Organic Chemistry ELEVENTH EDITION Solomons Fryhle Snyder Chapter















- Slides: 95

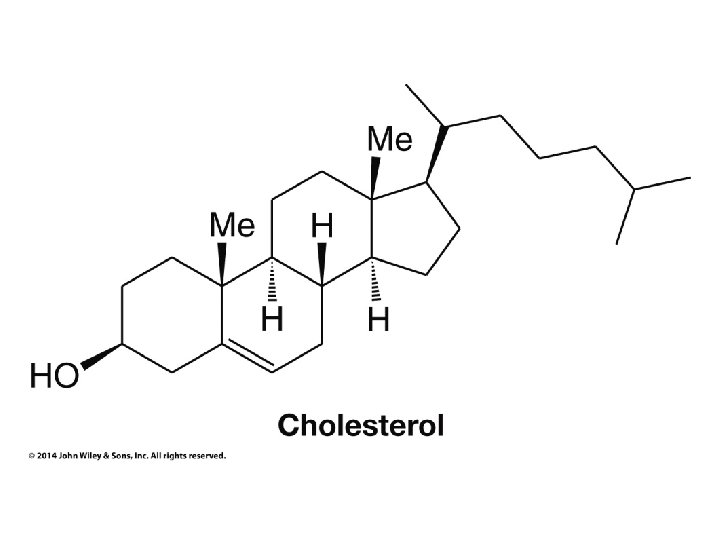
Organic Chemistry ELEVENTH EDITION Solomons • Fryhle • Snyder Chapter 5 Stereochemistry Copyright © 2014 by John Wiley & Sons, Inc. All rights reserved.

























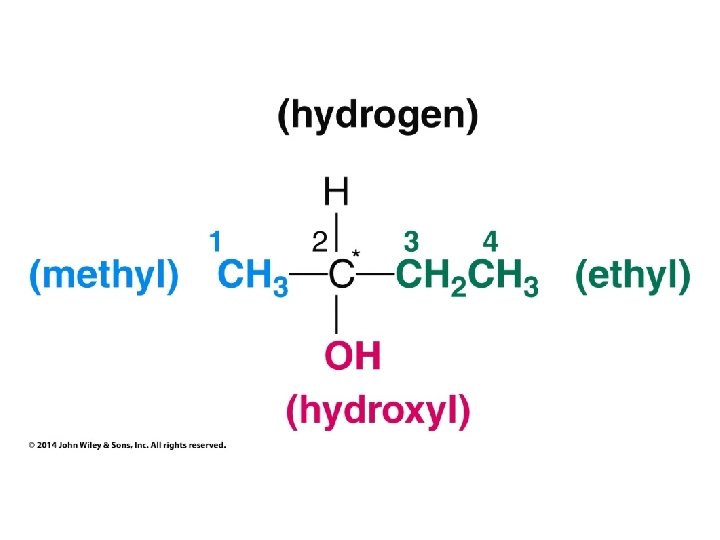
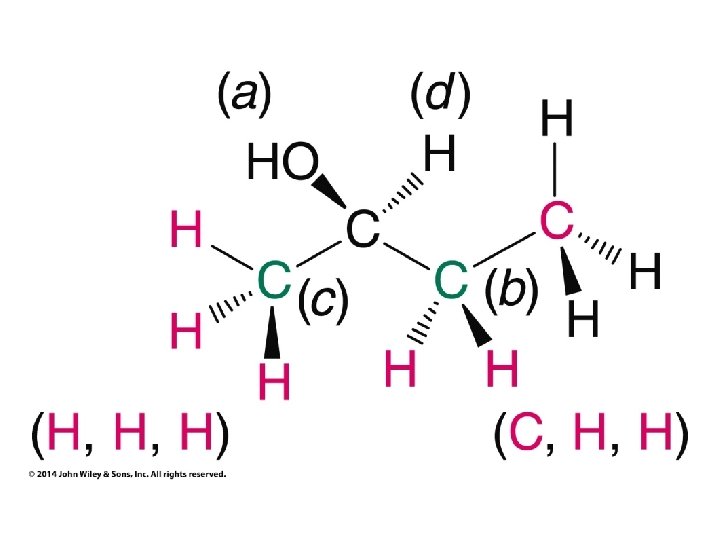
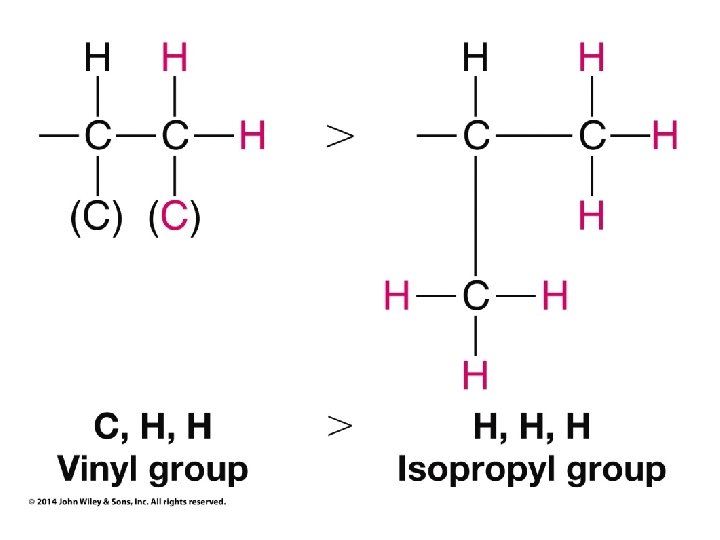
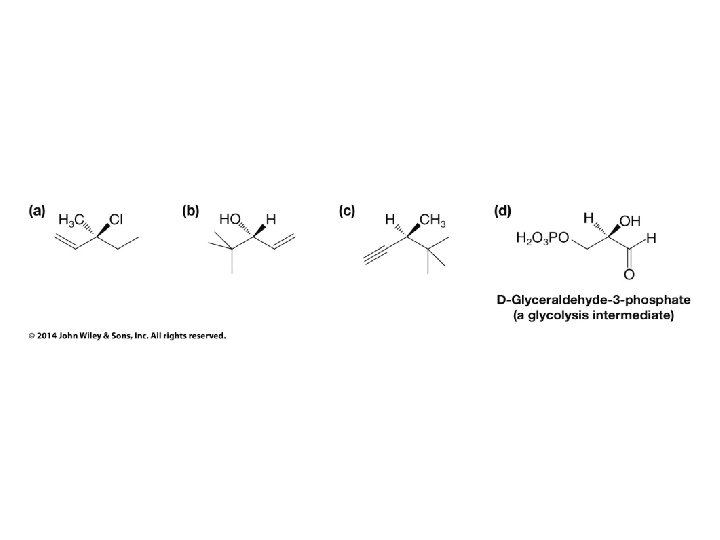
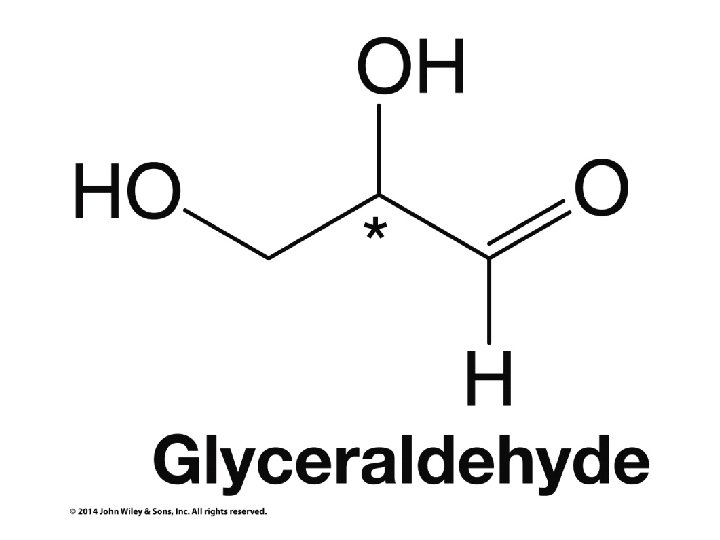
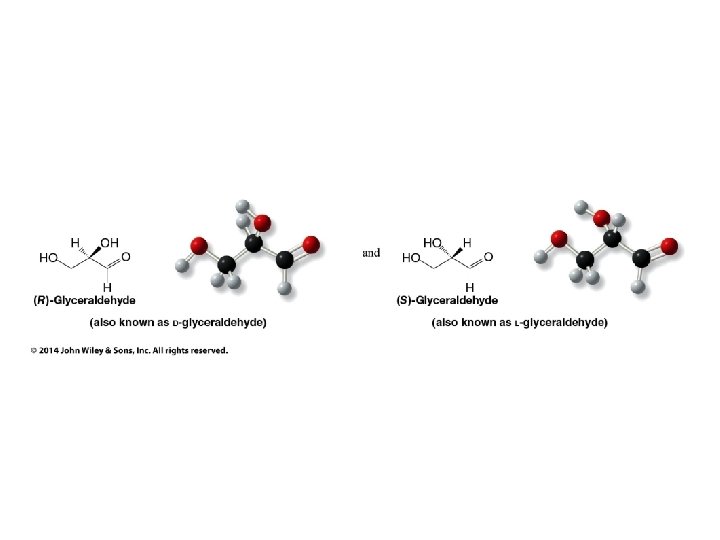
Stereochemistry Prioritization Rules • • Relative priorities depend first on the atomic number of the atom (not the formula weight of the group) bonded to the sp 2 carbon In the case of a tie, the atomic numbers of the atoms bonded to the tied atoms are considered next (e. g. C, C, & H beats C, H, & H)

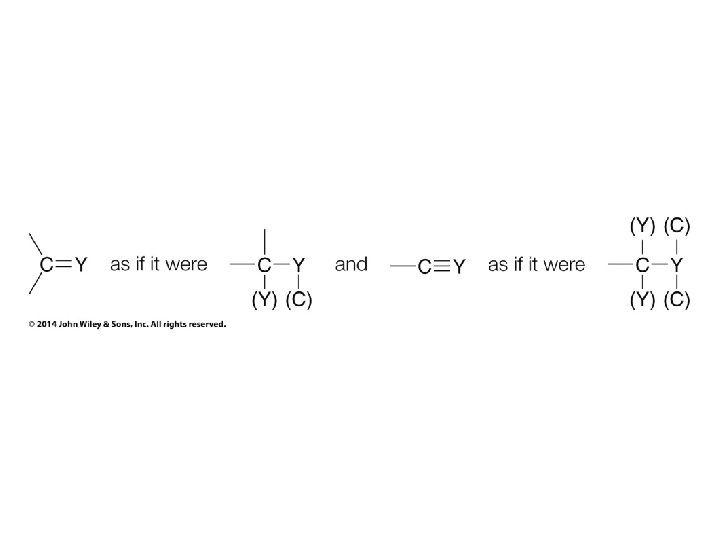
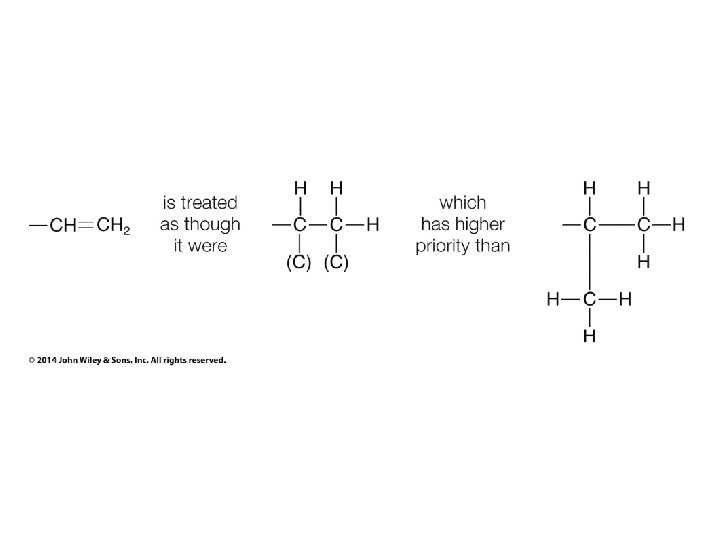
Stereochemistry Prioritization Rules • • If an atom is doubly bonded to another atom, the system treats it as if it were bonded to two such atoms In the case of isotopes, the isotope with the greater mass number has the higher priority




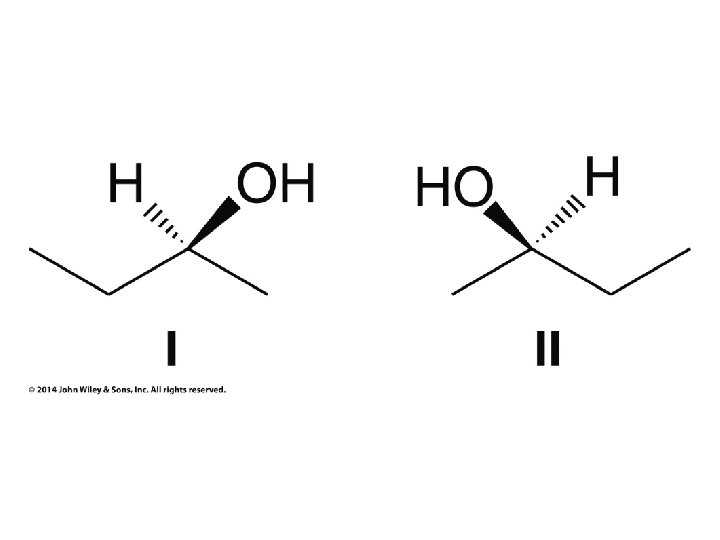
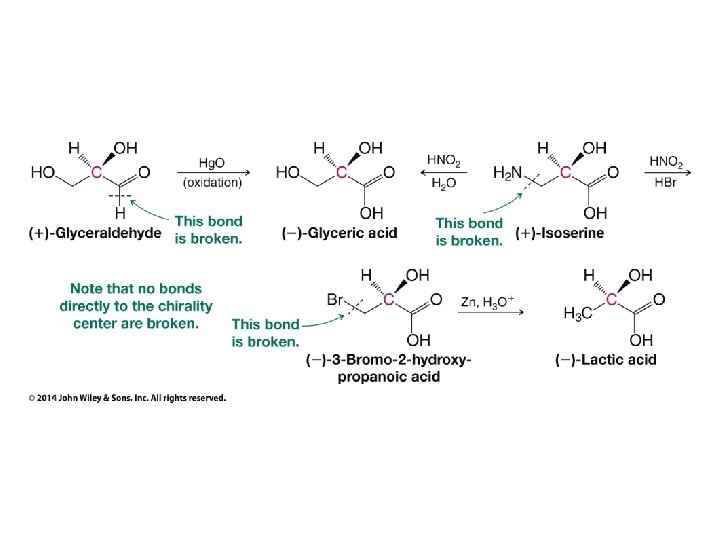
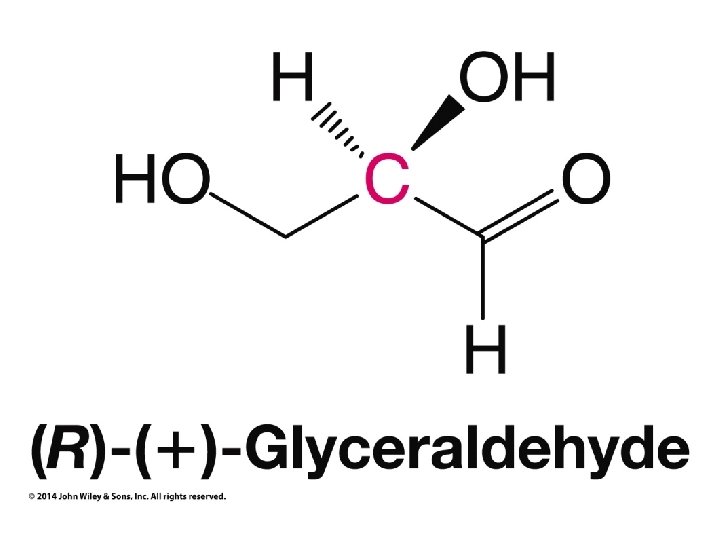
Hash and Wedge Tricks • Hash and wedge MUST be adjacent to each other!!! • If low priority group on hash ignore it, number other groups by priority, circle from 1 to 2 to 3 • If low priority group on wedge put minus below structure, ignore low priority group, circle from 1 to 2 to 3, and put result after minus sign (reverse result) • If low priority group in plane of paper (not hash OR wedge), number all groups by priority, swap numbers of low priority group and group on wedge, ignore number 4, and circle from 1 to 2 to 3 • Previous trick works because swapping low priority group gives opposite chirality, and ignoring low priority group on wedge gives opposite result of resulting chirality. Double opposite result is correct result.



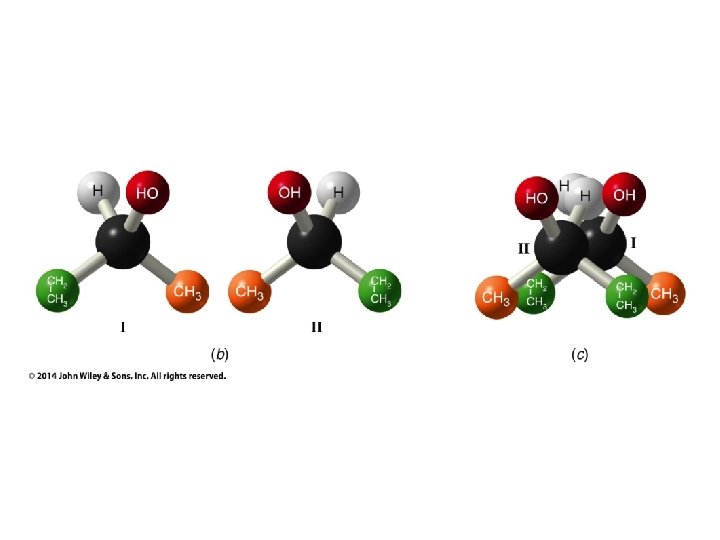
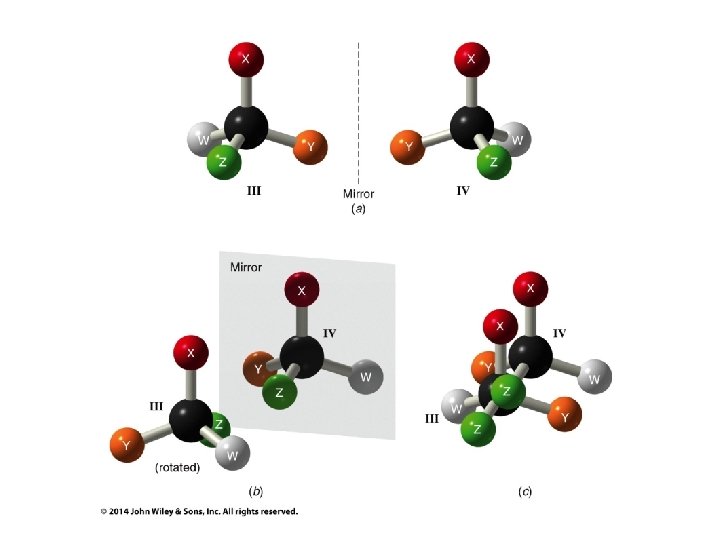
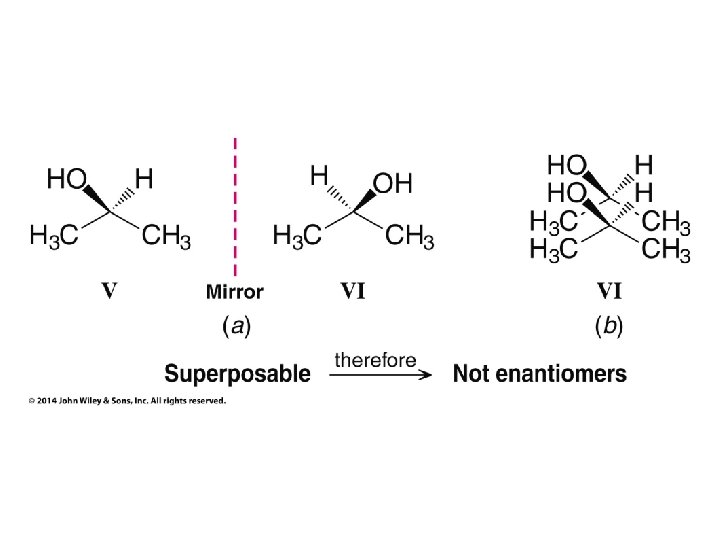
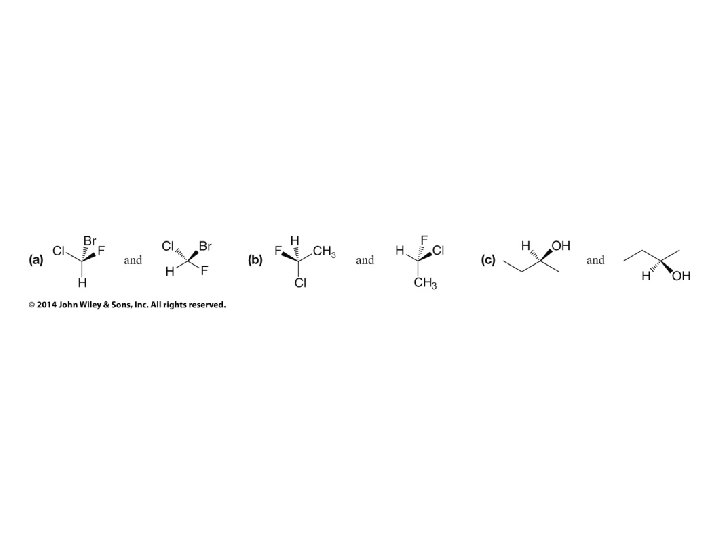
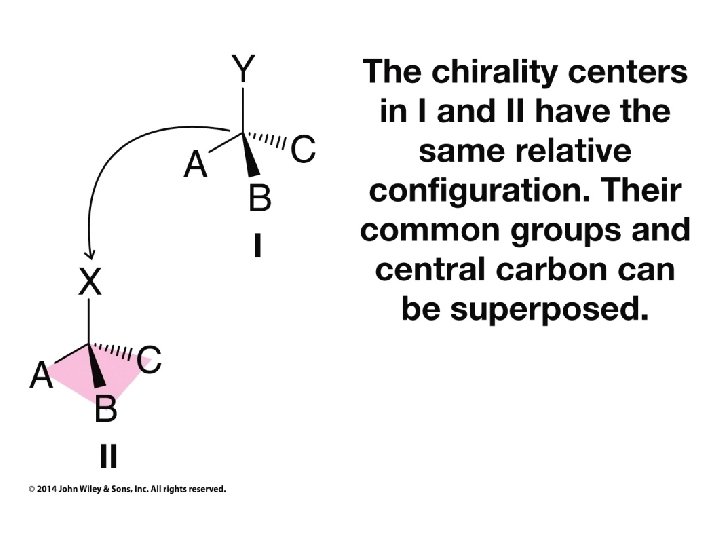
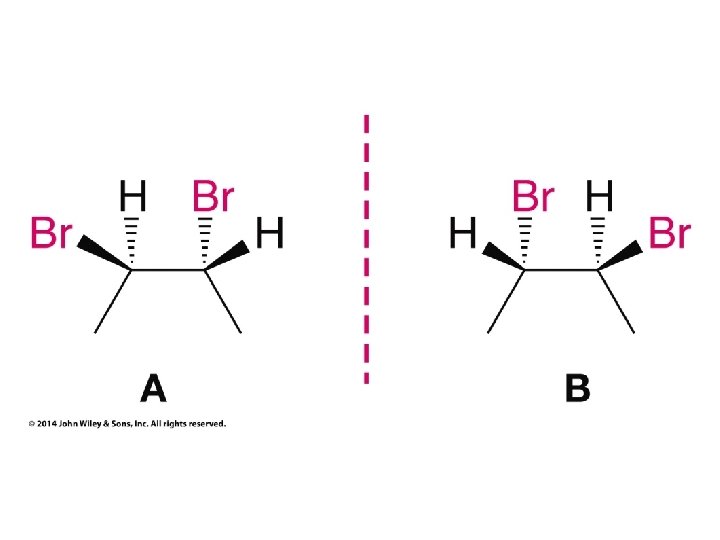
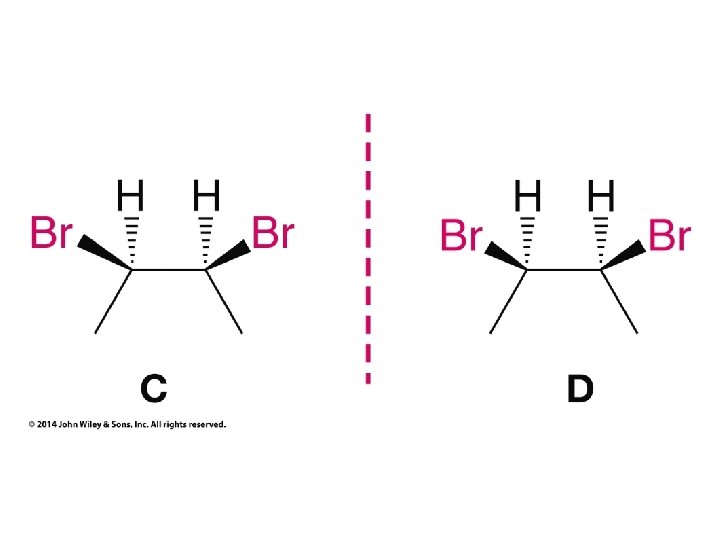
Hash/Wedge Comparisons • If two structures have all bonds in same positions and same groups attached to bonds, but groups attached differently comparison is done by swapping one group in one structure to be in same position in both structures • If both structures already have one group in same position and ALL THREE other groups in different positions structures are identical via rotation • If both groups have TWO AND ONLY TWO groups in same positions then structures have opposite chirality (other two groups are swapped) • If you need to swap a group in one structure to be in same position as in the other structure, give both structures labels and put a minus sign in front of label of structure where two groups have been swapped • Analyze resulting structures after making a necessary swap to put one group in common position in both structures; write an equation, ie, A = B, or -A ≠ B, etc.










































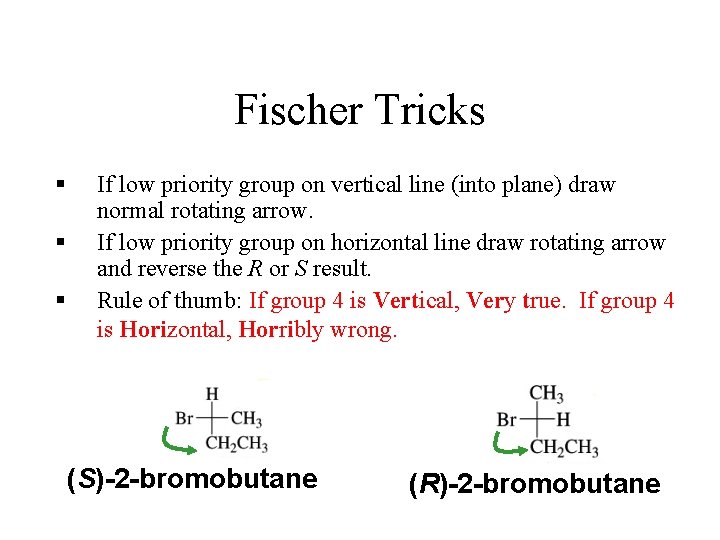
Fischer Tricks § § § If low priority group on vertical line (into plane) draw normal rotating arrow. If low priority group on horizontal line draw rotating arrow and reverse the R or S result. Rule of thumb: If group 4 is Vertical, Very true. If group 4 is Horizontal, Horribly wrong. (S)-2 -bromobutane (R)-2 -bromobutane

Fischer Tricks § § 90º rotation reverses all chiralities. 180º rotation maintains all chiralities.

Multi-Carbon Fischer Trick § § Can rotate 3 groups either CW or CCW without changing configuration If 3 grps have same rel CW or CCW sequence C’s same

Newman Projections § § For front C swap low priority group to back C if back C is not low priority group IF swap was NECESSARY curved arrow now gives reversed chirality

Newman Projections § § For back C swap low priority group to front C IF swap was NECESSARY curved arrow now gives correct chirality; otherwise reversed

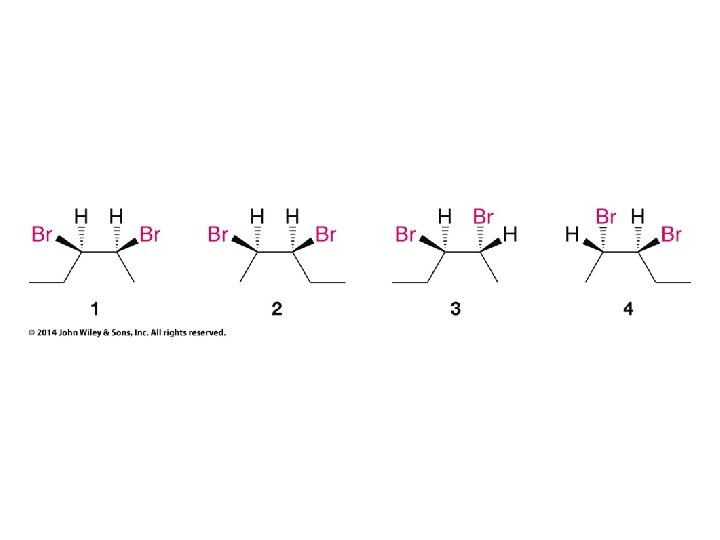
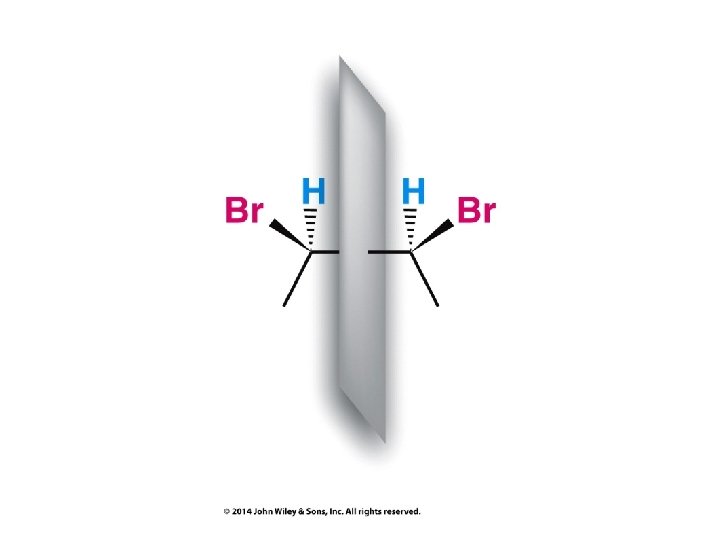
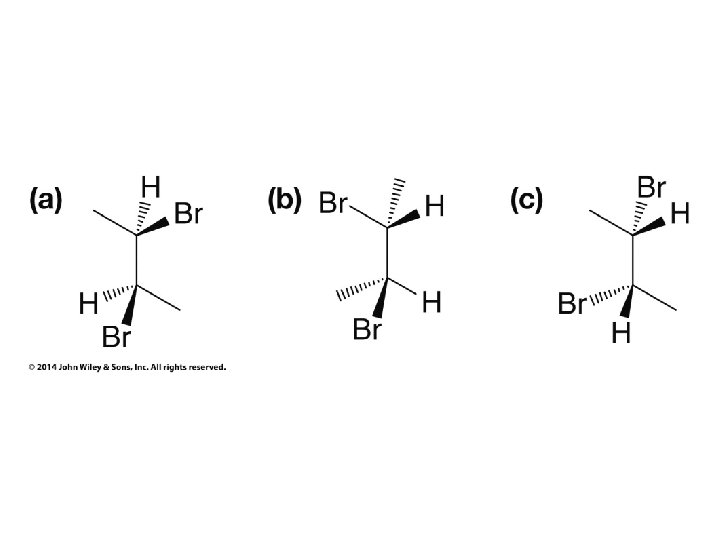
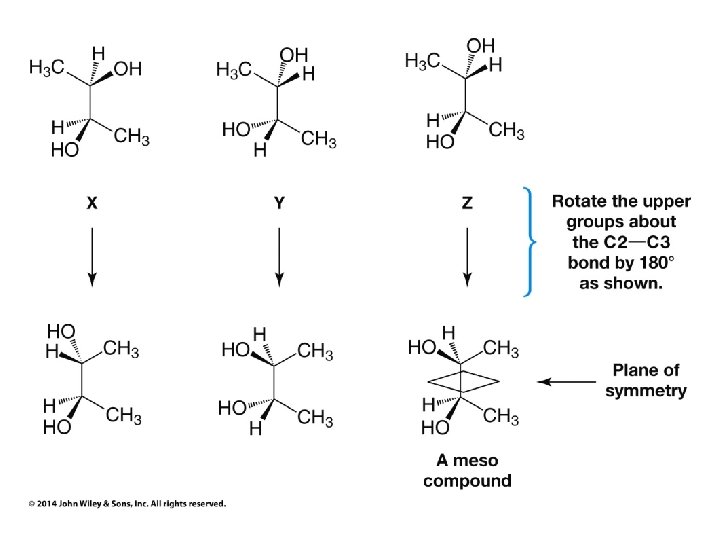
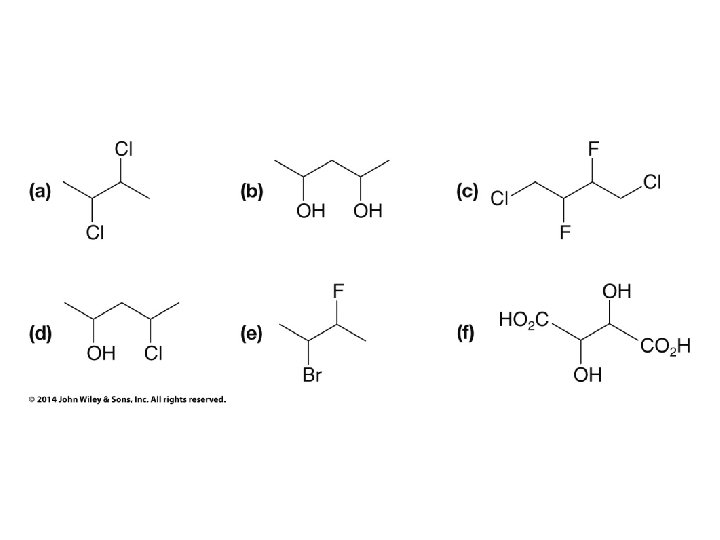
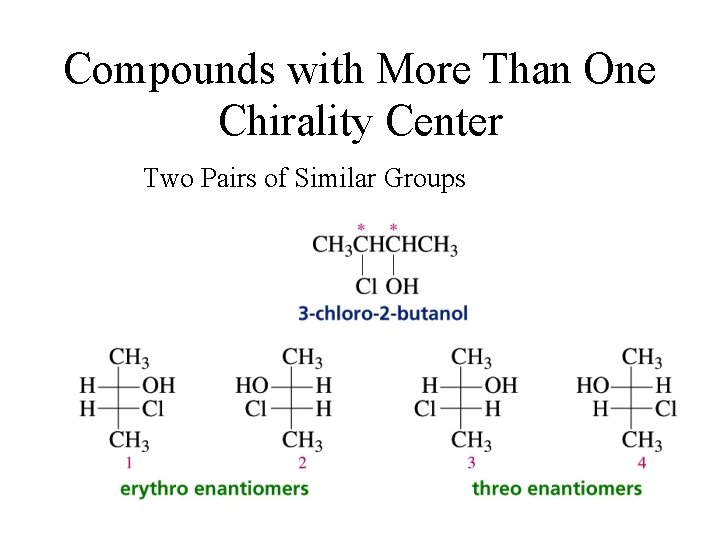
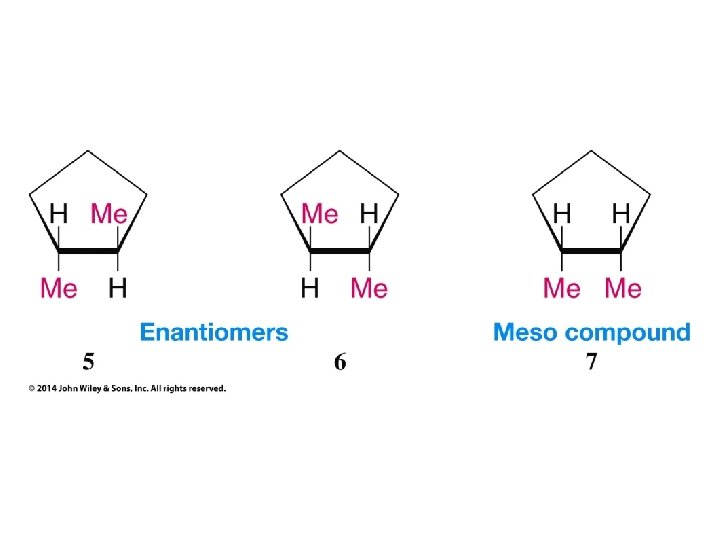
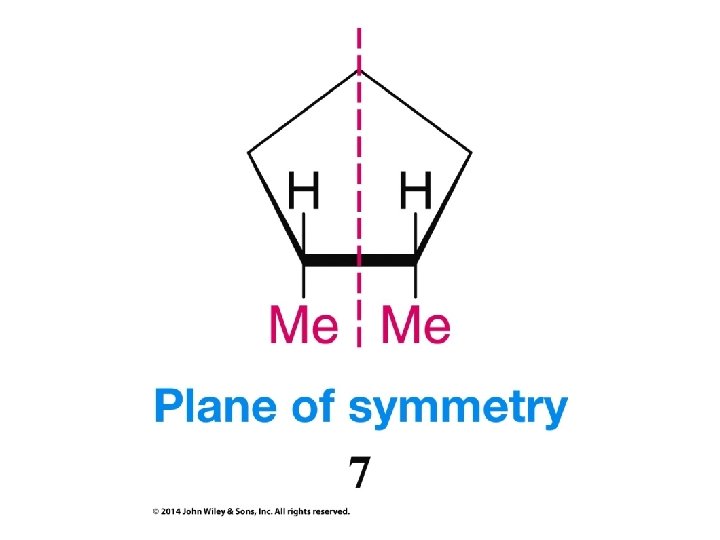
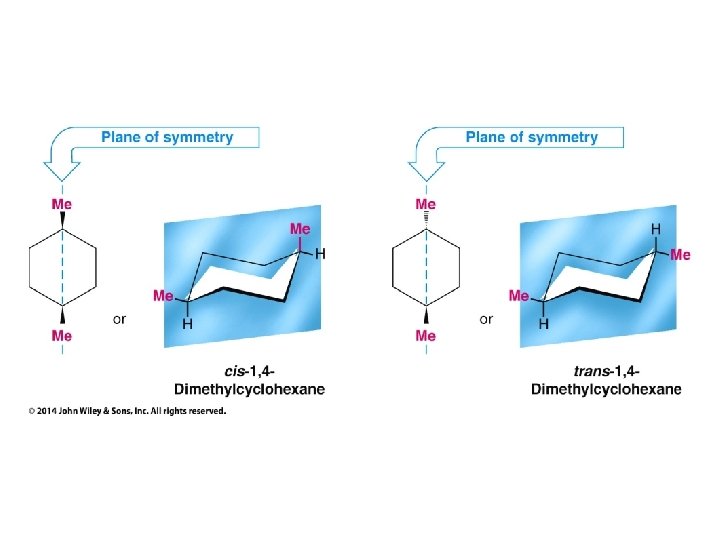
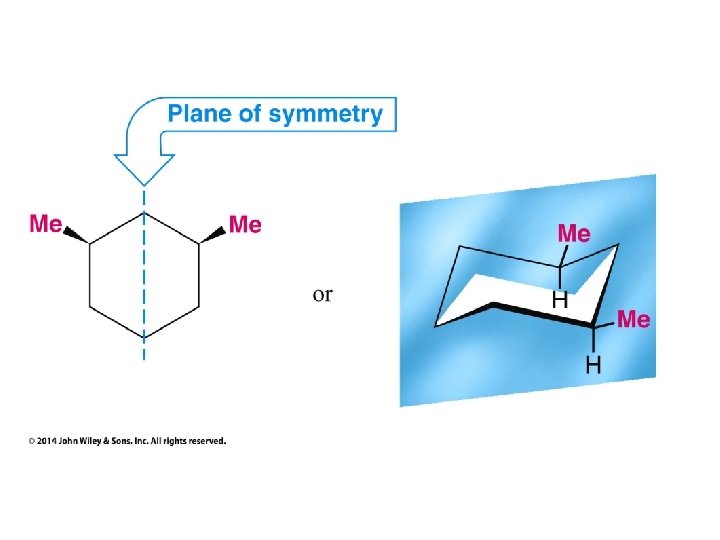
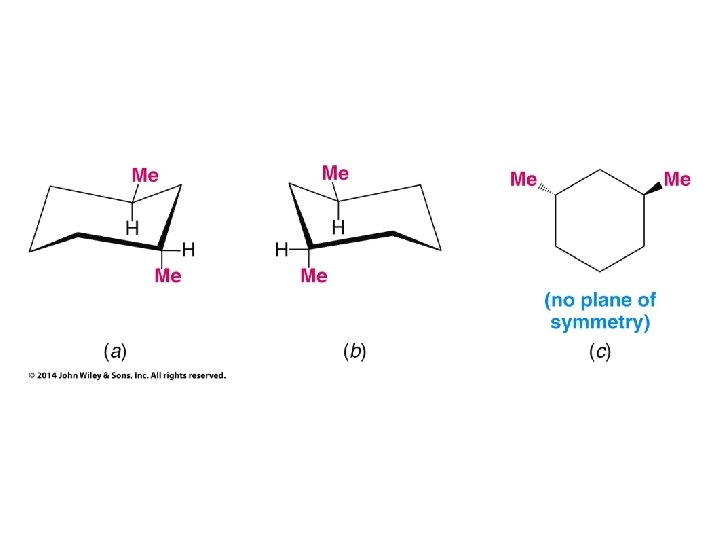
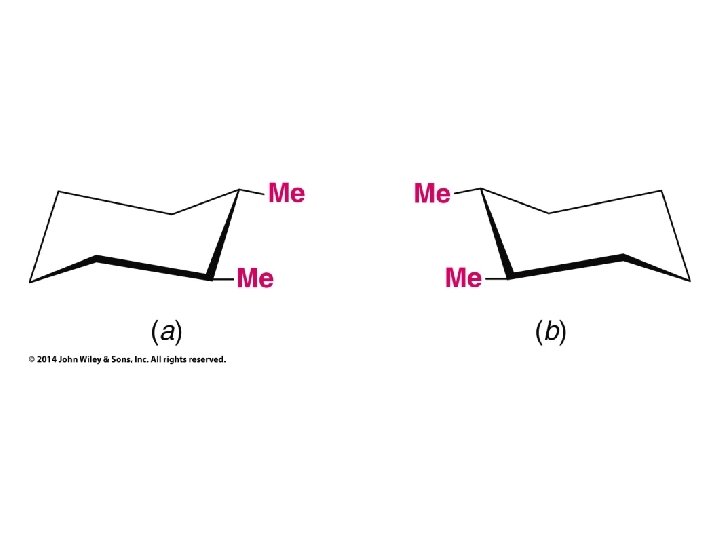
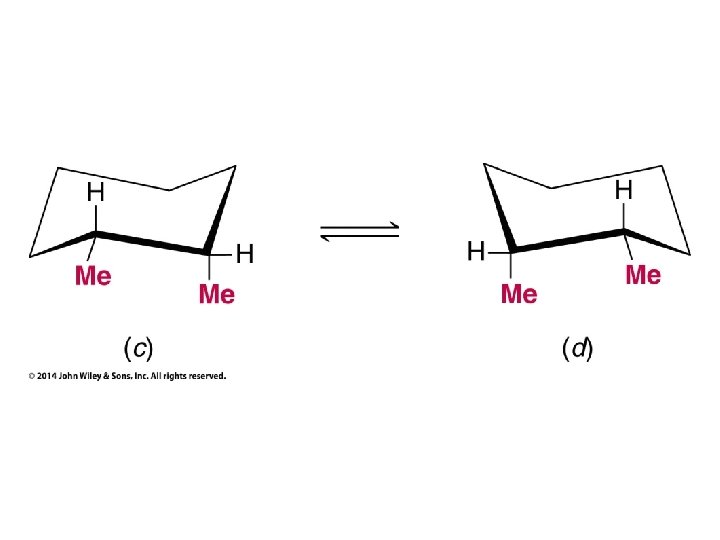
Compounds with More Than One Chirality Center Two Pairs of Similar Groups

Compounds with More Than One Chirality Center Three Pairs of Similar Groups

Topology of Stereoisomers With Two Chiral Centers











