Organic Chemistry Elements in Living Things Notes Carbon

Organic Chemistry Elements in Living Things Notes
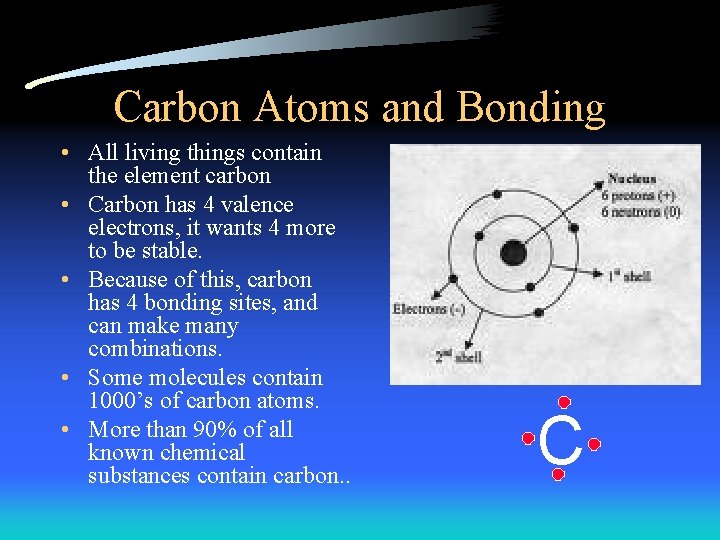
Carbon Atoms and Bonding • All living things contain the element carbon • Carbon has 4 valence electrons, it wants 4 more to be stable. • Because of this, carbon has 4 bonding sites, and can make many combinations. • Some molecules contain 1000’s of carbon atoms. • More than 90% of all known chemical substances contain carbon. . C
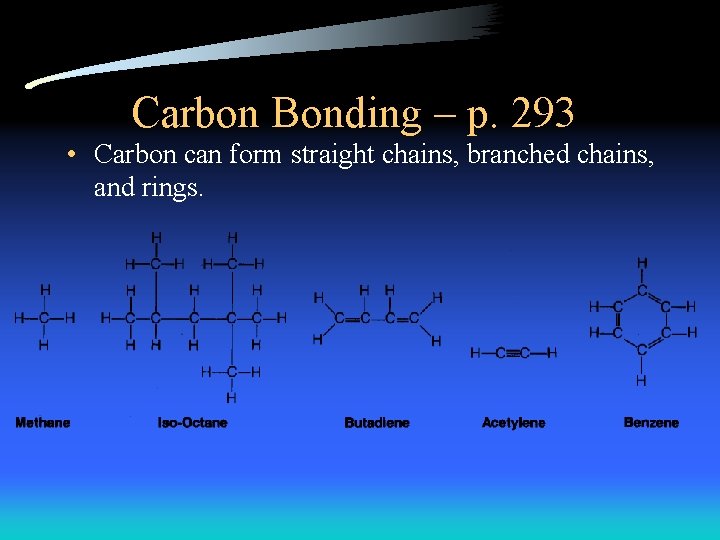
Carbon Bonding – p. 293 • Carbon can form straight chains, branched chains, and rings.

Diamonds – pure carbon • Carbon’s pure forms exist as diamonds, graphite, fullerenes, and nanotubes. • Diamonds form at really high temperatures and pressures. • Diamonds are extremely strong and nonreactive

Graphite – pure carbon • Graphite is carbon bonded to 3 other carbon atoms in flat layers. • Graphite is the ‘lead’ in your pencils.
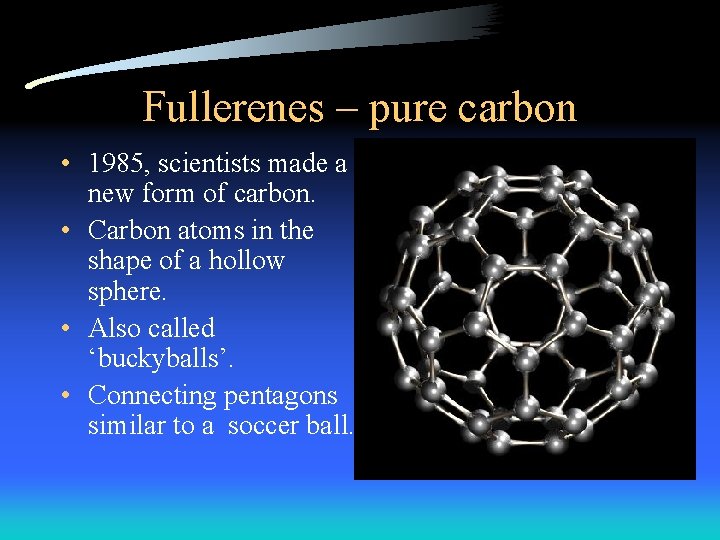
Fullerenes – pure carbon • 1985, scientists made a new form of carbon. • Carbon atoms in the shape of a hollow sphere. • Also called ‘buckyballs’. • Connecting pentagons similar to a soccer ball.

Nanotubes – pure carbon • In 1991, another form of carbon was made. • Nanotubes are carbon atoms arranged in the shape of a long, hollow cylinder. • Only a few nanometers wide. • Tiny, light, flexible, extremely strong, and good conductors of electricity and heat. • Nanotubes can make extremely strong cables.

Hydrocarbons • The simplest organic compounds are hydrocarbons – contains only hydrogen and carbon. • Examples: methane, propane, ethane, and butane • Properties: all flammable, and mostly used as fuels for stoves, heaters, cars, buses, and airplanes.
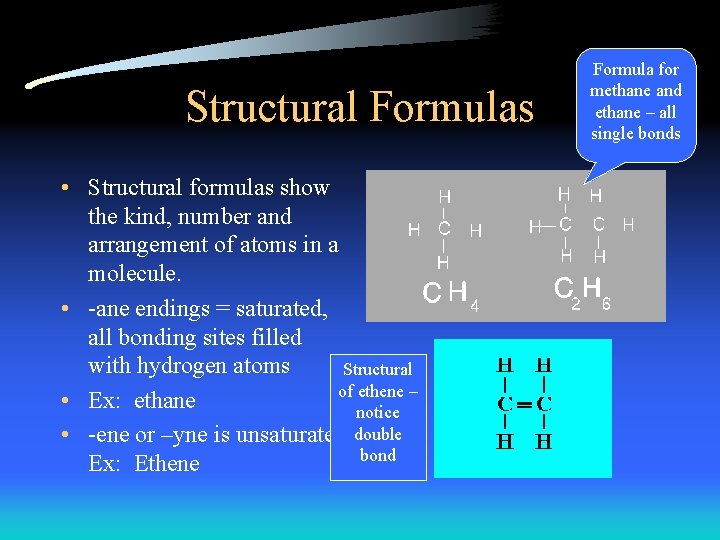
Structural Formulas • Structural formulas show the kind, number and arrangement of atoms in a molecule. • -ane endings = saturated, all bonding sites filled with hydrogen atoms Structural of ethene – • Ex: ethane notice • -ene or –yne is unsaturated. double bond Ex: Ethene Formula for methane and ethane – all single bonds

Polymers • Polymers are very large molecules made of a chain of many smaller molecules called ‘monomers’ bonded together. • Ex of polymers: DNA, cotton, silk, plastic, polyester, nylon, etc…
- Slides: 10