Organic Chemistry Carbohydrates Sugars Polyhydroxylated aldehydes and ketones
有机化学 Organic Chemistry 第十三章 糖 Carbohydrates


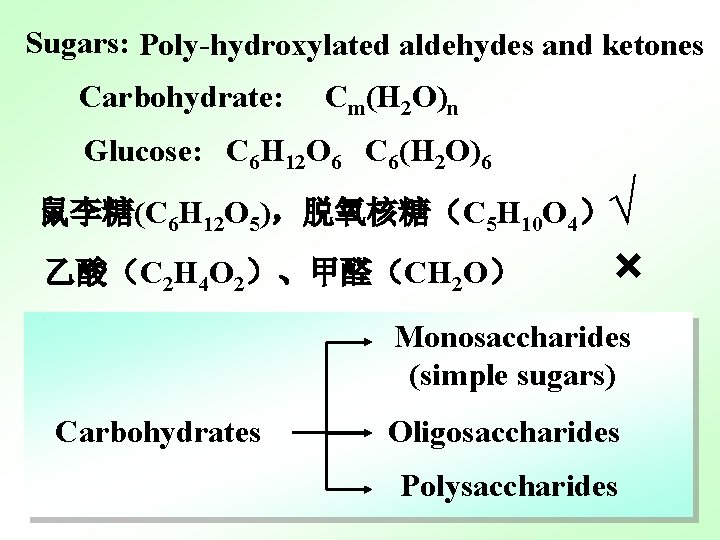
Sugars: Poly-hydroxylated aldehydes and ketones Carbohydrate: Cm(H 2 O)n Glucose: C 6 H 12 O 6 C 6(H 2 O)6 √ × 鼠李糖(C 6 H 12 O 5),脱氧核糖(C 5 H 10 O 4) 乙酸(C 2 H 4 O 2)、甲醛(CH 2 O) Monosaccharides (simple sugars) Carbohydrates Oligosaccharides Polysaccharides

Monosaccharides * * 己醛糖 (glucose) Aldoses Polyhydroxy aldehydes Ketoses Polyhydroxy ketones * * * 己酮糖 (fructose)
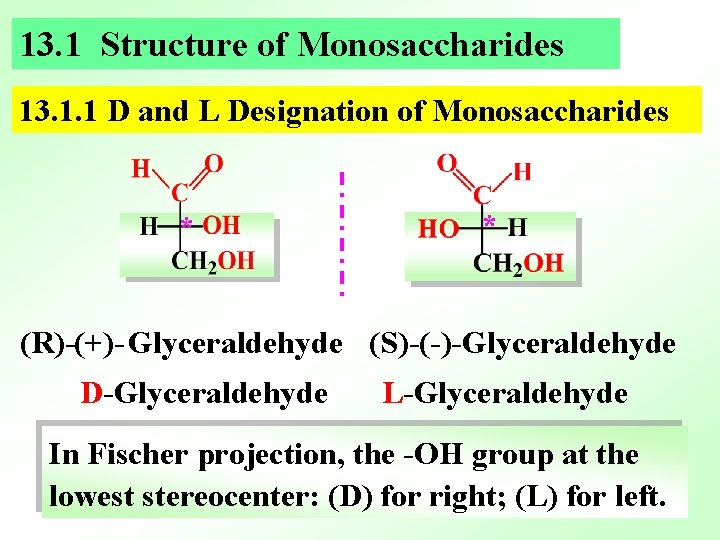
13. 1 Structure of Monosaccharides 13. 1. 1 D and L Designation of Monosaccharides * * (R)-(+)- Glyceraldehyde (S)-(-)-Glyceraldehyde D-Glyceraldehyde L-Glyceraldehyde In Fischer projection, the -OH group at the lowest stereocenter: (D) for right; (L) for left.
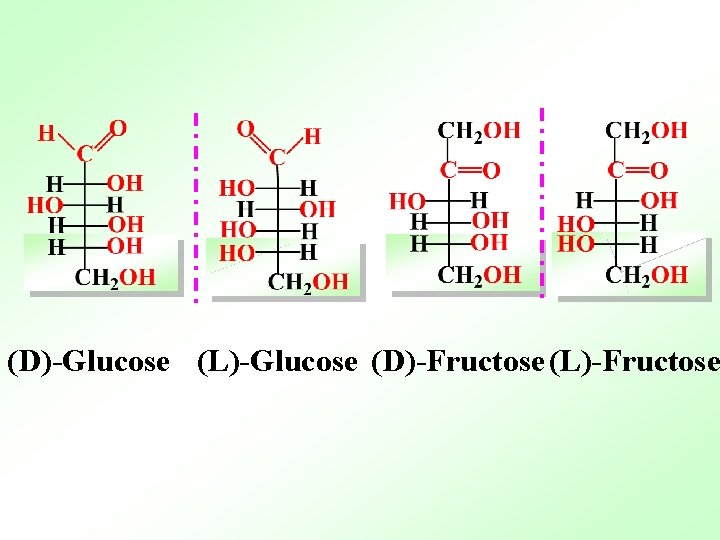
(D)-Glucose (L)-Glucose (D)-Fructose (L)-Fructose
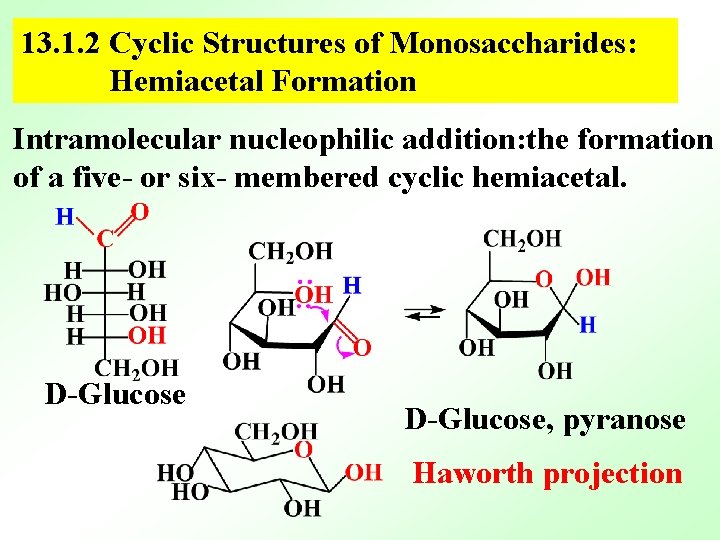
13. 1. 2 Cyclic Structures of Monosaccharides: Hemiacetal Formation Intramolecular nucleophilic addition: the formation of a five- or six- membered cyclic hemiacetal. D-Glucose, pyranose Haworth projection
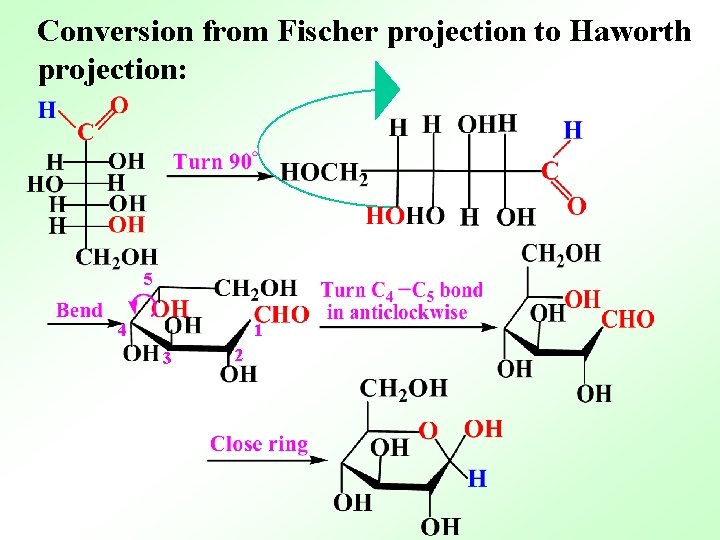
Conversion from Fischer projection to Haworth projection: 5 4 1 3 2

• Haworth式
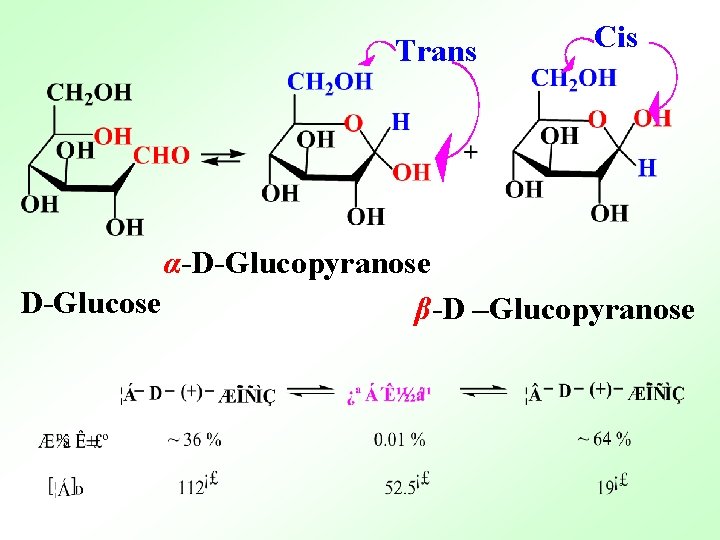
Trans Cis α-D-Glucopyranose D-Glucose β-D –Glucopyranose
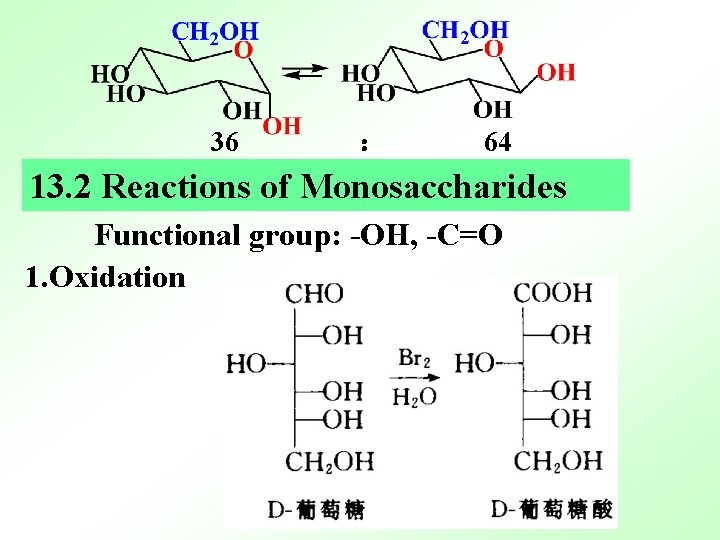
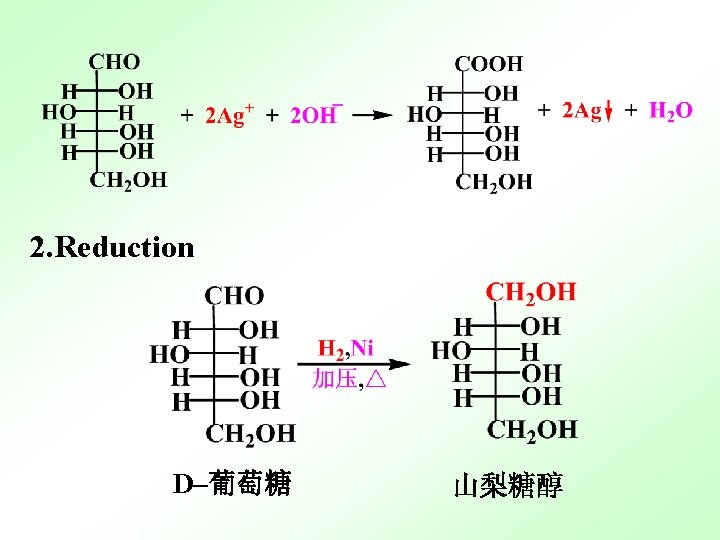
36 : 64 13. 2 Reactions of Monosaccharides Functional group: -OH, -C=O 1. Oxidation
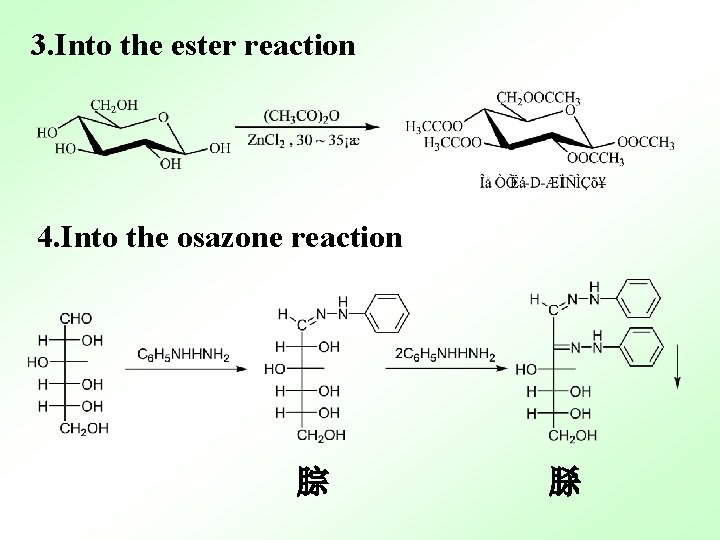
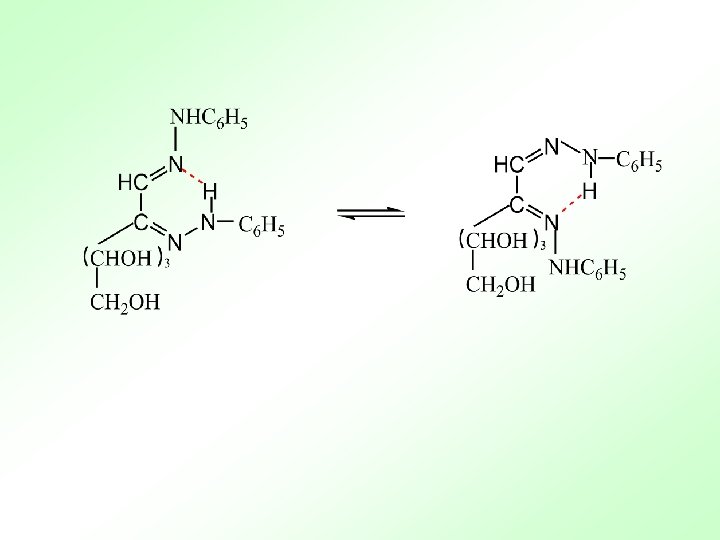
3. Into the ester reaction 4. Into the osazone reaction 腙 脎
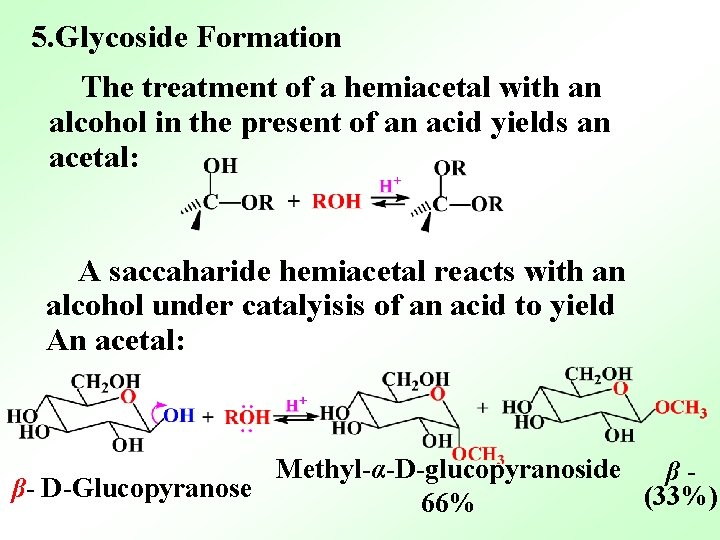
5. Glycoside Formation The treatment of a hemiacetal with an alcohol in the present of an acid yields an acetal: A saccaharide hemiacetal reacts with an alcohol under catalyisis of an acid to yield An acetal: Methyl-α-D-glucopyranoside ββ- D-Glucopyranose (33%) 66%

The features of the reaction: (1)The anomeric –OH has been replaced by an –OR group. (2) The products─glycosides are stable to neutral and basic water. (3)They aren’t in equibrium with an openchain form, and they don’t show mutarotation. (4) Can not be oxidized by Tollens、 Fehling reagents. (5)Can not change to osazone.
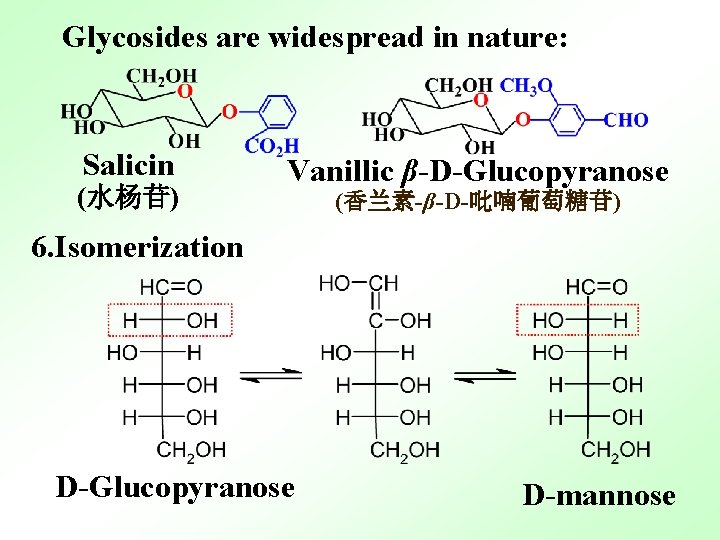
Glycosides are widespread in nature: Salicin (水杨苷) Vanillic β-D-Glucopyranose (香兰素-β-D-吡喃葡萄糖苷) 6. Isomerization D-Glucopyranose D-mannose
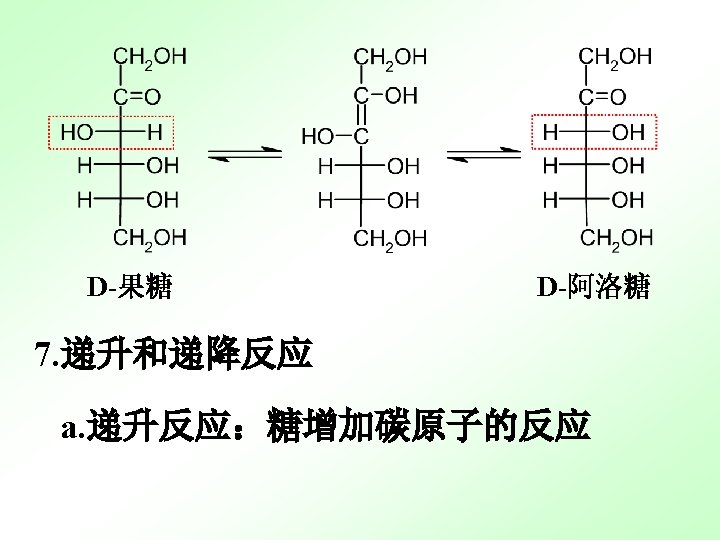
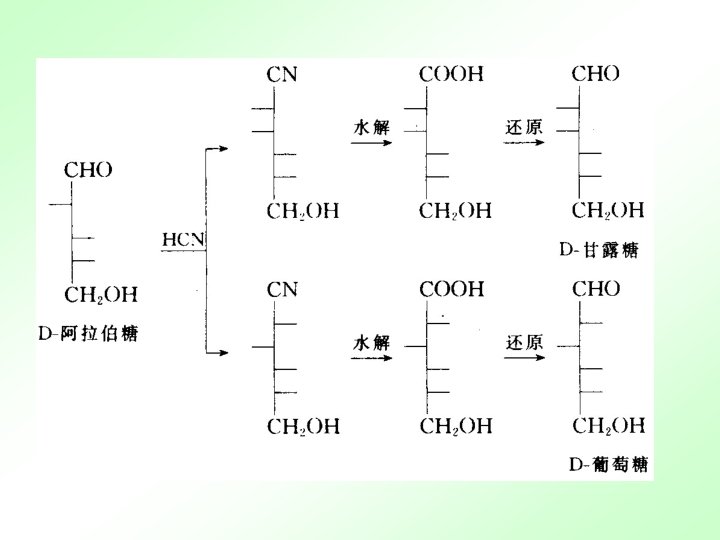
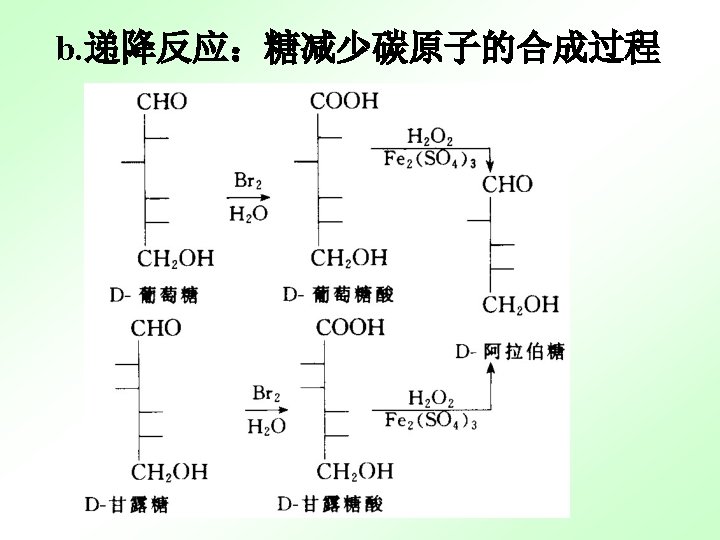
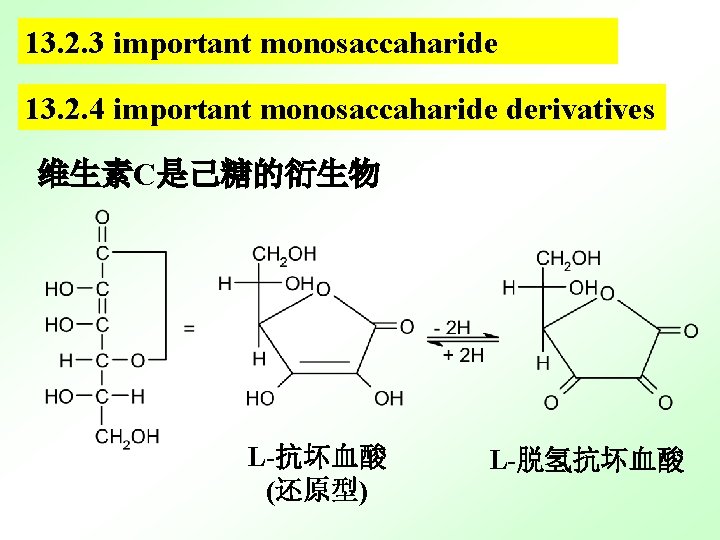
13. 2. 3 important monosaccaharide 13. 2. 4 important monosaccaharide derivatives 维生素C是己糖的衍生物 L-抗坏血酸 (还原型) L-脱氢抗坏血酸
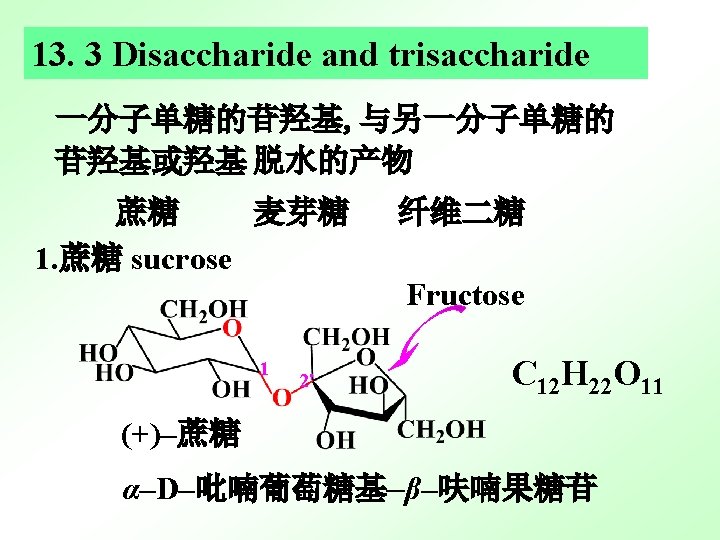
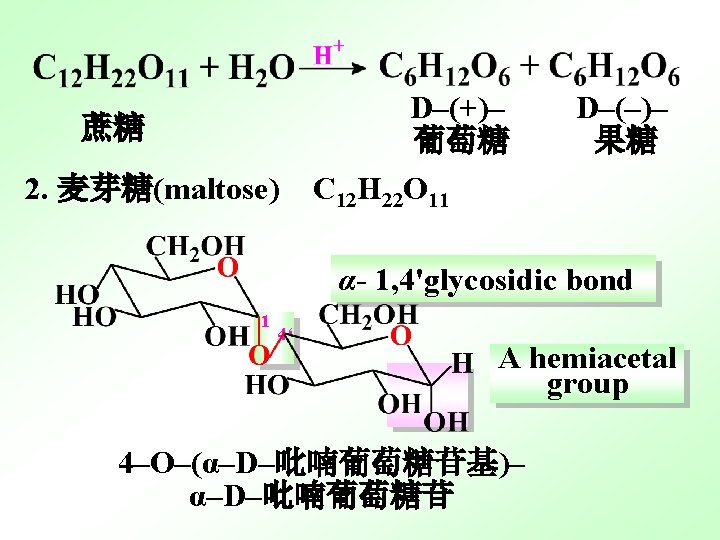
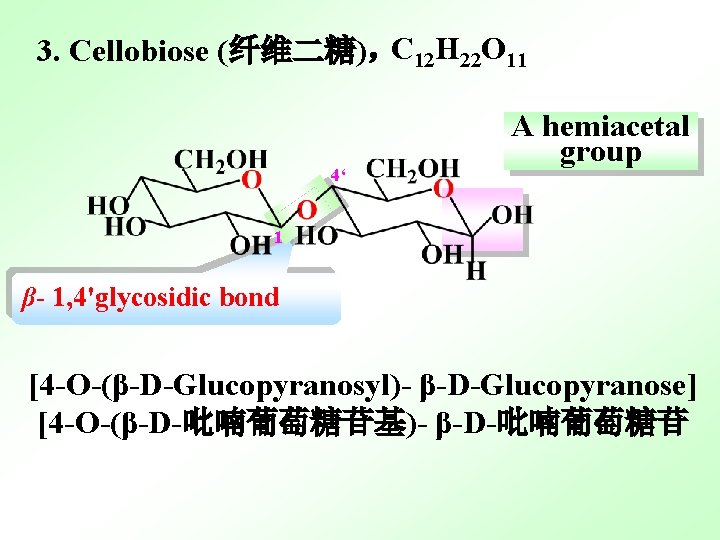
3. Cellobiose (纤维二糖),C 12 H 22 O 11 4‘ A hemiacetal group 1 β- 1, 4'glycosidic bond [4 -O-(β-D-Glucopyranosyl)- β-D-Glucopyranose] [4 -O-(β-D-吡喃葡萄糖苷基)- β-D-吡喃葡萄糖苷
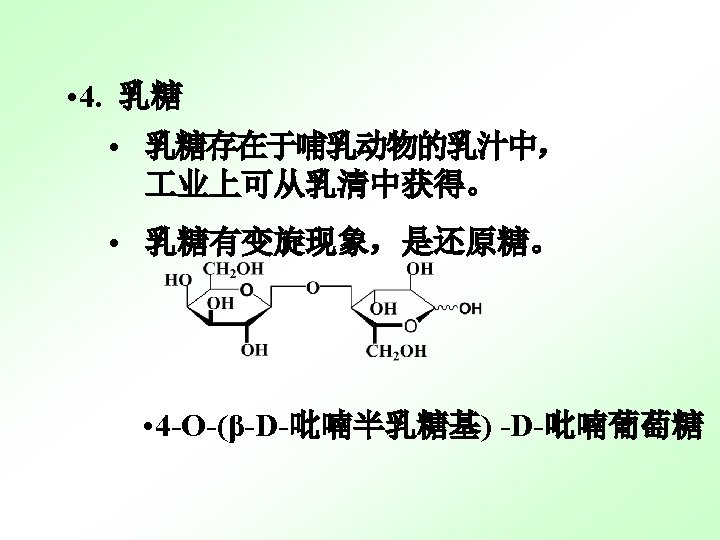



(1) Both Maltose and Cellobiose are reducing sugars. (2)Both are in equilibrium with aldehyde forms, which can reduce Tollens’ of Fehling’s reagent. (3) Both exhibit mutarotation. (4)Both have dramatic different biological properties.


• THANK YOU!
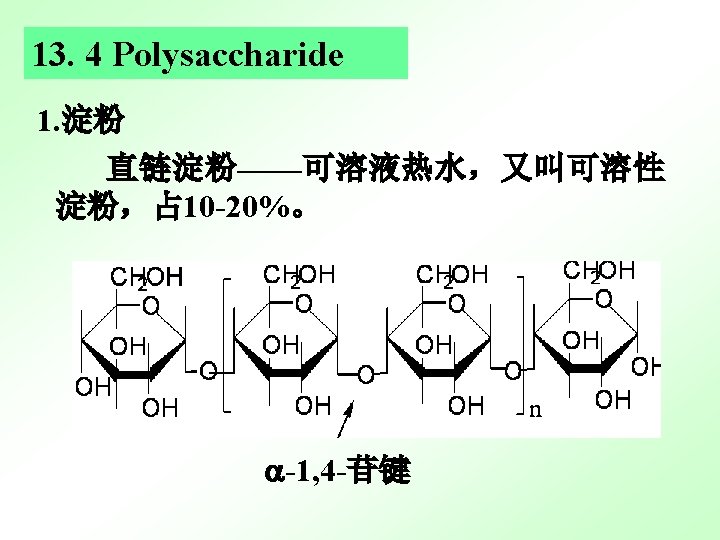
- Slides: 36