragment from the nucleophile to the – epoxide O; add the – fragment from the nucleophile to the + carbon of the epoxide ring

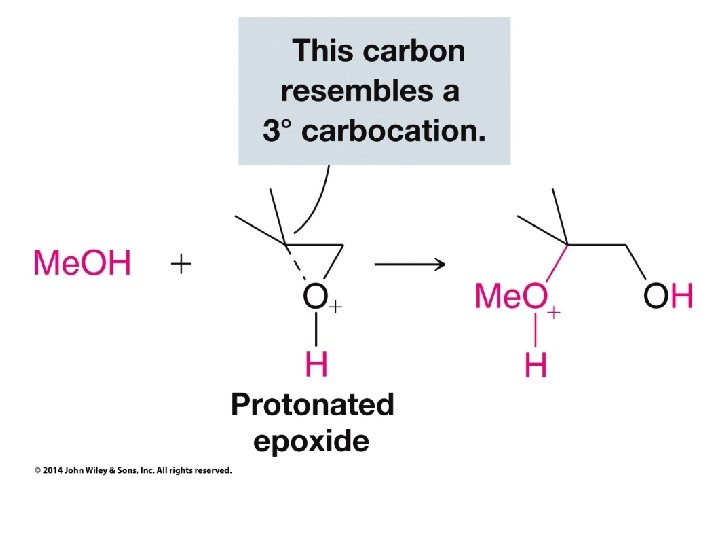
• Under acidic conditions the C which supports the most + charge gets the + polarization • Polarize everything else in the usual fashion and swap partners as usual with polar reactions • The correct bond to break in the epoxide is the one which connects the negativelypolarized O to the carbon which you polarized positive






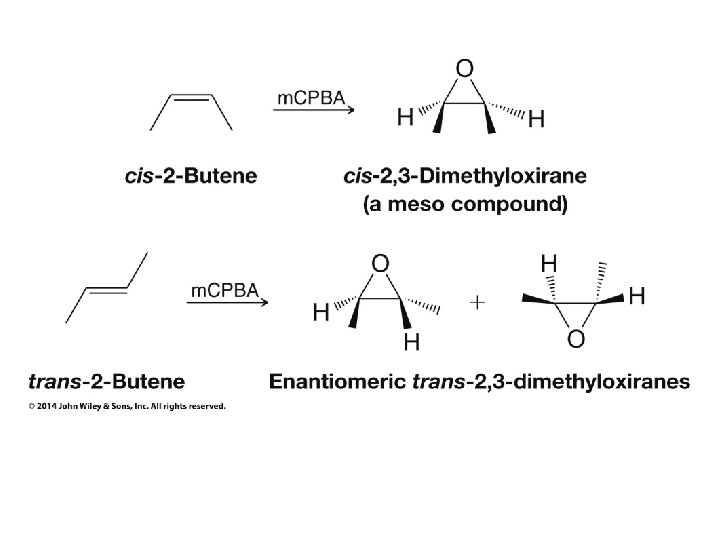
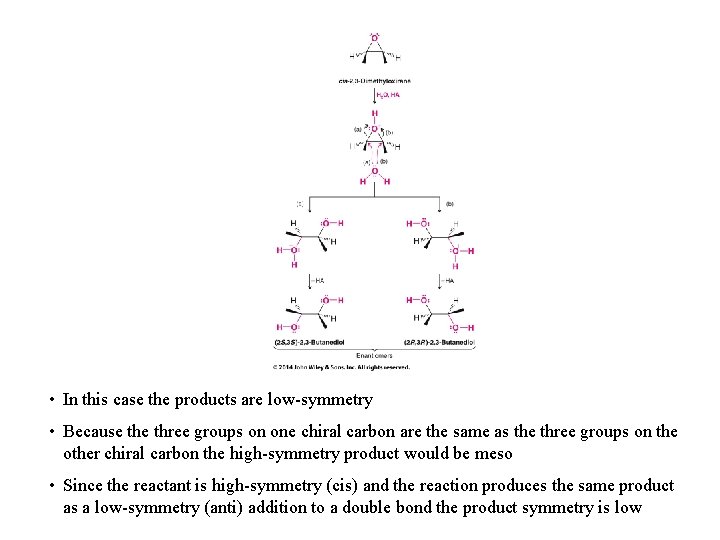
• In this case the products are low-symmetry • Because three groups on one chiral carbon are the same as the three groups on the other chiral carbon the high-symmetry product would be meso • Since the reactant is high-symmetry (cis) and the reaction produces the same product as a low-symmetry (anti) addition to a double bond the product symmetry is low

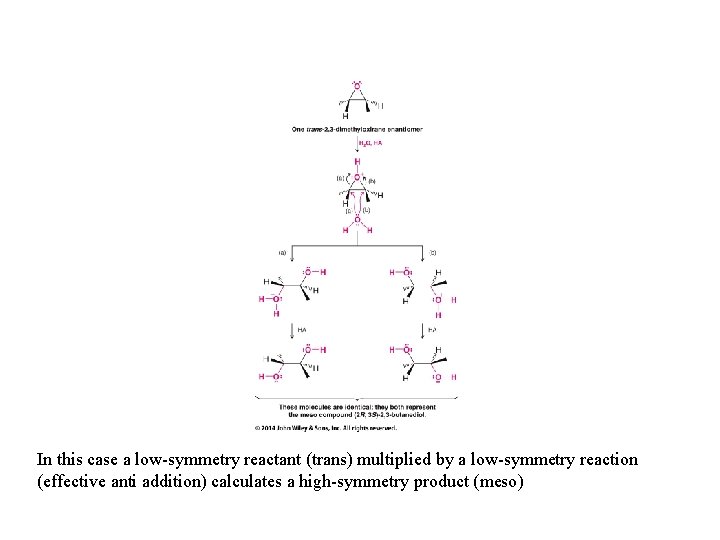
In this case a low-symmetry reactant (trans) multiplied by a low-symmetry reaction (effective anti addition) calculates a high-symmetry product (meso)

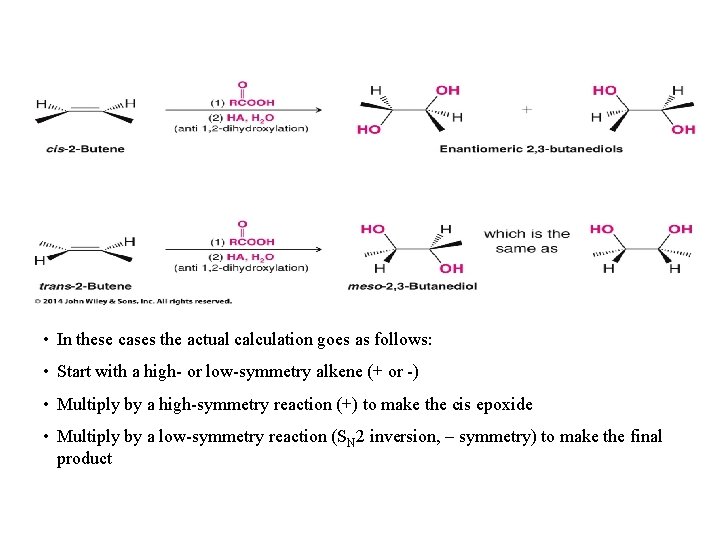
• In these cases the actual calculation goes as follows: • Start with a high- or low-symmetry alkene (+ or -) • Multiply by a high-symmetry reaction (+) to make the cis epoxide • Multiply by a low-symmetry reaction (SN 2 inversion, – symmetry) to make the final product

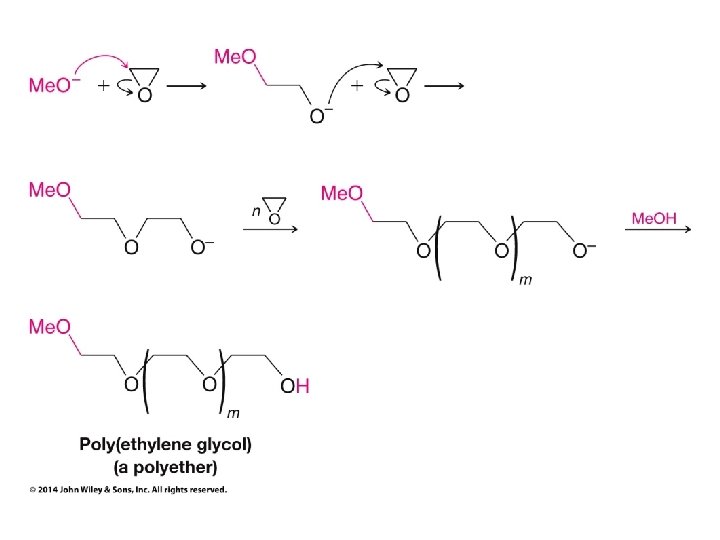
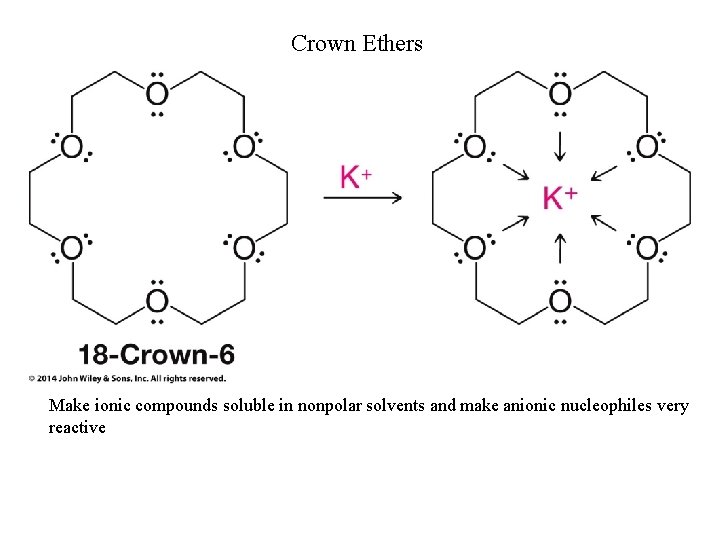
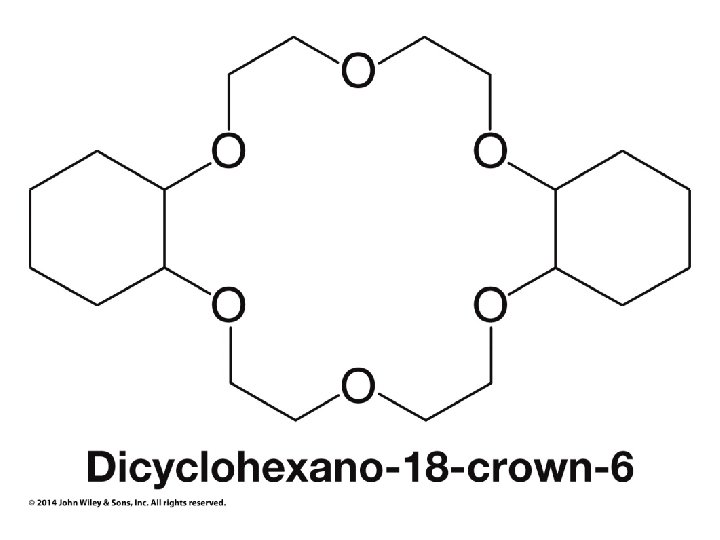
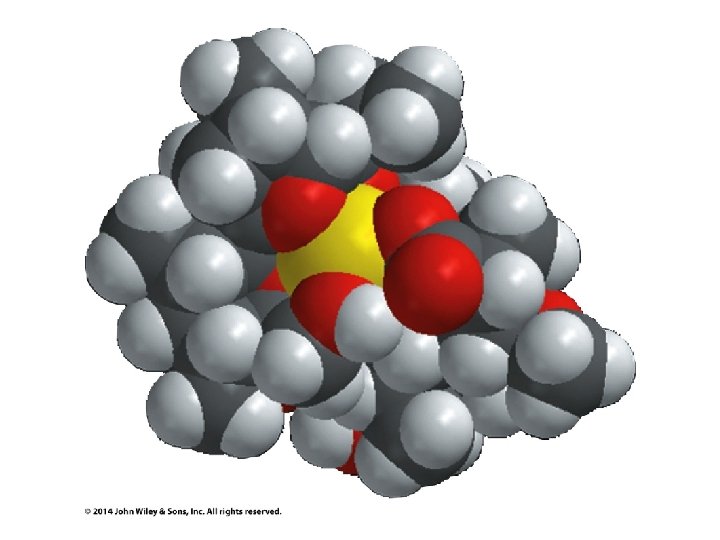
Crown Ethers Make ionic compounds soluble in nonpolar solvents and make anionic nucleophiles very reactive

