Organic Chemistry Chapter 4 Carbon Chemistry Organic Chemistry
Organic Chemistry Chapter 4: Carbon Chemistry
Organic Chemistry: The part of chemistry that deals with compounds made by living organisms. Berzelius: first to make the distinction between organic and inorganic compounds in 19 th century. Built on a foundation of vitalism: the belief in a force outside the jurisdiction of physical and chemical laws.

Organic Chemistry = Carbon Chemistry Organic: compounds containing carbon that are found in living organisms. Exceptions: oxides of carbon (i. e. carbon monoxide, carbon dioxide), carbonates, and hydrogencarbonates are not regarded as organic.

Many organic compounds form spontaneously l l l Chemical reactions give rise to organic compounds. Miller-Urey experiments. Evidence that organic compounds can be made from inorganic substances. Modeled primitive earth’s atmosphere and synthesized urea and amino acids and organic acids.
Carbon Chemistry a) b) Richer than the chemistry of other elements: able to support life because of the unique associative properties of carbon atoms. Biological diversity reflects molecular diversity. Most organisms have same % composition of C, H, O, N, P, S, but uniqueness is assured because of the diversity of molecules.
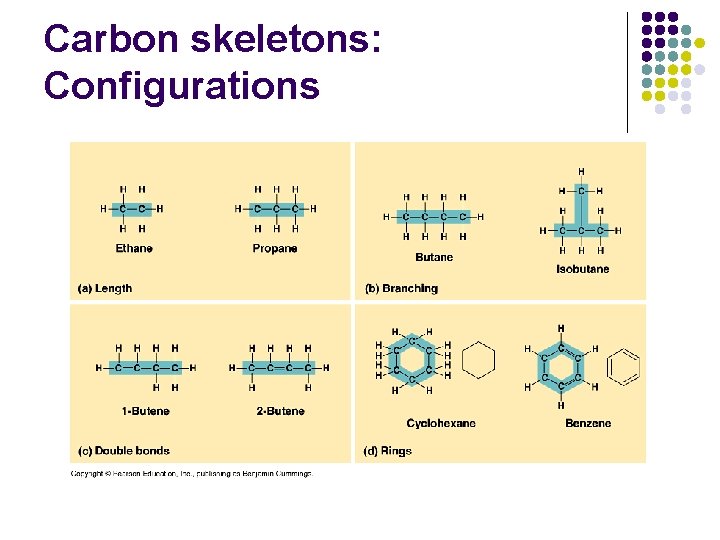
Carbon skeletons: Configurations
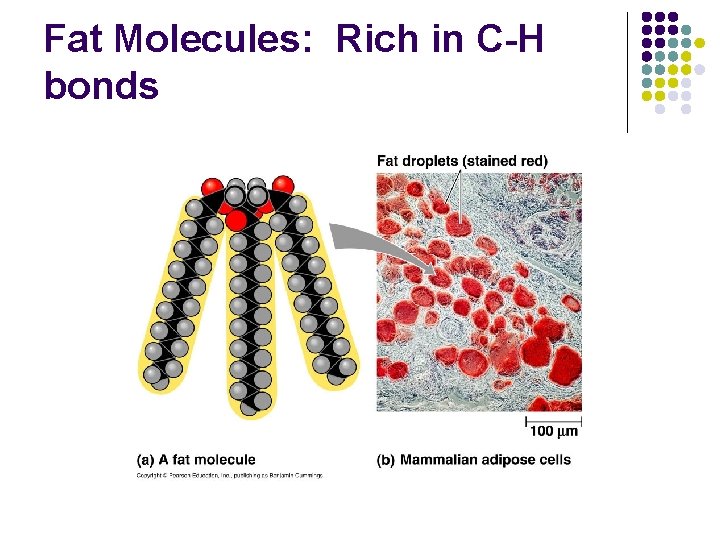
Fat Molecules: Rich in C-H bonds
More characteristics 6. 7. Form isomers: molecules with same chemical formula but with different structures. Many functional groups can be attached to a carbon skeleton, making each variation a new compound with new and different properties. E. g. -NH 2 = amino group; -OH = hydroxyl group, -C=O is carbonyl group.
Isomers 1. Structural: different covalent arrangement of atoms or position of the double bond. e. g. Glucose and Fructose, both C 6 H 12 O 6.
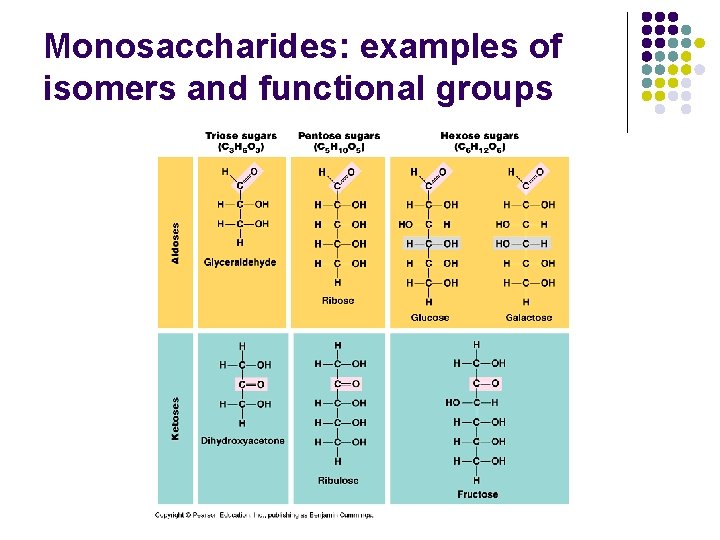
Monosaccharides: examples of isomers and functional groups
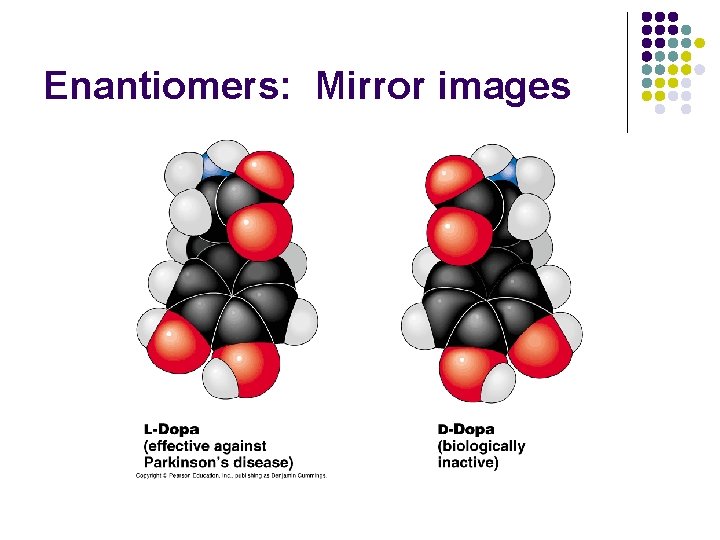
Enantiomers: Mirror images
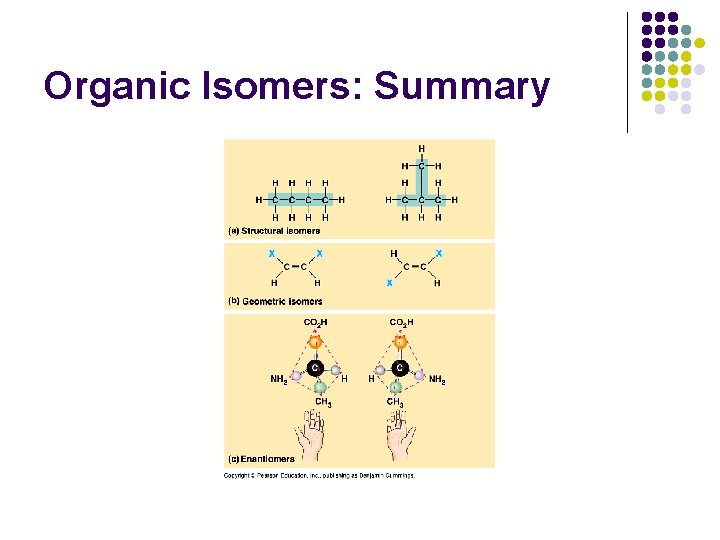
Organic Isomers: Summary
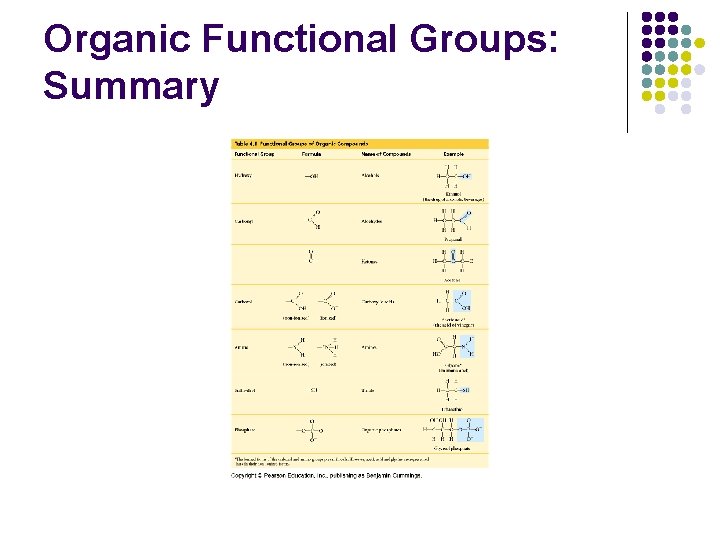
Functional Groups Functional Group: Region of an organic molecule most commonly involved in chemical reactions. We see transfer of the functional group from 1 molecule to another or the breaking of C-C bonds.

Functional Groups: 1 -7 1. Methyl Group: -CH 3. Found in fats, oils and waxes. It is non-polar and hydrophobic. Important in turning on and turning off of genes. 2. Hydroxyl Group: -OH. Found in sugars. It is polar and hydrophilic. Water is attracted to it and can form H bonds. Molecules with –OH can be dissolved in water. Organic compounds are called alcohols.
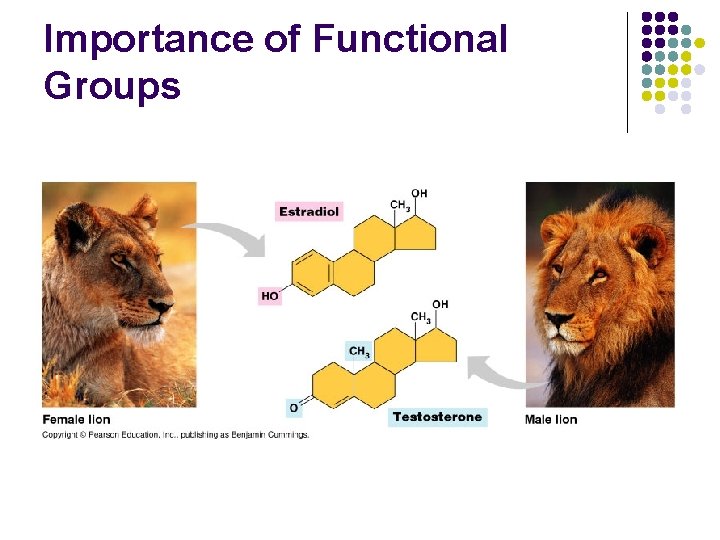
Importance of Functional Groups
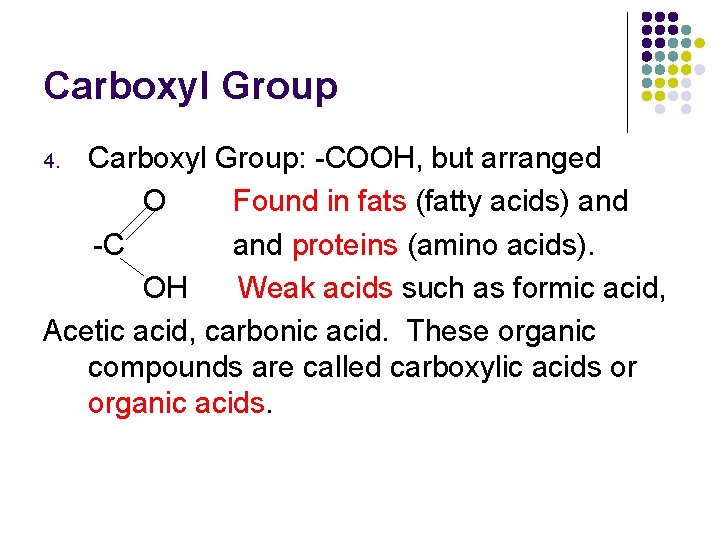
Carboxyl Group: -COOH, but arranged O Found in fats (fatty acids) and -C and proteins (amino acids). OH Weak acids such as formic acid, Acetic acid, carbonic acid. These organic compounds are called carboxylic acids or organic acids. 4.
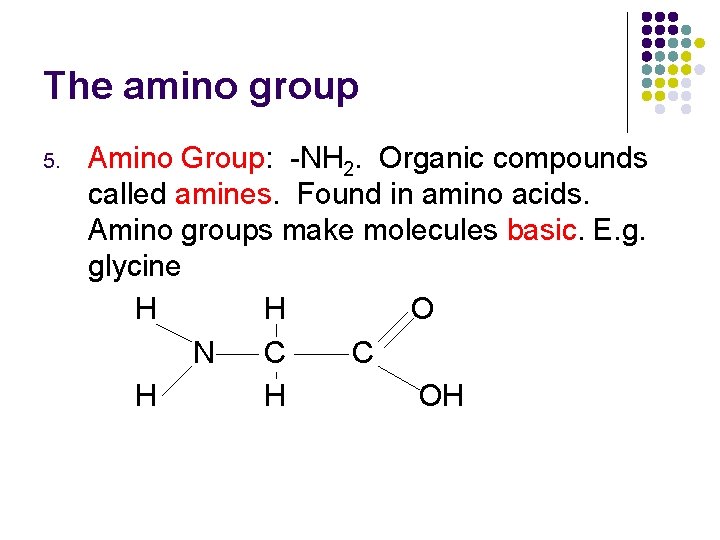
The amino group 5. Amino Group: -NH 2. Organic compounds called amines. Found in amino acids. Amino groups make molecules basic. E. g. glycine H H O N C C H H OH
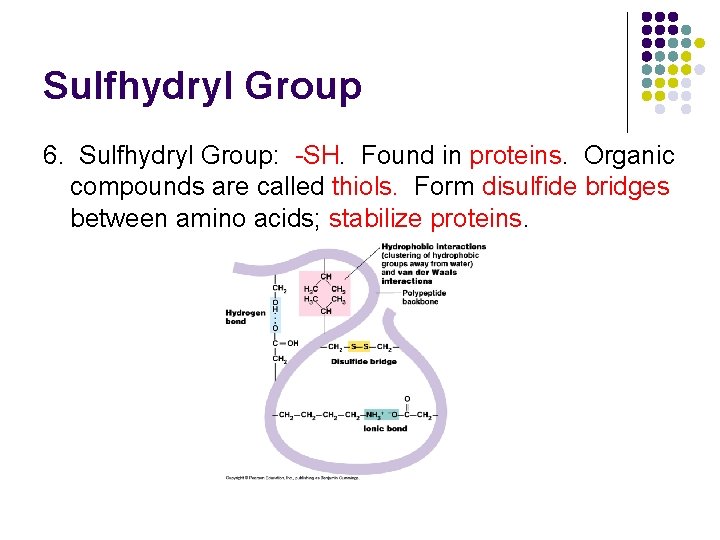
Sulfhydryl Group 6. Sulfhydryl Group: -SH. Found in proteins. Organic compounds are called thiols. Form disulfide bridges between amino acids; stabilize proteins.
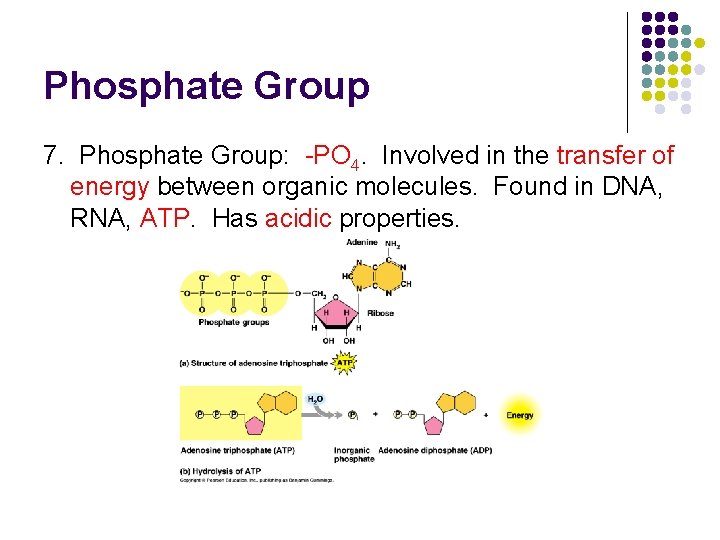
Phosphate Group 7. Phosphate Group: -PO 4. Involved in the transfer of energy between organic molecules. Found in DNA, RNA, ATP. Has acidic properties.
Organic Functional Groups: Summary
- Slides: 20