Organic Chemistry Dr Walker What is Organic Chemistry






- Slides: 21

Organic Chemistry Dr. Walker

What is Organic Chemistry? • Organic chemistry is the study of carbon compounds. – Organic compounds have carbon AND hydrogen – Carbon Dioxide is NOT organic • The versatility and stability of carbon’s molecular structures provides the enormous range of properties of its compounds. • Carbon can bond to other carbons – Reason for the degree of structural complexity

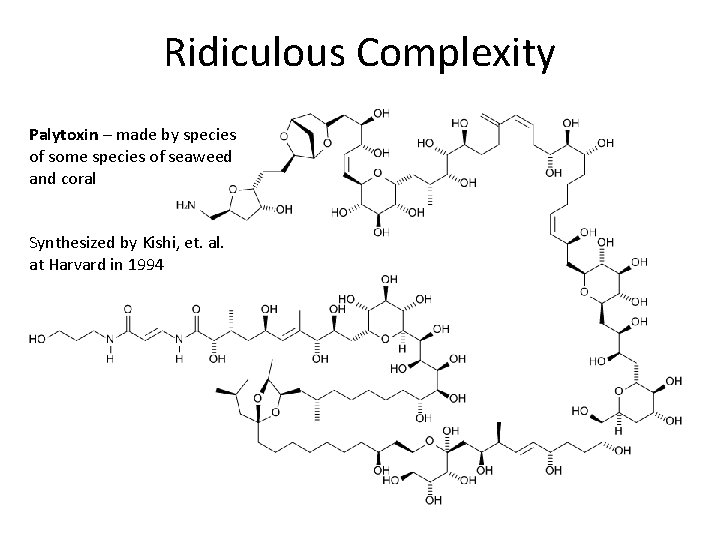
Ridiculous Complexity Palytoxin – made by species of some species of seaweed and coral Synthesized by Kishi, et. al. at Harvard in 1994

Organic Chemistry • Carbon – Has 4 valence electrons – Makes 4 covalent bonds to fill its octet • Can include double and triple bonds – Hydrocarbon • Compound with only carbon and hydrogen

Organic Chemistry in Everyday Life • • Smells & tastes: fruits, fish, mint Medications: aspirin, Tylenol, decongestants, sedatives, insulin Addictive substances: caffeine, nicotine, alcohol, narcotics Hormones/Neurotransmitters: adrenaline, dopamine, serotonin Food: carbohydrates, protein, fat, vitamins Genetics: DNA, RNA Consumer products: plastics, nylon, rayon, polyester

Organic Materials • Addition to previous notes – Before, it was stated that covalent compounds were mostly liquid/gas at room temp – This is primarily for binary compounds (2 elements) – More complicated structures can be solid at room temp

General Formulas • Alkanes – Contains only single bonds – Cx H 2 x+2 • Alkenes – Contains at least one double bond • Alkynes – Contains at least one triple bond • Alkenes and Alkynes are more reactive than alkanes

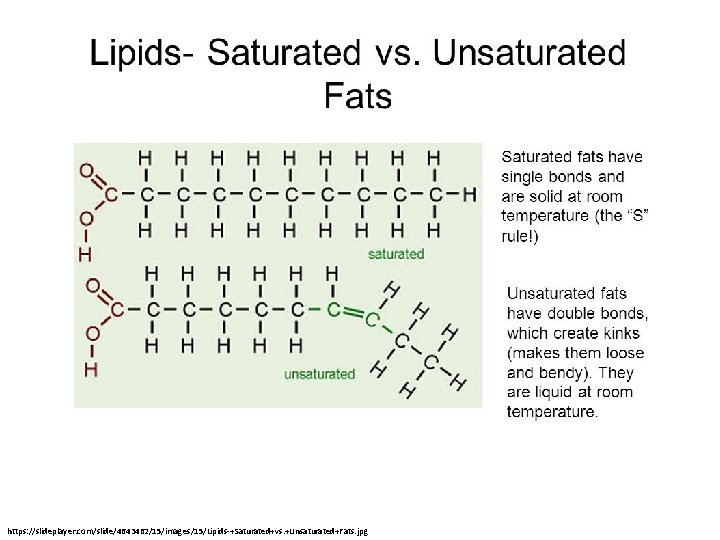
Saturation • Saturated: a carbon chain contains as many hydrogens as possible. • Saturated compounds contain only single C-H bonds. Alkanes are saturated. • Unsaturated means that a carbon chain contains at least one multiple (double or triple) bond. – Alkene = double bond – Alkyne = triple bond

https: //slideplayer. com/slide/4643462/15/images/15/Lipids-+Saturated+vs. +Unsaturated+Fats. jpg

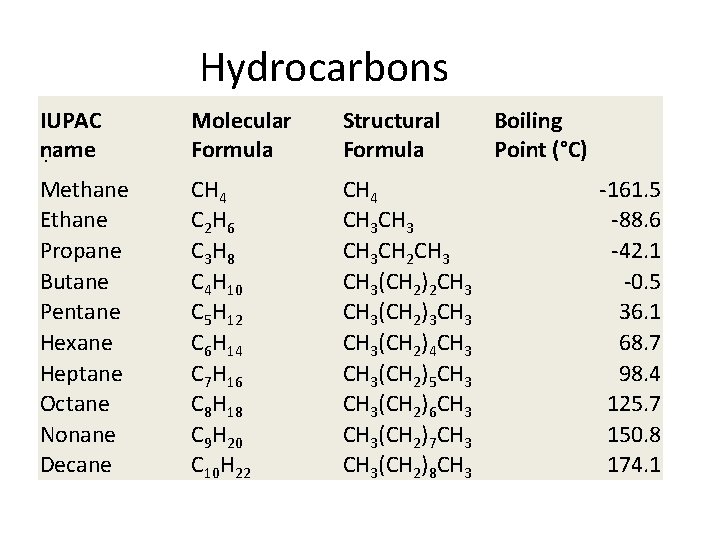
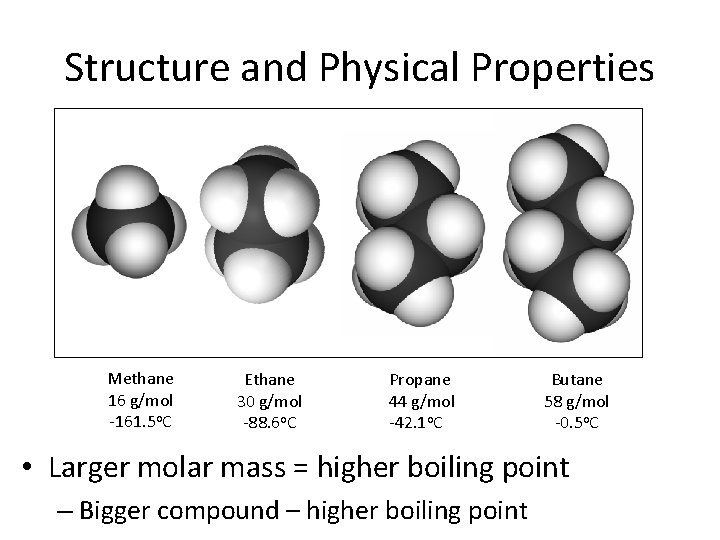
Hydrocarbons IUPAC name. Molecular Formula Structural Formula Methane Ethane Propane Butane Pentane Hexane Heptane Octane Nonane Decane CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 C 9 H 20 C 10 H 22 CH 4 CH 3 CH 2 CH 3(CH 2)3 CH 3(CH 2)4 CH 3(CH 2)5 CH 3(CH 2)6 CH 3(CH 2)7 CH 3(CH 2)8 CH 3 Boiling Point (°C) -161. 5 -88. 6 -42. 1 -0. 5 36. 1 68. 7 98. 4 125. 7 150. 8 174. 1

Structure and Physical Properties Methane 16 g/mol -161. 5 o. C Ethane 30 g/mol -88. 6 o. C Propane 44 g/mol -42. 1 o. C Butane 58 g/mol -0. 5 o. C • Larger molar mass = higher boiling point – Bigger compound – higher boiling point

Petrochemicals • Simple, small hydrocarbons are petrochemicals – Chemicals isolated from crude oil (petroleum) • Octane – used in gasoline • Propane – used in grills, heaters, etc. • Butane – lighter fluid

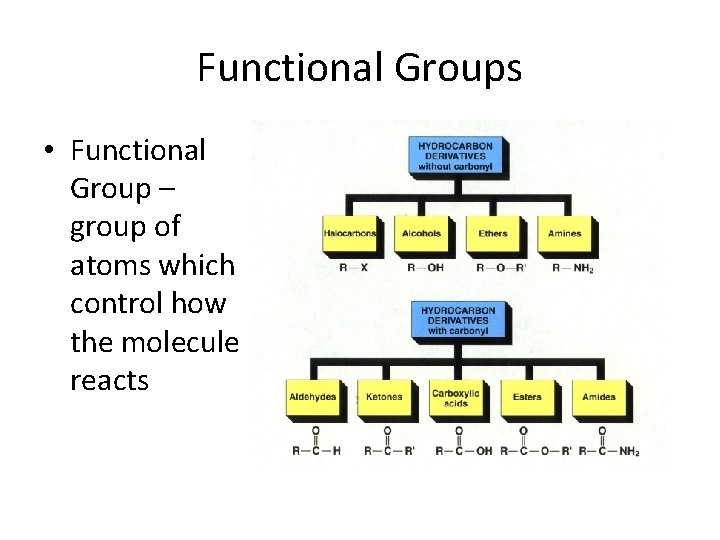
Functional Groups • Functional Group – group of atoms which control how the molecule reacts

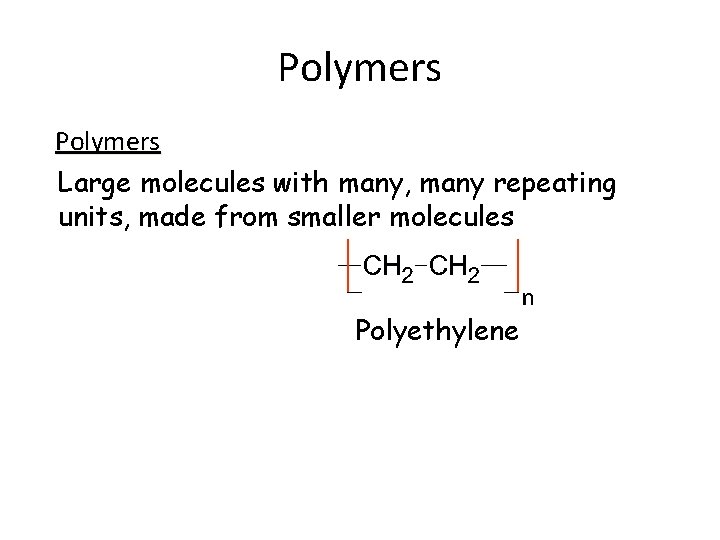
Polymers Large molecules with many, many repeating units, made from smaller molecules Polyethylene

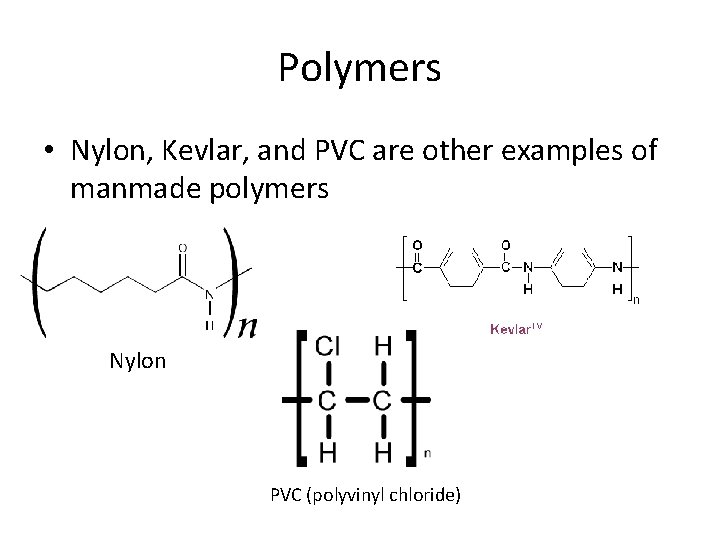
Polymers • Nylon, Kevlar, and PVC are other examples of manmade polymers Nylon PVC (polyvinyl chloride)

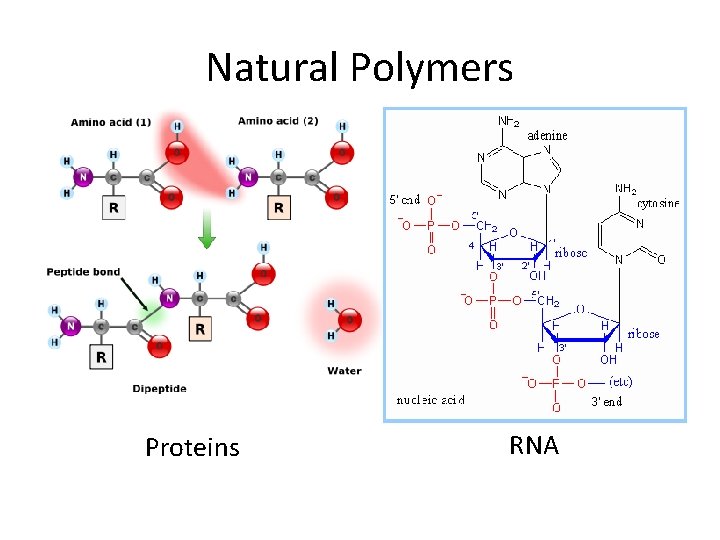
Natural Polymers Proteins RNA

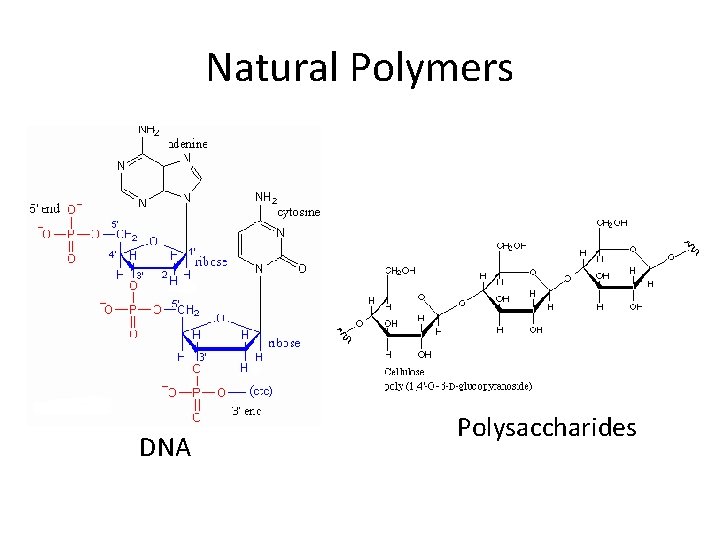
Natural Polymers DNA Polysaccharides

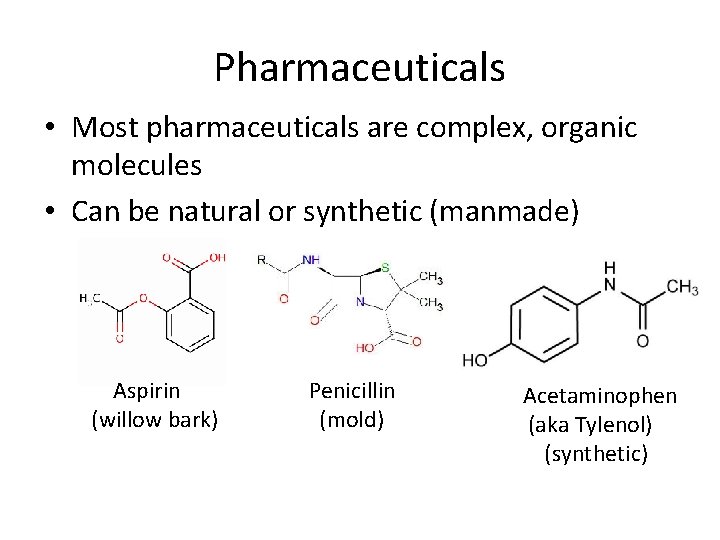
Pharmaceuticals • Most pharmaceuticals are complex, organic molecules • Can be natural or synthetic (manmade) Aspirin (willow bark) Penicillin (mold) Acetaminophen (aka Tylenol) (synthetic)

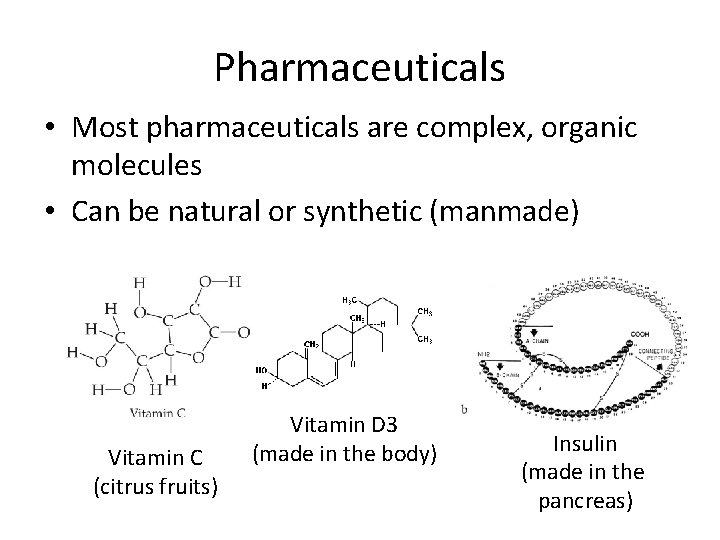
Pharmaceuticals • Most pharmaceuticals are complex, organic molecules • Can be natural or synthetic (manmade) Vitamin C (citrus fruits) Vitamin D 3 (made in the body) Insulin (made in the pancreas)

Terms To Know • • • Hydrocarbon Alkane Alkene Alkyne Saturated Unsaturated Petrochemicals Functional Group Polymers

Skills To Master • Differentiating alkanes, alkenes, and alkynes • Differentiating saturated and unsaturated compounds • Differentiating natural polymers and manmade polymers • Recognizing common pharmaceuticals
 Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Britannica.com
Britannica.com Oxidation of carbohydrates
Oxidation of carbohydrates Organic chemistry nomenclature
Organic chemistry nomenclature Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 C5h12
C5h12 Objective lab report example
Objective lab report example Extraction of caffeine from vivarin tablets lab report
Extraction of caffeine from vivarin tablets lab report Organic chemistry
Organic chemistry Hono organic chemistry
Hono organic chemistry Ee organic chemistry
Ee organic chemistry Organic chemistry
Organic chemistry Kiliani fischer synthesis
Kiliani fischer synthesis Organic chemistry
Organic chemistry Mind map organic chemistry
Mind map organic chemistry Organic chemistry
Organic chemistry Organic chemistry chapter 1 problem 59pp
Organic chemistry chapter 1 problem 59pp Ferrox test for oxygen
Ferrox test for oxygen How to calculate percentage yield in organic chemistry
How to calculate percentage yield in organic chemistry Hydrocarbon prefixes
Hydrocarbon prefixes