Organic Chemistry CHEM 145 Chemistry 2 Credit hrs






- Slides: 25

Organic Chemistry CHEM 145 Chemistry 2 Credit hrs Department College of Science King Saud By University Prof. Mohamed El-Newehy

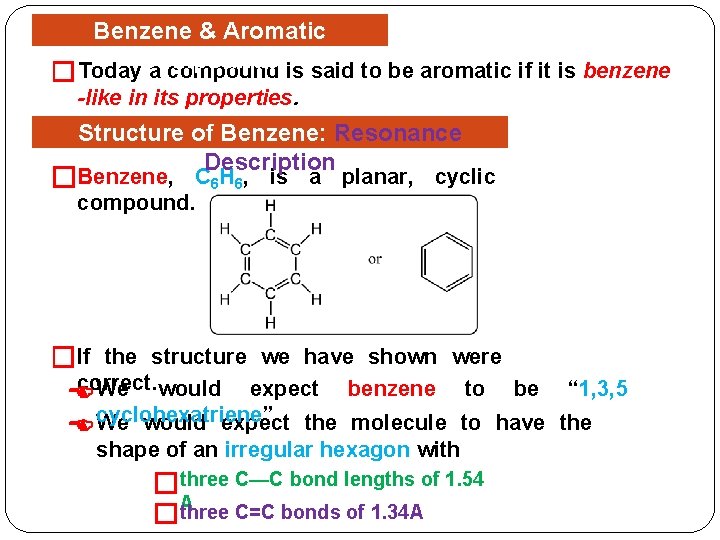
� Benzene & Aromatic Today. Compounds a compound is said to be aromatic if it is benzene -like in its properties. Structure of Benzene: Resonance Description �Benzene, C 6 H 6, is a planar, cyclic compound. �If the structure we have shown were correct. We would expect benzene to be cyclohexatriene” We would expect the molecule to have shape of an irregular hexagon with �three C—C bond lengths of 1. 54 three C=C bonds of 1. 34 A �A “ 1, 3, 5 the

� X-ray diffraction experiments reveals that all carbon bonds in benzene are of equal length, 1. 39 A. � Expect benzene to undergo addition reactions the typical reaction of benzene is substitution, rather than addition. �The true structure of benzene can be explained by the concept of resonance. Structure of Benzene: Molecular Orbital Description �We expect benzene to be A planar molecule having the shape of a regular hexagon, with bond angles of 120°. All the carbon-carbon bond distances to be intermediate (1. 39 A) between the usual C-C single bond (1. 54 A) and the typical C=C double bond (1. 34 A).

Stability of Benzene: Resonance Energy �The heat of hydrogenation �Actually, when benzene is hydrogenated to cyclohexane, only 49. 8 kcal/mole of energy is liberated. �Recall that we defined aromatic compounds as those with benzene-like benzene, aromatic compounds tend to undergo �Likeproperties. substitution rather than addition.

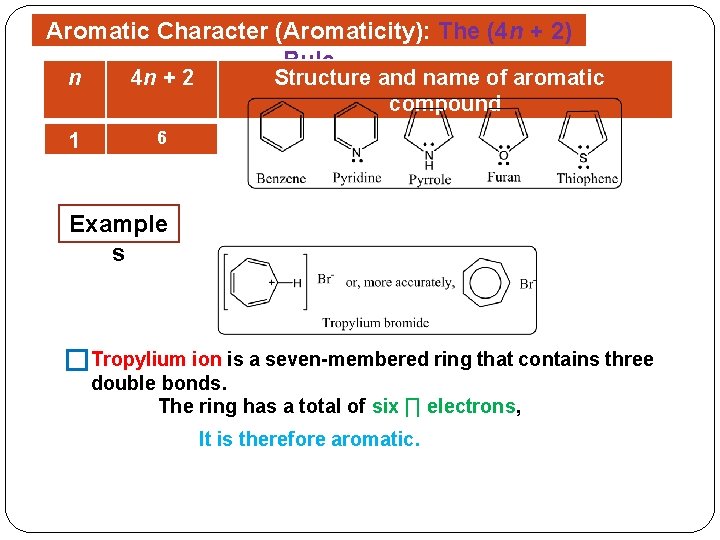
Aromatic Character (Aromaticity): The (4 n + 2) Rule � Aromatic character (Aromaticity) is associated with several structural requirements. Aromatic compounds are cyclic structures that contain what looks like a continuous system of alternating double and single bonds. Aromatic compounds must be planar. Aromaticity is possible only if i. e. obey Hückel’s rule the number of ∏ electrons in the compound = (4 n + 2) Where (n = 1, 2, 3, and so on).

Aromatic Character (Aromaticity): The (4 n + 2) Rule n 4 n + 2 1 6 Structure and name of aromatic compound Example s �Tropylium ion is a seven-membered ring that contains three double bonds. The ring has a total of six ∏ electrons, It is therefore aromatic.

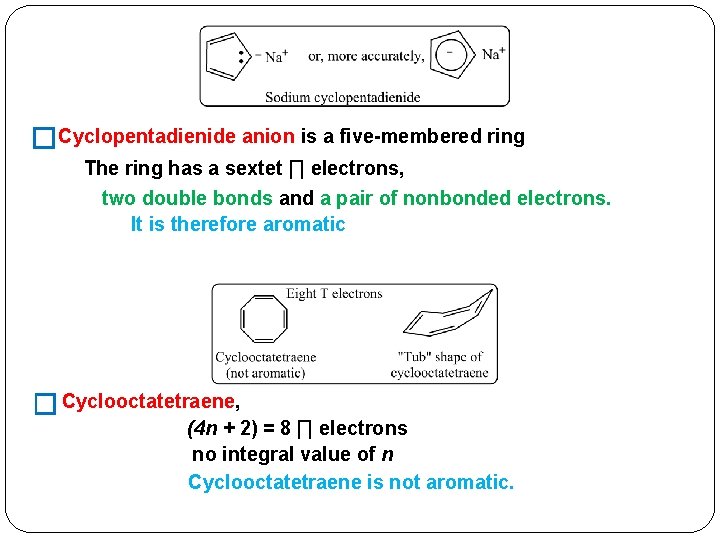
�Cyclopentadienide anion is a five-membered ring The ring has a sextet ∏ electrons, two double bonds and a pair of nonbonded electrons. It is therefore aromatic � Cyclooctatetraene, (4 n + 2) = 8 ∏ electrons no integral value of n Cyclooctatetraene is not aromatic.

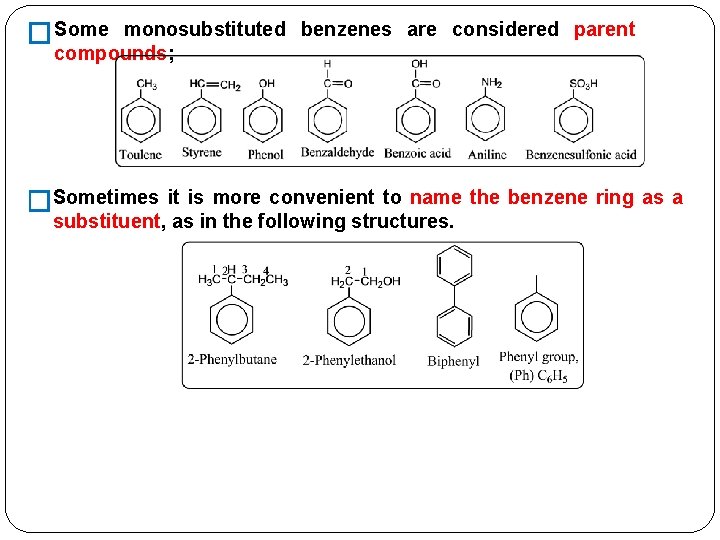
Nomenclature of Aromatic Compounds Monosubstituted Benzenes Because all six positions in benzene are � there is no need to specify by a number the position of a equivalent, substituent for monosubstituted benzenes.

�Some monosubstituted benzenes are considered parent compounds; �Sometimes it is more convenient to name the benzene ring as a substituent, as in the following structures.

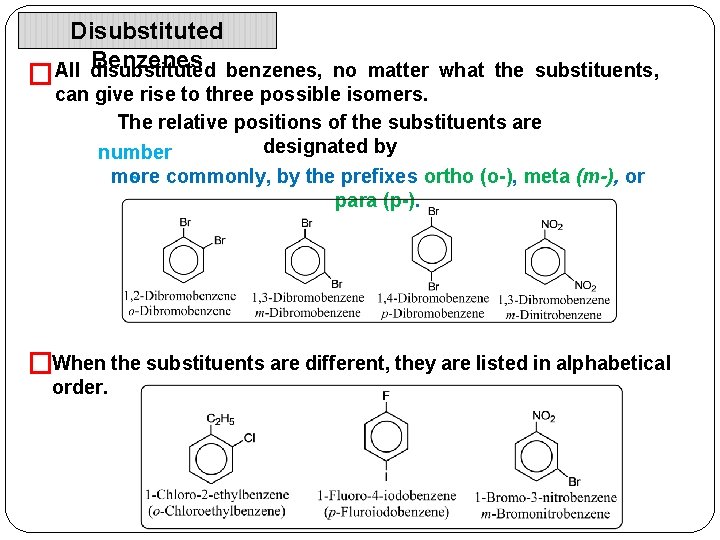
Disubstituted Benzenes benzenes, no matter what the substituents, All disubstituted � can give rise to three possible isomers. The relative positions of the substituents are designated by number more s commonly, by the prefixes ortho (o-), meta (m-), or para (p-). �When the substituents are different, they are listed in alphabetical order.

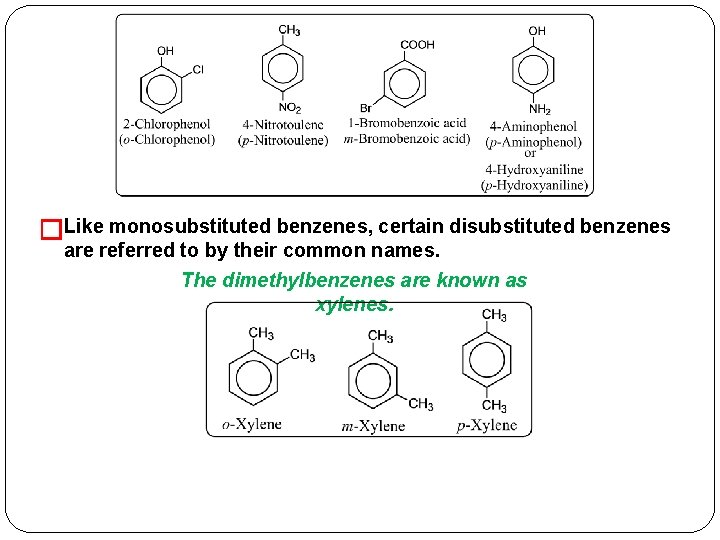
�Like monosubstituted benzenes, certain disubstituted benzenes are referred to by their common names. The dimethylbenzenes are known as xylenes.

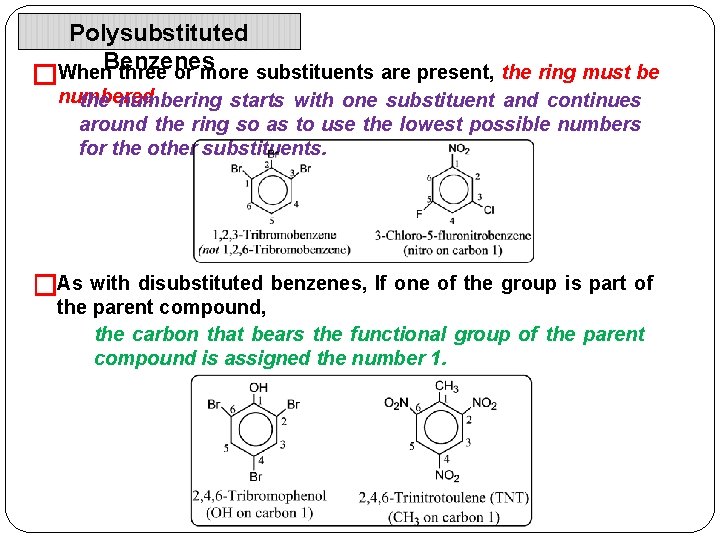
Polysubstituted When. Benzenes three or more substituents are present, the ring must be � numbered the numbering starts with one substituent and continues around the ring so as to use the lowest possible numbers for the other substituents. �As with disubstituted benzenes, If one of the group is part of the parent compound, the carbon that bears the functional group of the parent compound is assigned the number 1.

Electrophilic Aromatic Substitution General The. Mechanism aromatic ring, with its delocalized electrons is an electron-rich � system. �Attack on the ring takes place by means of an electon-difficient species (Electrophile) (E+). Step 1. The electrophile E+ approaches the cloud of the aromatic ring and forms a bond to carbon, creating a +ve charge in the ring. Step 2. The removal of the proton by the nucleophile Nu, which leads to the restoration of the aromatic ring

�The net overall result is the substitution of the group E+ for a proton H+. i. e. electrophilic aromatic substitution. � The Role of The Catalysts catalysts are Lewis acids or a protonic acid. The purpose of these catalysts is to generate powerful electrophiles. Bromination of benzene It requires the presence of a Lewis acid catalyst, ferric bromide. Ferric bromide will help to generate a highly reactive electrophile, the bromonium ion (Cl+).

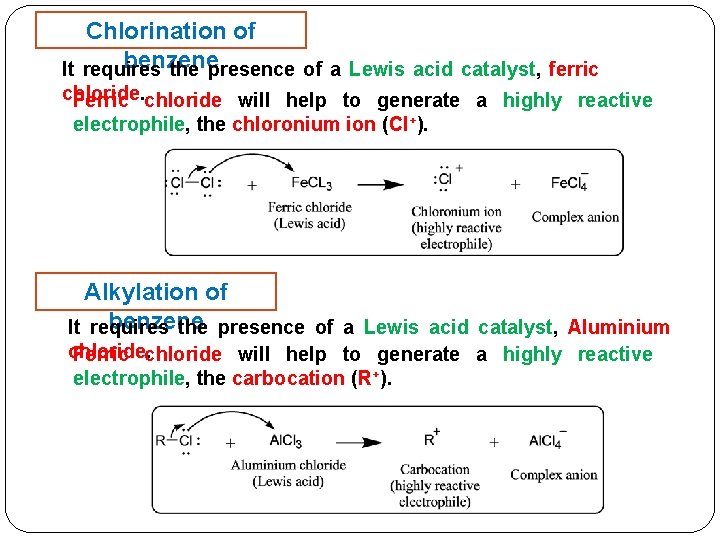
Chlorination of benzene It requires the presence of a Lewis acid catalyst, ferric chloride. Ferric chloride will help to generate a highly reactive electrophile, the chloronium ion (Cl+). Alkylation of benzene It requires the presence of a Lewis acid catalyst, Aluminium chloride. Ferric chloride will help to generate a highly reactive electrophile, the carbocation (R+).

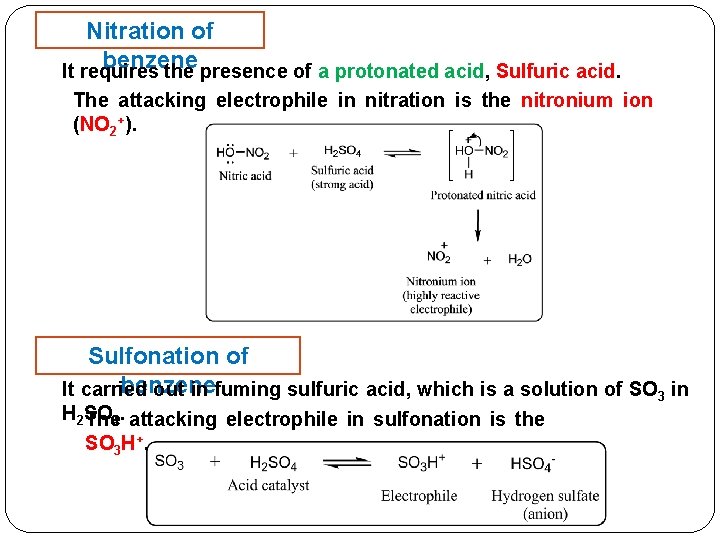
Nitration of benzene It requires the presence of a protonated acid, Sulfuric acid. The attacking electrophile in nitration is the nitronium ion (NO 2+). Sulfonation of benzene It carried out in fuming sulfuric acid, which is a solution of SO 3 in H 2 SO The 4. attacking electrophile in sulfonation is the SO 3 H+.

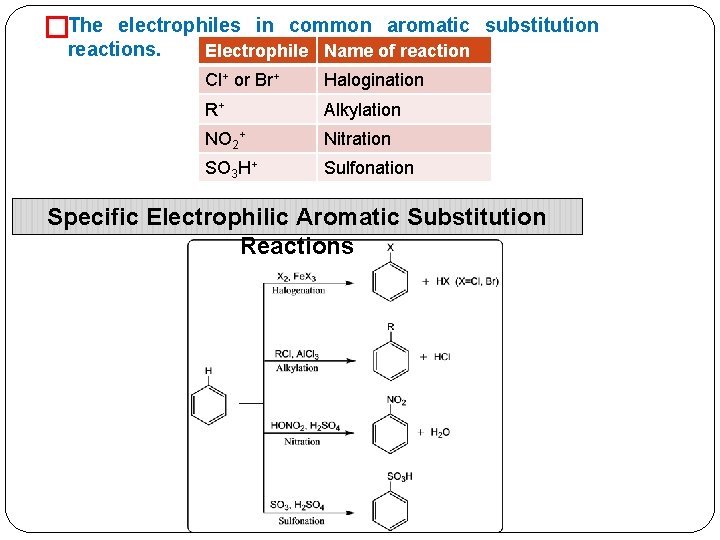
�The electrophiles in common aromatic substitution reactions. Electrophile Name of reaction Cl+ or Br+ Halogination R+ Alkylation NO 2+ Nitration SO 3 H+ Sulfonation Specific Electrophilic Aromatic Substitution Reactions

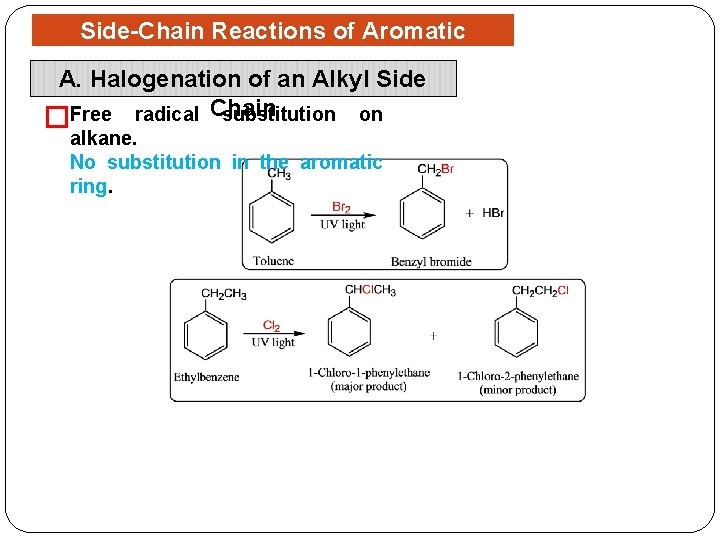
Side-Chain Reactions of Aromatic Compounds A. Halogenation of an Alkyl Side substitution on �Free radical Chain alkane. No substitution in the aromatic ring.


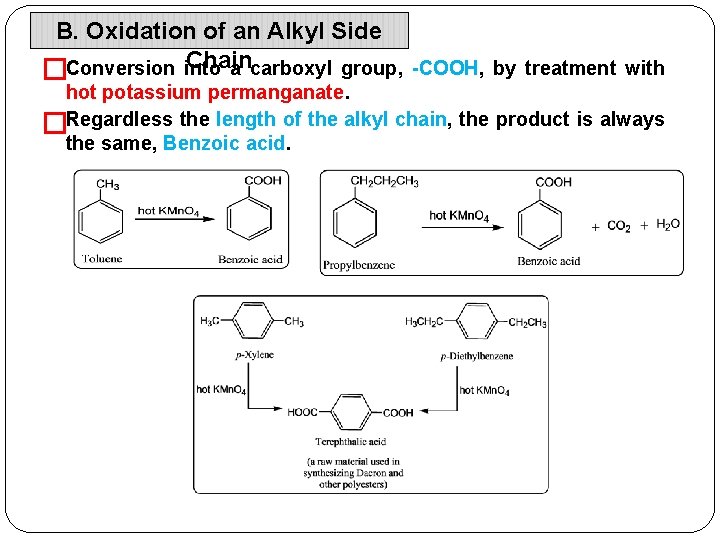
B. Oxidation of an Alkyl Side Chain a carboxyl group, -COOH, by treatment with �Conversion into hot potassium permanganate. �Regardless the length of the alkyl chain, the product is always the same, Benzoic acid.

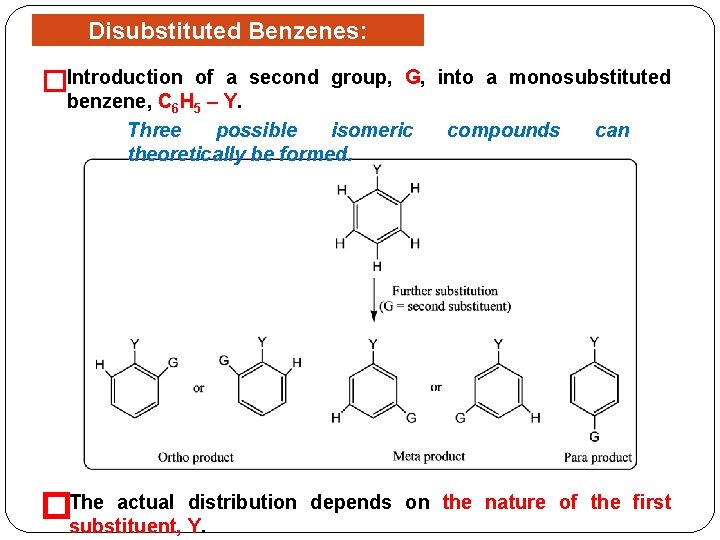
Disubstituted Benzenes: Orientation �Introduction of a second group, G, into a monosubstituted benzene, C 6 H 5 – Y. Three possible isomeric compounds can theoretically be formed. actual distribution �The substituent, Y. depends on the nature of the first

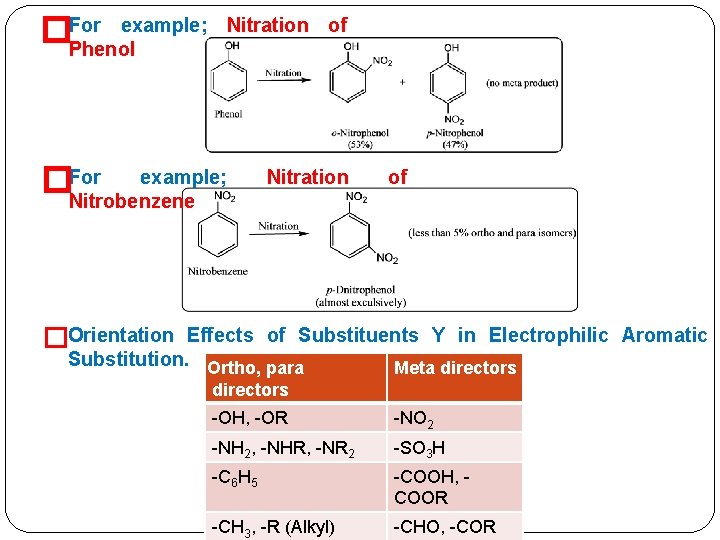
example; �For Phenol Nitration of �For example; Nitrobenzene Nitration of �Orientation Effects of Substituents Y in Electrophilic Aromatic Substitution. Ortho, para Meta directors -OH, -OR -NO 2 -NH 2, -NHR, -NR 2 -SO 3 H -C 6 H 5 -COOH, COOR -CH 3, -R (Alkyl) -CHO, -COR

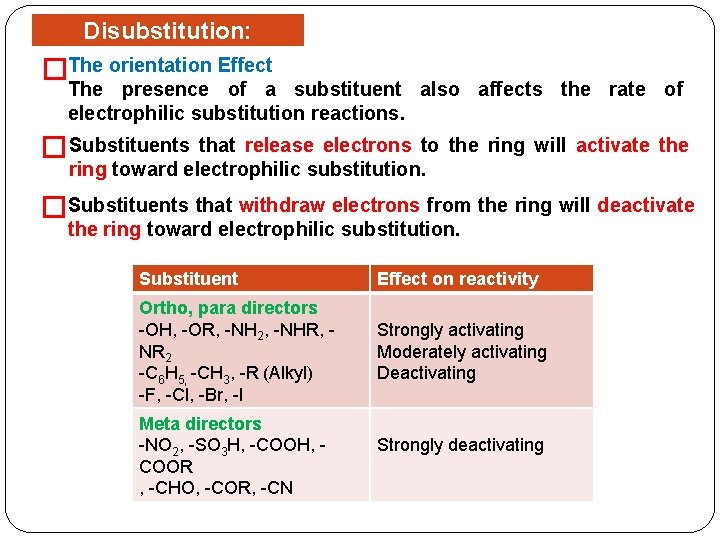
Disubstitution: Reactivity. Effect �The orientation The presence of a substituent also affects the rate of electrophilic substitution reactions. � Substituents that release electrons to the ring will activate the ring toward electrophilic substitution. �Substituents that withdraw electrons from the ring will deactivate the ring toward electrophilic substitution. Substituent Effect on reactivity Ortho, para directors -OH, -OR, -NH 2, -NHR, NR 2 -C 6 H 5, -CH 3, -R (Alkyl) -F, -Cl, -Br, -I Strongly activating Moderately activating Deactivating Meta directors -NO 2, -SO 3 H, -COOH, COOR , -CHO, -COR, -CN Strongly deactivating

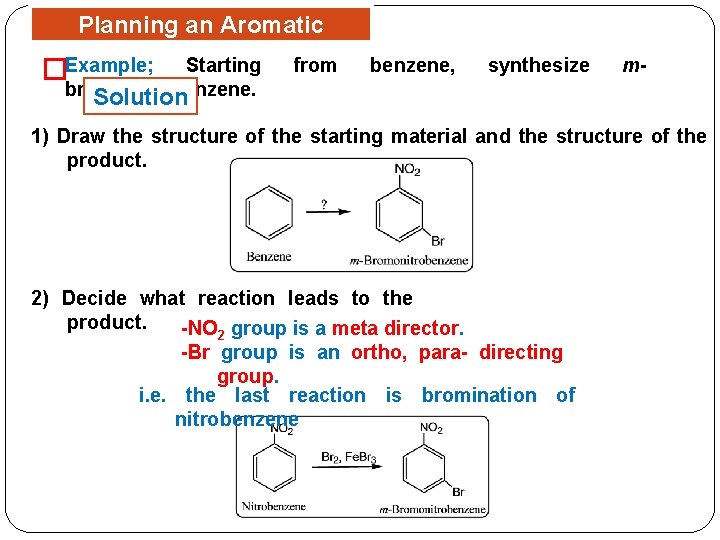
Planning an Aromatic Synthesis Example; Starting from � benzene, synthesize m- bromonitrobenzene. Solution 1) Draw the structure of the starting material and the structure of the product. 2) Decide what reaction leads to the product. -NO 2 group is a meta director. -Br group is an ortho, para- directing group. i. e. the last reaction is bromination of nitrobenzene

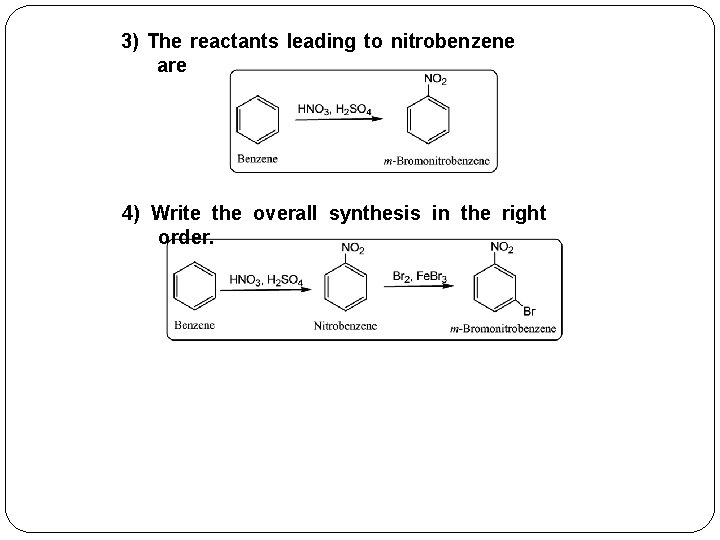
3) The reactants leading to nitrobenzene are 4) Write the overall synthesis in the right order.
 Why the day has 24 hours
Why the day has 24 hours Ario organic chem
Ario organic chem Alkanes formula
Alkanes formula Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Wake up stroke definition
Wake up stroke definition Cdmvt navigation
Cdmvt navigation Hrs rand
Hrs rand Decimal time
Decimal time Hrs taiwan
Hrs taiwan 1300 hrs
1300 hrs Bitgain 24 hrs
Bitgain 24 hrs Hrs
Hrs Hrs types
Hrs types 1300 hrs
1300 hrs Hrs
Hrs How to write time in 24 hour clock
How to write time in 24 hour clock When dealing with a complaint it is vital to remember that
When dealing with a complaint it is vital to remember that Nh road cross section
Nh road cross section This can be avoided by giving credit where credit is due.
This can be avoided by giving credit where credit is due. Organic chemistry class 11 notes
Organic chemistry class 11 notes Saponifiable lipids example
Saponifiable lipids example Alkene alcohol naming
Alkene alcohol naming C6h14 structural isomers
C6h14 structural isomers Alkane cracking
Alkane cracking Bu organic chemistry
Bu organic chemistry