Organic Chemistry Chp 18 Names Chp 19 Names























































- Slides: 64

Organic Chemistry

Chp 18 Names Chp 19 Names 18 19 Random

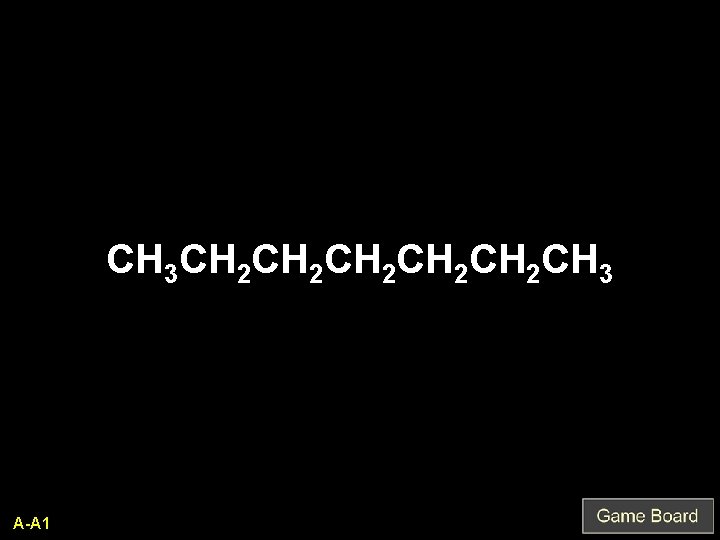
CH 3 CH 2 CH 2 CH 2 CH 3 A-A 1

heptane Q-A 1

CH 3 C(CH 3)2 CH 3 A-A 2

2, 2 -dimethylbutane Q-A 2

CH 2=CHCH(CH 3)2 A-A 3

3, 4 -dimethyl-1 -pentene Q-A 3

CH 3 CH(CH 3)CH(CH 2 CH 3)C(CH 3)3 A-A 4

3 -ethyl-2, 2, 4 -trimethylpentane Q-A 4

(CH 3 CH 2)2 CHCH=CHCH 3 A-A 5

4 -ethyl-2 -hexene Q-A 5

HCHO A-B 1

methanal or Formaldehyde Q-B 1

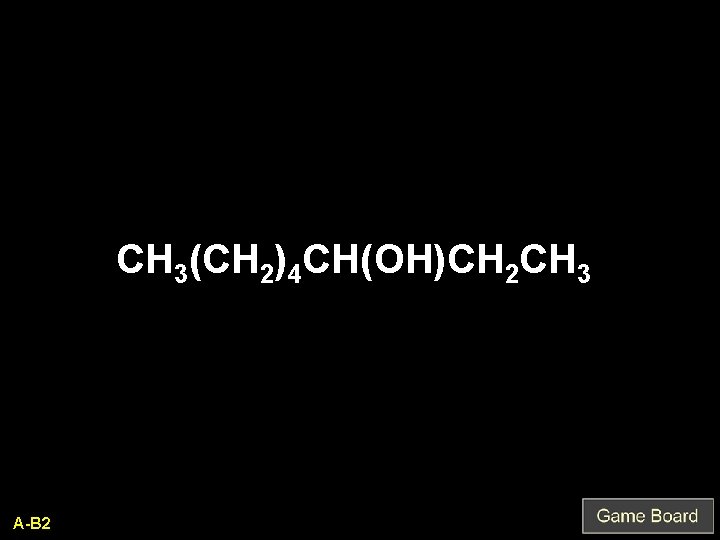
CH 3(CH 2)4 CH(OH)CH 2 CH 3 A-B 2

octanol Q-B 2

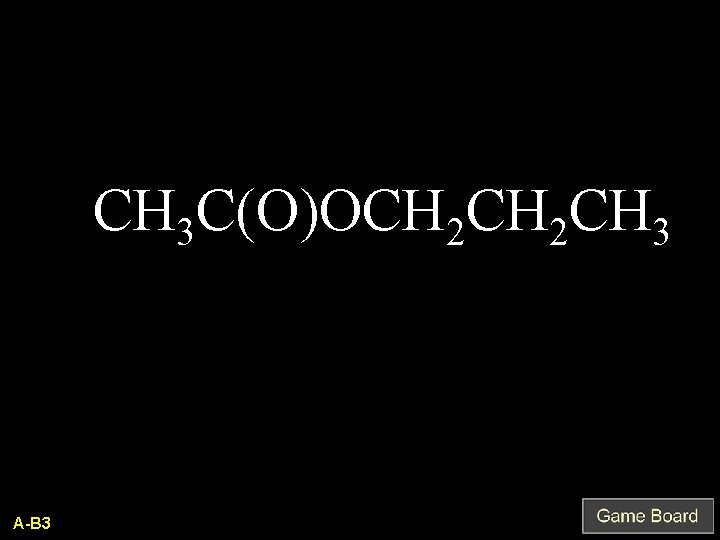
CH 3 C(O)OCH 2 CH 3 A-B 3

Propylethanoate Q-B 3

Name: CH 2 C(CH 3)2 CH 2 COOH A-B 4

3, 3 -dimethyl-butanoic acid Q-B 4

C 6 H 5 C(O)CH 3 A-B 5

Phenylethanone common name acetophenone used for flavor and fragrance in gum and cigarettes Q-B 5

The hybridization on the carbon atoms in the ethene molecule H 2 C=CH 2 is A- C 1

sp 2 Q-C 1

All of the following are structural isomers of C 6 H 14 except a) CH 3(CH 2)4 CH 3 b) CH 3 CH 2 C(CH 3)3 c) (CH 3)2 CHCH(CH 3)2 d) (CH 3)2 CHCH 2 CH 3 A-C 2

d) (CH 3)2 CHCH 2 CH 3 Q-C 2

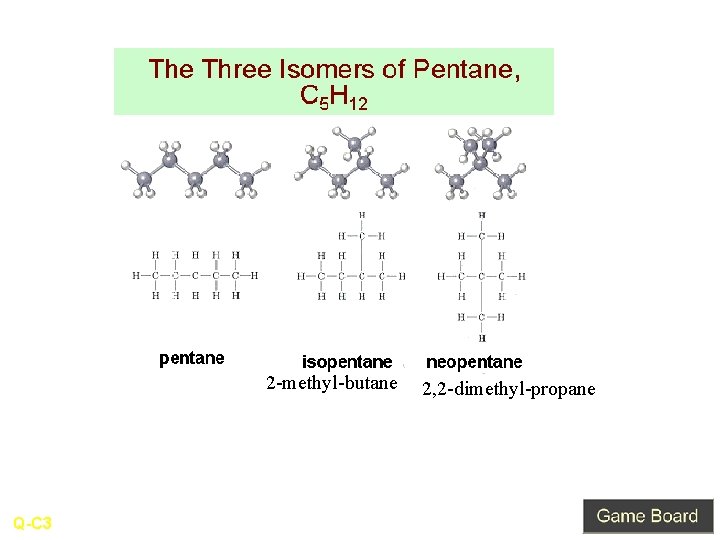
Double Name and Draw all structural isomers are there for the molecular formula C 5 H 12 A-C 3

2 -methyl-butane Q-C 3 2, 2 -dimethyl-propane

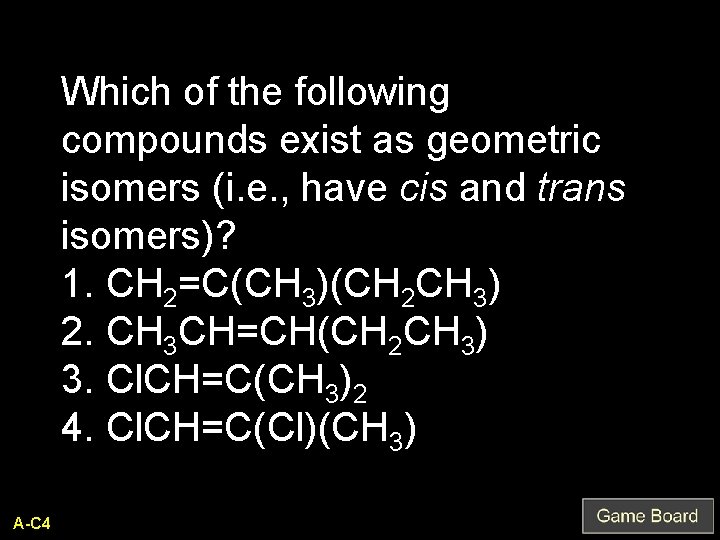
Which of the following compounds exist as geometric isomers (i. e. , have cis and trans isomers)? 1. CH 2=C(CH 3)(CH 2 CH 3) 2. CH 3 CH=CH(CH 2 CH 3) 3. Cl. CH=C(CH 3)2 4. Cl. CH=C(Cl)(CH 3) A-C 4

2 and 4 Q-C 4

Which of the following compounds contain a chiral carbon (i. e. , optical isomers)? 1. CH 2 C(CH 3)(CH 2 CH 3) 2. CH 3 C(CH 2 CH 3)(Cl)CH 3 3. CH(CH 2 CH 3)(Cl)CH 3 4. CH 3 C(CH 2 CH 3)(Cl)F A-C 5

3, 4 Q-C 5

The compound CH 3 CH 2 OCH 2 CH 3 is A-D 1

Ether Q-D 1

What are the building blocks of carbohydrates? A-D 2

monosaccharides Q-D 2

Starch and cellulose are A-D 3

polysaccharides Q-D 3

What is the oxidation number of carbon in the following? a) methane, CH 4 b) methanol, CH 3 OH c) formaldehyde, HCHO d) formic acid, HCOOH A-D 4

a) methane, CH 4 -4 b) methanol, CH 3 OH -2 c) formaldehyde, HCHO 0 d) formic acid, HCOOH +2 Q-D 4

Which of the following have bond angles of 120? A) CH 3 C(CH 2 CH 3)(OH)CH 3 B) CH 3 COOH C) CH 3 CH 2 OCH 2 CH 3 D) CH 3 COCH 2 CH 3 E) H 2 CO A-D 5

B, D, E Q-D 5

What is the general formula for saturated hydrocarbons? A-E 1

Cn. H 2 n+2 alkane (all single bonds) Cn. H 2 n alkene (one double bond) or cycloalkane Cn. H 2 n-2 alkyne (one triple bond) or diene (two double bonds) or cycloalkane with a double bond Q-E 1

Which of the following is not a proper name of an isomer of dimethylbenzene (xylene)? a) 1, 2 -dimethylbenzene b) 1, 4 -dimethylbenzene c) 1, 5 -dimethylbenzene d) 1, 3 -dimethylbenzene A-E 2

c) 1, 5 -dimethylbenzene Q-E 2

What is the hybridization of the middle carbon and oxygen? CH(O)CH 2 CH 3 A-E 3

d) CH 3 CH(CH 3)(CH 2)3 CH 3 Q-E 3

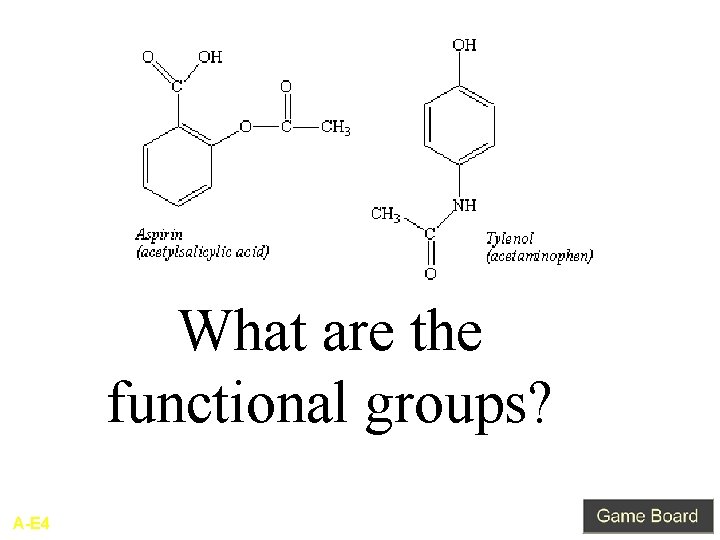
Type answer here What are the functional groups? A-E 4

Aspirin: Carboxylic Acid Ester Tylenol: phenol Amide Q-E 4

Which ones contain a carbonyl? A)propanol B)propanal C)propanone D)propanoic acid E)methoxyethane A-E 5

B, C, D Q-E 5

Final Jeopardy Building blocks Q’est-ce que c’est que. S-Final

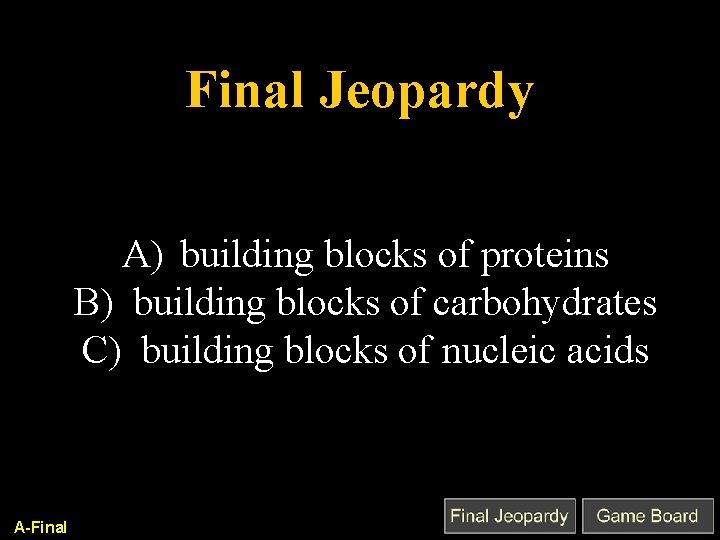
Final Jeopardy A) building blocks of proteins B) building blocks of carbohydrates C) building blocks of nucleic acids A-Final

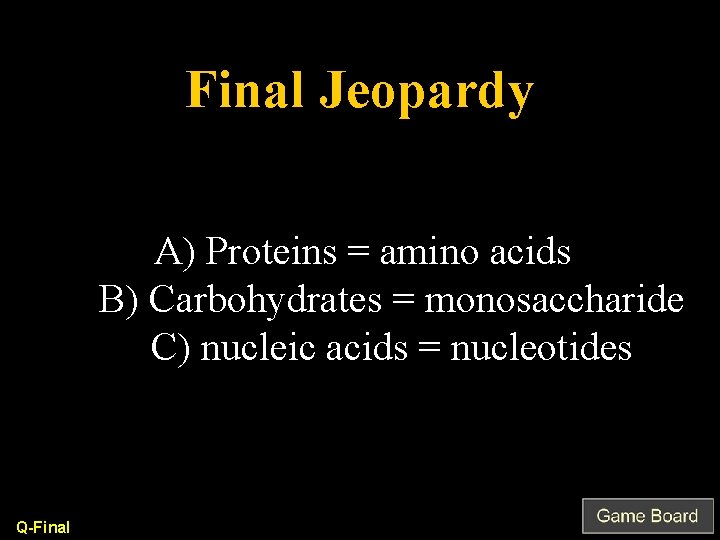
Final Jeopardy A) Proteins = amino acids B) Carbohydrates = monosaccharide C) nucleic acids = nucleotides Q-Final

Daily Double

Power. Point Jeopardy Ver 2. 2 Written by Winston Riley IV (Winston. Riley@Wonder. Dog. Programs. com) From Wonder Dog Programs (www. Wonder. Dog. Programs. com)

INSTRUCTIONS

How To Set Up A New Game (Page 1) You will need to come up with 25 answers followed by 25 questions in five different categories. All the updates to this file are like any other changes you can make in a Power. Point slideshow. There are 25 pairs of pages waiting for you to enter your answers and questions. Each slide has a unique number in the lower left corner to help you keep track of which slide you are on. Since there are five categories the number starts with an A, B, C, D, or E, followed by a number within that category (1, 2, 3, 4, 5). The A- or Q- prefix indicates the position within a pair. So a slide that has A-C 3 is the third category, third answer: it is the one in the very middle of the board. You can page down or page up to the desired slide and change the text that is already there with your answer. The category headings must also be changed on the game board slide. Do this by selecting the slide and clicking in the text you want to change. Note that the headings are in a table More

How To Set Up A New Game (Page 2) The first slide also has a title on it which should be changed to reflect the topic of the game you are making. If you want to show a double Jeopardy answer select the slide and right-click on the black background. Choose ‘Background’ and select a red color for the background. Apply the background only to that slide. Repeat for the second slide. That’s it. You are now ready to play Power. Point Jeopardy. IMPORTANT NOTE: Do not rearrange the slides or delete them. There is VBA programming code within this slide show that relies on the slides being exactly where they are.

How To Play Jeopardy (Page 1) Jeopardy is unusual in that the host (teacher) reads an answer and the players (students) must give the question. This means that the answers must clearly point to a unique question, and the players must phrase their responses with a “what is. . . ” or “Who is. . . ” etc. A player asks for a category and a number of any available answers. The host reads the answer and the first person to raise their hand, once the question is finished being read, gets to respond with the question. If they are correct then they receive the number of points for that question (as shown on the game board) and they get to select the next answer. If they are wrong then any remaining players may raise their hand respond. (NOTE: If you have a particular way that this game works well in a classroom situation, please e-mail me at rriley. und 5. umd. edu, so that I can include that here. Thanks)

F. A. Q. (Frequently Asked Questions) (Page 1) Q) I keep getting error messages when I play, or the game board goes to the wrong slide. A) You have rearranged the slide and the program code within this game is no longer valid. Try setting up the game using a new template. Q) Nothing happens when I press the buttons. A) You must ‘Enable Macros’ when the slide show is opened. This game relies on the macro code that lies behind each slide. (NOTE: If you have any questions or comments about how this game works, please e-mail me at rriley. und 5. umd. edu, so that I can include that here. Thanks)

thinkmusic. wav dailydouble. wav thinkmusic. wav
