Organic Chemistry E 1 Reactions E 1 Elimination



- Slides: 21

Organic Chemistry E 1 Reactions

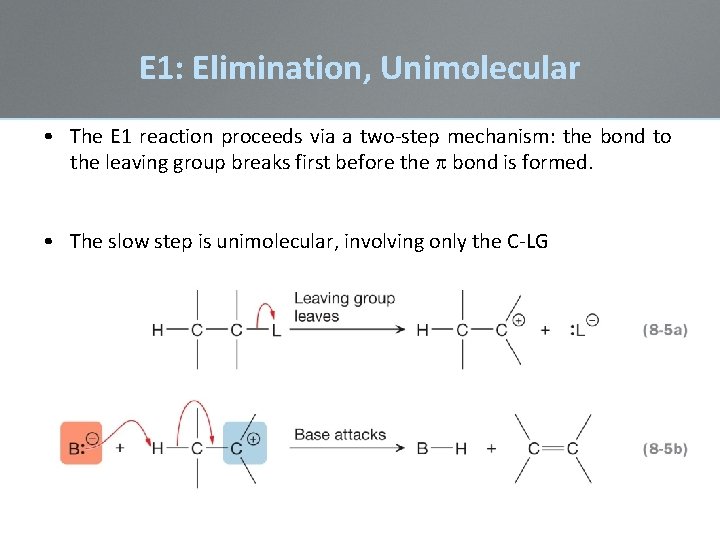
E 1: Elimination, Unimolecular • The E 1 reaction proceeds via a two-step mechanism: the bond to the leaving group breaks first before the bond is formed. • The slow step is unimolecular, involving only the C-LG

Four-way Rate Competition S N 1 S N 2 E 1

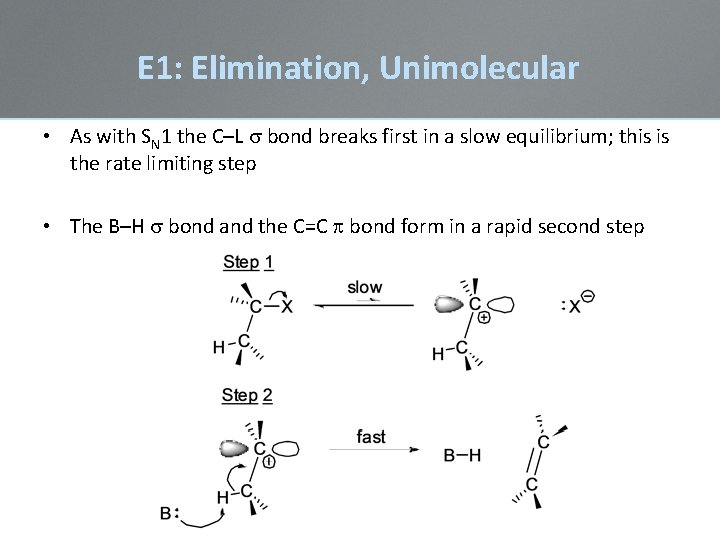
E 1: Elimination, Unimolecular • As with SN 1 the C–L s bond breaks first in a slow equilibrium; this is the rate limiting step • The B–H s bond and the C=C bond form in a rapid second step

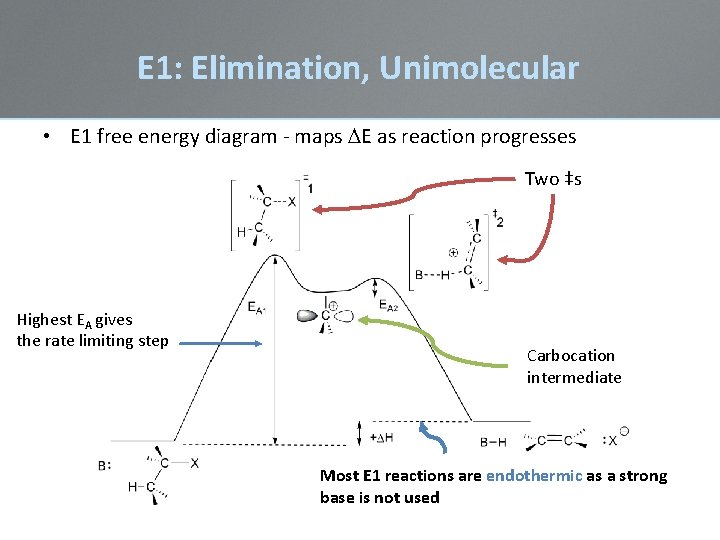
E 1: Elimination, Unimolecular • E 1 free energy diagram - maps DE as reaction progresses Two ‡s Highest EA gives the rate limiting step Carbocation intermediate Most E 1 reactions are endothermic as a strong base is not used

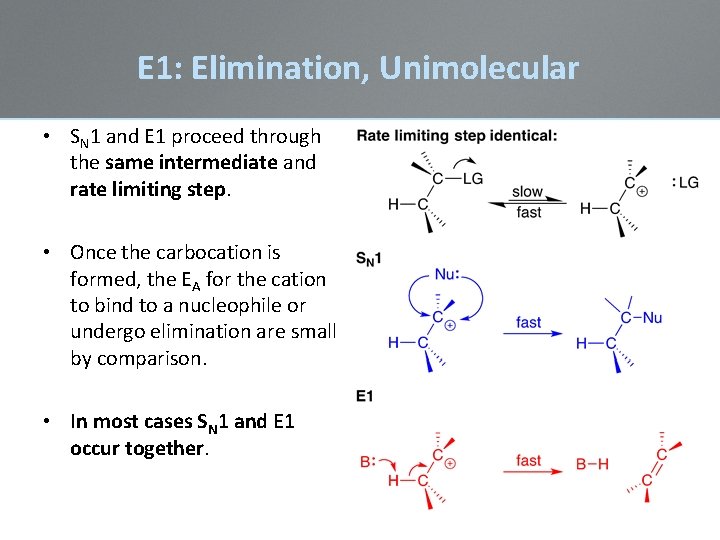
E 1: Elimination, Unimolecular • SN 1 and E 1 proceed through the same intermediate and rate limiting step. • Once the carbocation is formed, the EA for the cation to bind to a nucleophile or undergo elimination are small by comparison. • In most cases SN 1 and E 1 occur together.

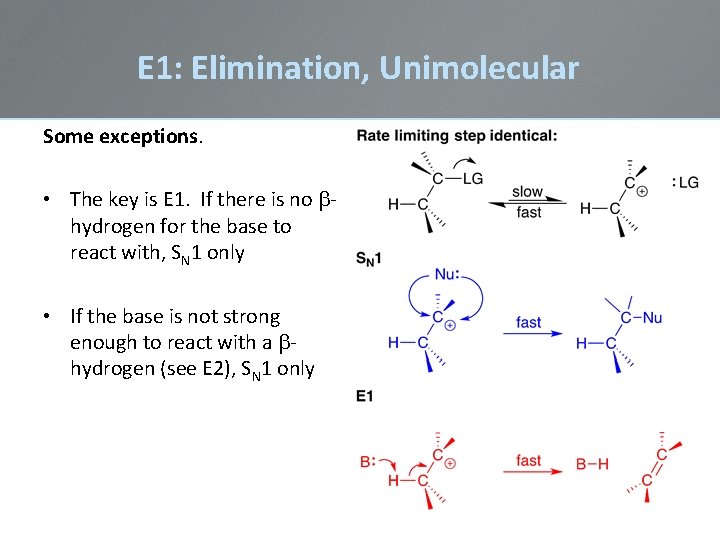
E 1: Elimination, Unimolecular Some exceptions. • The key is E 1. If there is no bhydrogen for the base to react with, SN 1 only • If the base is not strong enough to react with a bhydrogen (see E 2), SN 1 only

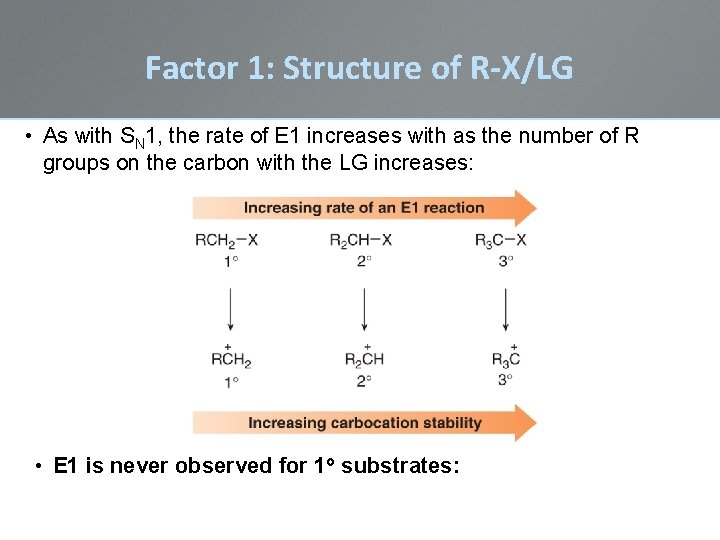
Factor 1: Structure of R-X/LG • As with SN 1, the rate of E 1 increases with as the number of R groups on the carbon with the LG increases: • E 1 is never observed for 1 o substrates:

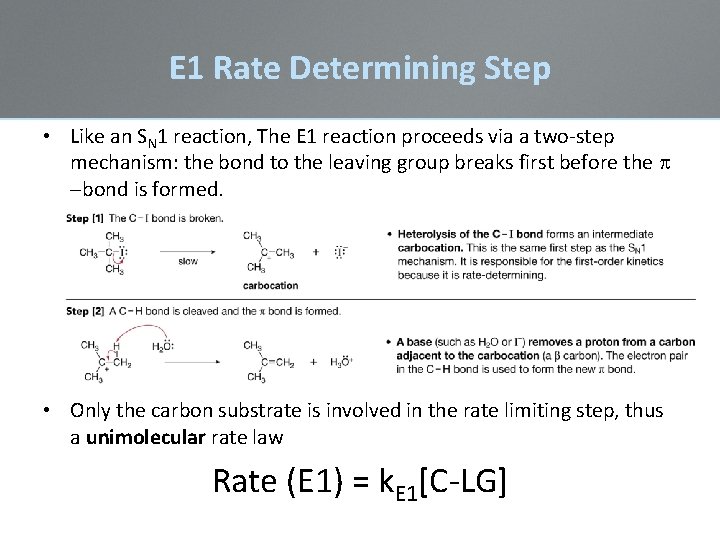
E 1 Rate Determining Step • Like an SN 1 reaction, The E 1 reaction proceeds via a two-step mechanism: the bond to the leaving group breaks first before the -bond is formed. • Only the carbon substrate is involved in the rate limiting step, thus a unimolecular rate law Rate (E 1) = k. E 1[C-LG]

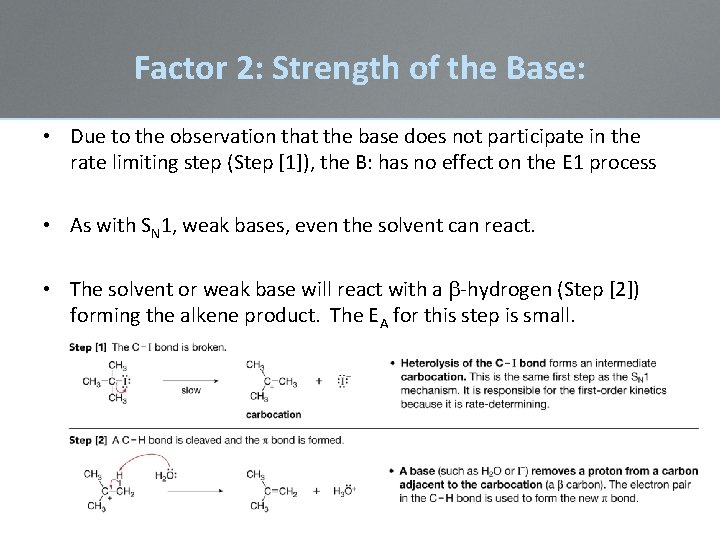
Factor 2: Strength of the Base: • Due to the observation that the base does not participate in the rate limiting step (Step [1]), the B: has no effect on the E 1 process • As with SN 1, weak bases, even the solvent can react. • The solvent or weak base will react with a b-hydrogen (Step [2]) forming the alkene product. The EA for this step is small.

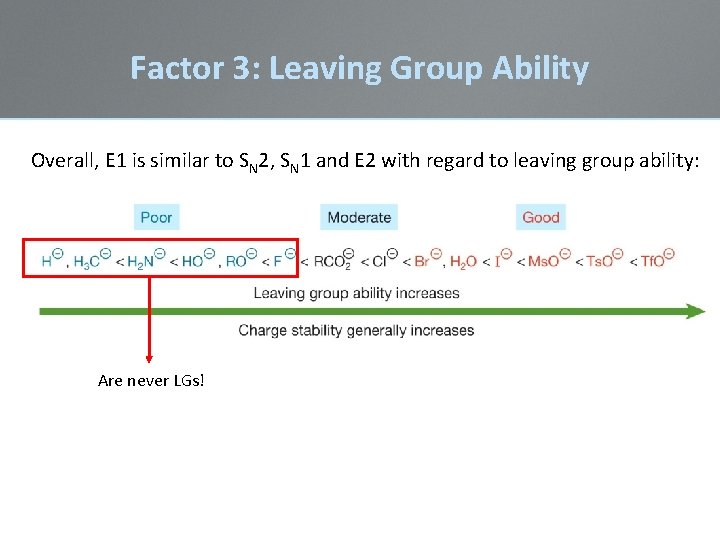
Factor 3: Leaving Group Ability • A leaving group must leave in the rate-determining step of an SN 2, SN 1, E 2, or E 1 reaction. • The identity of the leaving group has an effect on the rate of each reaction. • A good leaving group is necessary for the reaction to be exothermic (and spontaneous) via a –DH • Leaving group ability strongly affects E 1 reactions

Factor 3: Leaving Group Ability Overall, E 1 is similar to SN 2, SN 1 and E 2 with regard to leaving group ability: Are never LGs!

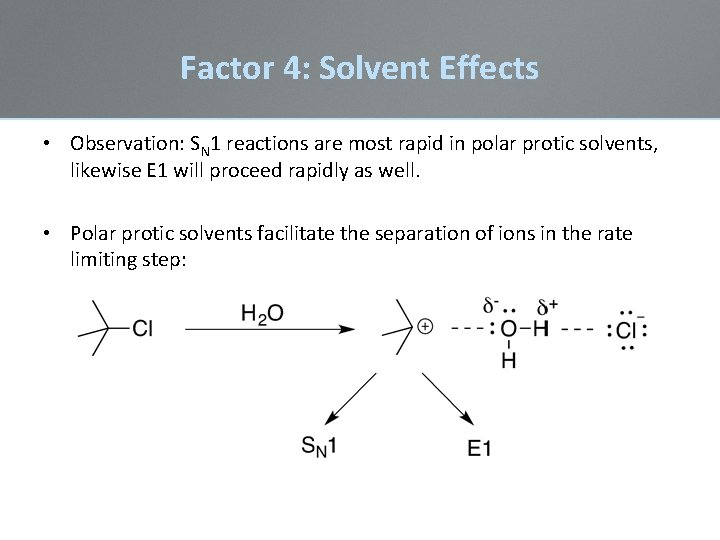
Factor 4: Solvent Effects • Observation: SN 1 reactions are most rapid in polar protic solvents, likewise E 1 will proceed rapidly as well. • Polar protic solvents facilitate the separation of ions in the rate limiting step:

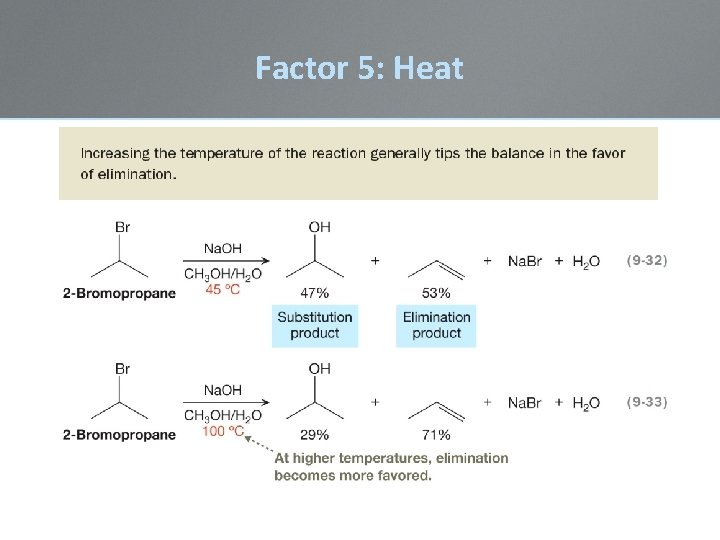
Factor 5: Heat • When substitution and elimination reactions are both favored under a specific set of conditions, it is often possible to influence the outcome by changing the temperature under which the reactions take place. • All of these reactions have an EA that needs to be surmounted. • Heat will accelerate the rate of all reactions; the object is not to overheat to allow higher EA reaction pathways to compete • E 1 reactions are more strongly accelerated by heat than SN 1

Factor 5: Heat

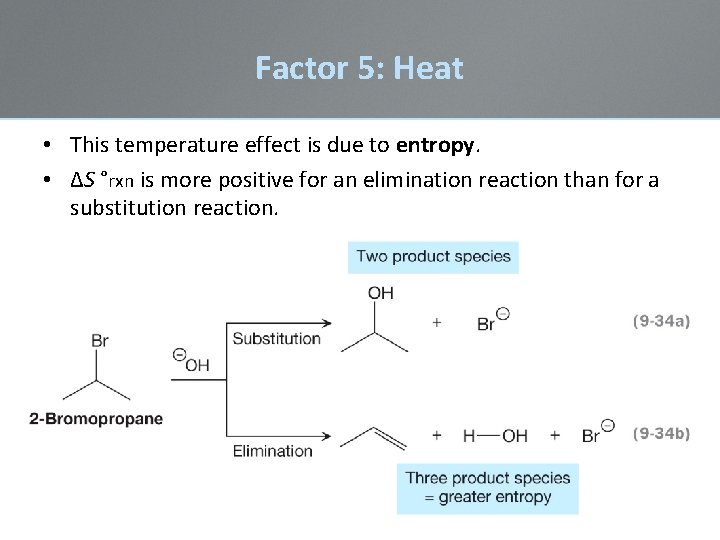
Factor 5: Heat • This temperature effect is due to entropy. • ∆S °rxn is more positive for an elimination reaction than for a substitution reaction.

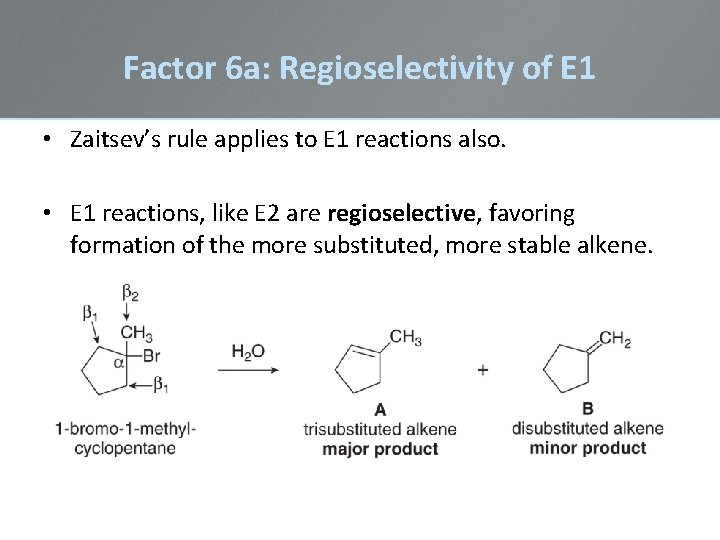
Factor 6 a: Regioselectivity of E 1 • Zaitsev’s rule applies to E 1 reactions also. • E 1 reactions, like E 2 are regioselective, favoring formation of the more substituted, more stable alkene.

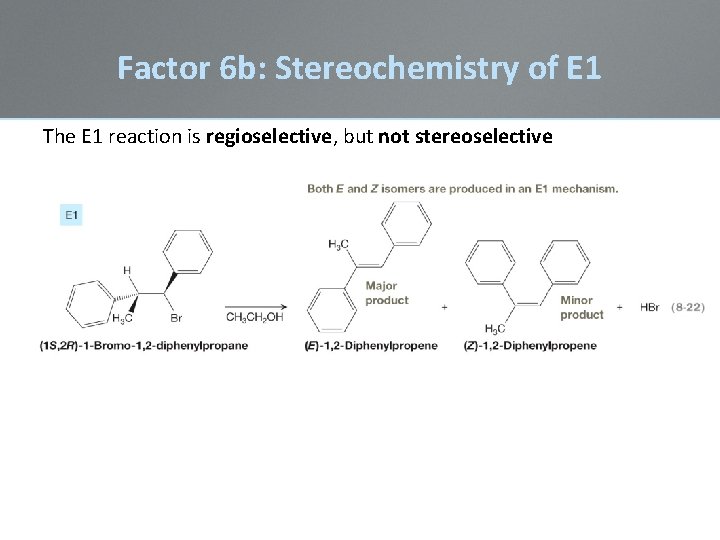
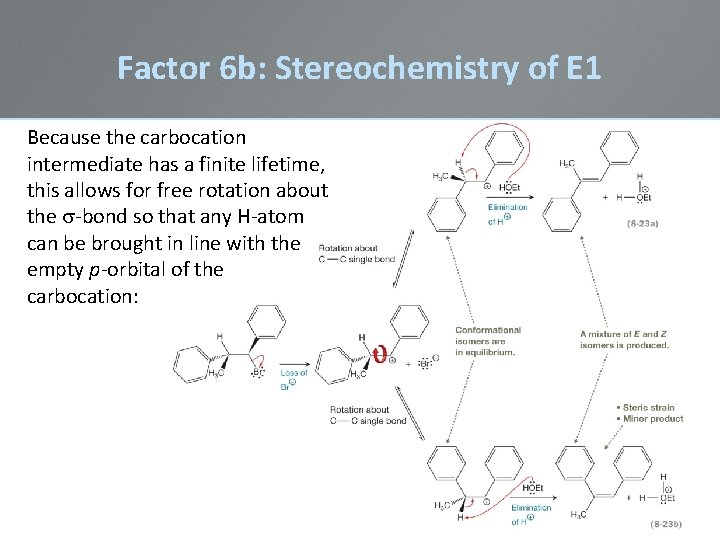
Factor 6 b: Stereochemistry of E 1 The E 1 reaction is regioselective, but not stereoselective Because the carbocation intermediate has a finite lifetime, there is time for free rotation about the s-bond so that any H-atom can be brought in line with the empty p-orbital of the carbocation:

Factor 6 b: Stereochemistry of E 1 The E 1 reaction is regioselective, but not stereoselective

Factor 6 b: Stereochemistry of E 1 Because the carbocation intermediate has a finite lifetime, this allows for free rotation about the s-bond so that any H-atom can be brought in line with the empty p-orbital of the carbocation:

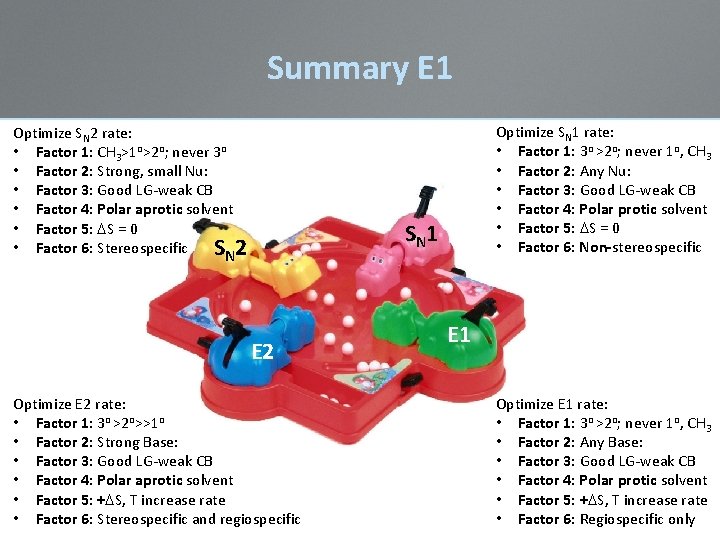
Summary E 1 Optimize SN 2 rate: • Factor 1: CH 3>1 o>2 o; never 3 o • Factor 2: Strong, small Nu: • Factor 3: Good LG-weak CB • Factor 4: Polar aprotic solvent • Factor 5: DS = 0 • Factor 6: Stereospecific S N 2 Optimize SN 1 rate: • Factor 1: 3 o >2 o; never 1 o, CH 3 • Factor 2: Any Nu: • Factor 3: Good LG-weak CB • Factor 4: Polar protic solvent • Factor 5: DS = 0 • Factor 6: Non-stereospecific S N 1 E 2 Optimize E 2 rate: • Factor 1: 3 o >2 o>>1 o • Factor 2: Strong Base: • Factor 3: Good LG-weak CB • Factor 4: Polar aprotic solvent • Factor 5: +DS, T increase rate • Factor 6: Stereospecific and regiospecific E 1 Optimize E 1 rate: • Factor 1: 3 o >2 o; never 1 o, CH 3 • Factor 2: Any Base: • Factor 3: Good LG-weak CB • Factor 4: Polar protic solvent • Factor 5: +DS, T increase rate • Factor 6: Regiospecific only