Organic chemistry BC Science Probe 10 Section 8

Organic chemistry BC Science Probe 10 Section 8. 3
Organic Chemistry • Organic means living • Hydrocarbons contain hydrogen and carbon atoms • Organic Chemistry is the study of carbon chemicals that are natural and artificial. • The bonding in hydrocarbons is covalent so bonding between molecules is weak. • The structure is molecular.
Percentage of Carbon • To be considered organic, a compound must have a high percentage of carbon by mass: – To calculate the percentage of carbon, you take the mass of carbon in the molecule divided by the mass of the whole molecule and multiply it by 100% – If it is higher than 50%, it’s organic!
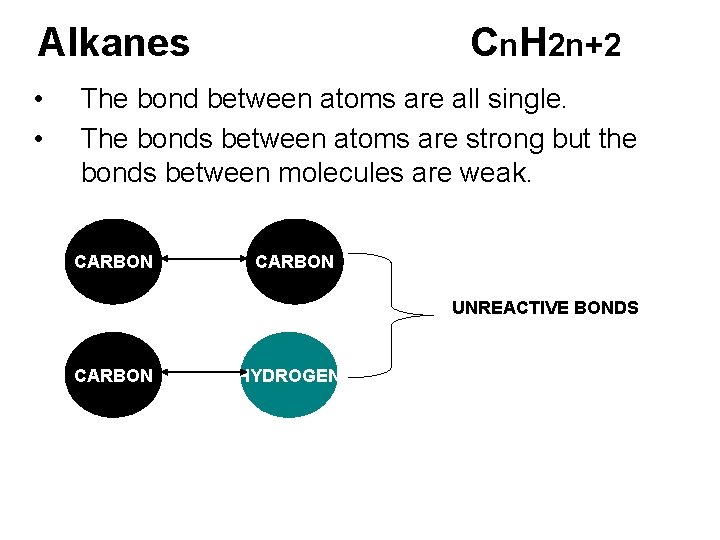
Alkanes • • Cn. H 2 n+2 The bond between atoms are all single. The bonds between atoms are strong but the bonds between molecules are weak. CARBON UNREACTIVE BONDS CARBON HYDROGEN
Properties 1. Good fuels: - lots of heat energy made - COMPLETE COMBUSTION H 2 O + CO 2 - Short chains are best (cleaner + less energy to break) 2. Do not dissolve or react with water or reactants dissolved in water: - no energy to break the unreactive C-C or C-H bonds.
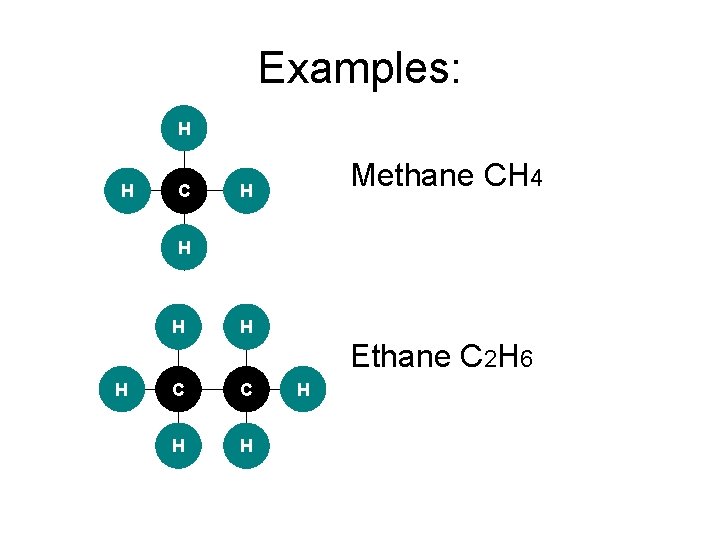
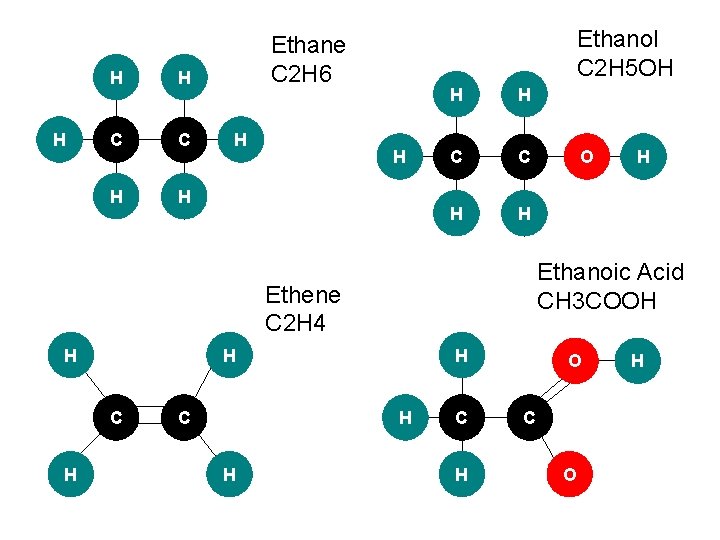
Examples: H H C Methane CH 4 H H Ethane C 2 H 6 H C C H H H
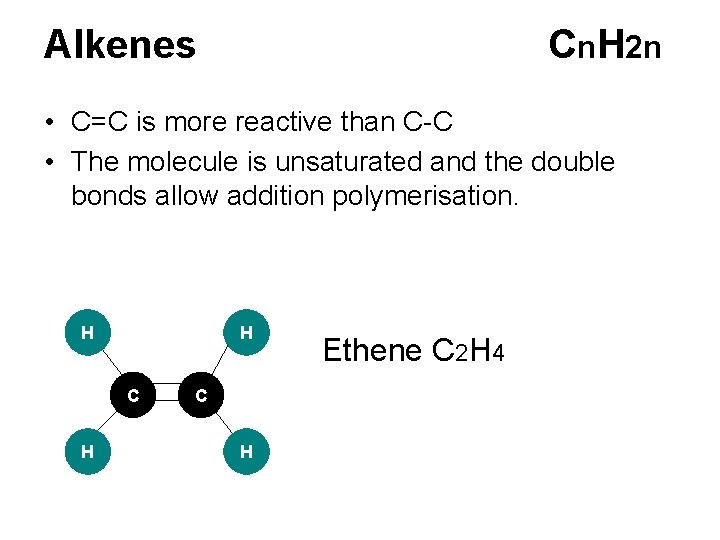
Alkenes Cn. H 2 n • C=C is more reactive than C-C • The molecule is unsaturated and the double bonds allow addition polymerisation. H H C H Ethene C 2 H 4
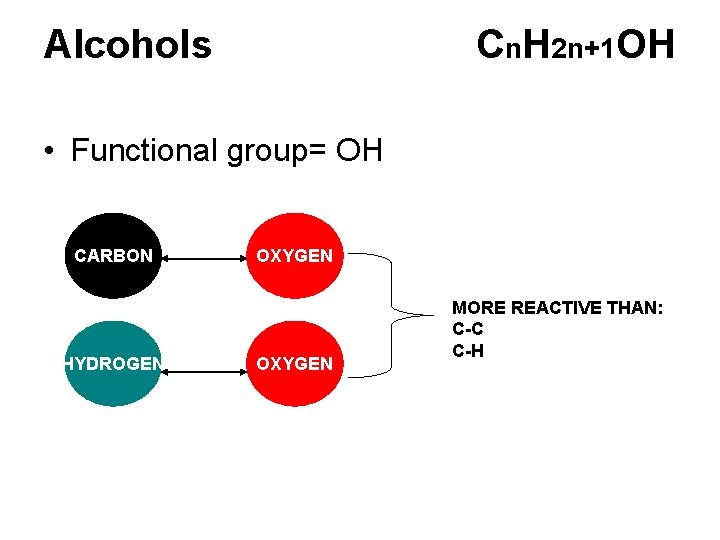
Alcohols Cn. H 2 n+1 OH • Functional group= OH CARBON HYDROGEN OXYGEN MORE REACTIVE THAN: C-C C-H
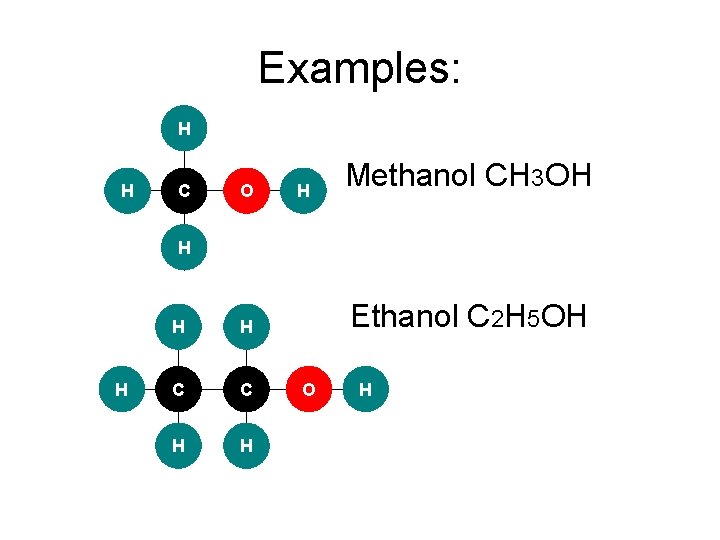
Examples: H H C O H Methanol CH 3 OH H H C C H H Ethanol C 2 H 5 OH O H

Physical Properties 1. Forces between molecules are stronger than between alkane molecules so alcohols are liquid at room temp. 2. Alcohol molecules have a tendency to stick together because they have OH, like water but not as much. 3. Short chains mix with water. 4. Long chains are oily because more CH than OH.
Chemical Properties 1. CH part burns CO 2 + H 2 O 2. React with Na like water but slower: OH reacts and CH is inert. sodium + ethanol sodium ethanoxide + water

Organic Acids (Carboxylic) Cn. H 2 n-1 COOH • • Functional group= COOH Made by oxidising Alcohols: 1. Alcohol heated with catalyst 2. Reflux condenser used to vapours are not lost but drip back into the solution. 3. Organic acid collected. • • The oxidation reaction is used in breathalyser tests ethanoic acid (in water) H+ and ethanoate ions This is reversible as the ions react and reform ethanoic acid.
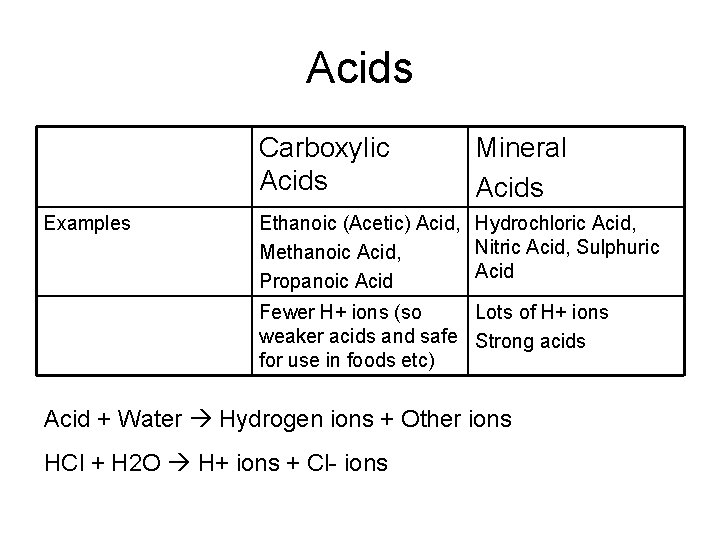
Acids Carboxylic Acids Examples Mineral Acids Ethanoic (Acetic) Acid, Hydrochloric Acid, Nitric Acid, Sulphuric Methanoic Acid, Acid Propanoic Acid Fewer H+ ions (so Lots of H+ ions weaker acids and safe Strong acids for use in foods etc) Acid + Water Hydrogen ions + Other ions HCl + H 2 O H+ ions + Cl- ions
REACTIVITY Alcohols Alkenes Alkanes
H H H C C H H Ethanol C 2 H 5 OH Ethane C 2 H 6 H H C C H H H C H H Ethanoic Acid CH 3 COOH Ethene C 2 H 4 H O C O H

Esters Organic Acid + Alcohol Ester + Water This is a CONDENSATION REACTION as a molecule of water is lost. Alcohol loses OH and Acid loses H = H 2 O This is HYDROLYSIS because water is used to split the ester molecules. Sped up when heated &/or acid/alkali added
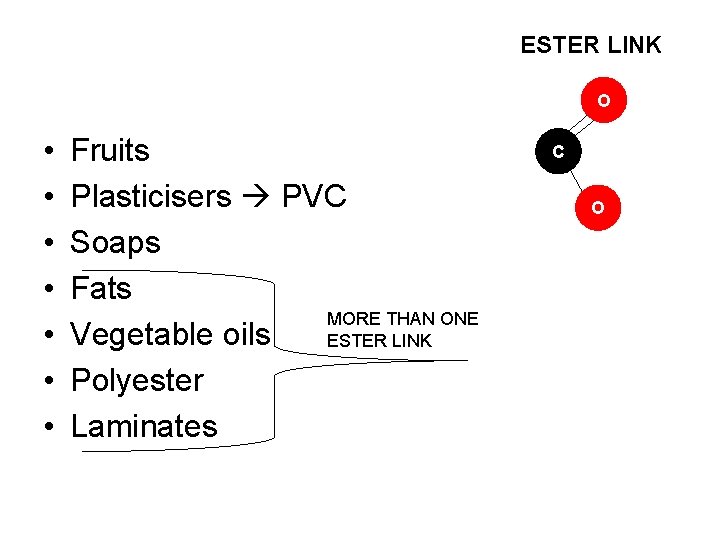
ESTER LINK O • • Fruits Plasticisers PVC Soaps Fats MORE THAN ONE Vegetable oils ESTER LINK Polyester Laminates C O

Esterification Ester + Alcohol Pure Ester Sample + Impurities Esterification is this reaction when heated with some concentrated sulphuric acid to make a pure Ester. 1. Heated + reflux condenser 2. Keep heating after reflux + impurities condense and out. . 3. Separating method: shaken alcohols, acids react with aqueous reagents and dissolve but Ester does not. 4. Calcium chloride crystals absorb moisture dry Ester 5. Heat, only Ester evaporates and then condenses PURE DRY ESTER

Fats and Oils • Fats and Oils have more than one Ester link in a single molecule. • Fats and Oils release 5 x more energy when oxidised than carbs. They are better energy store. Fats Acids + Alcohol Fats Fatty Acids + Glycerol (Alcohol)
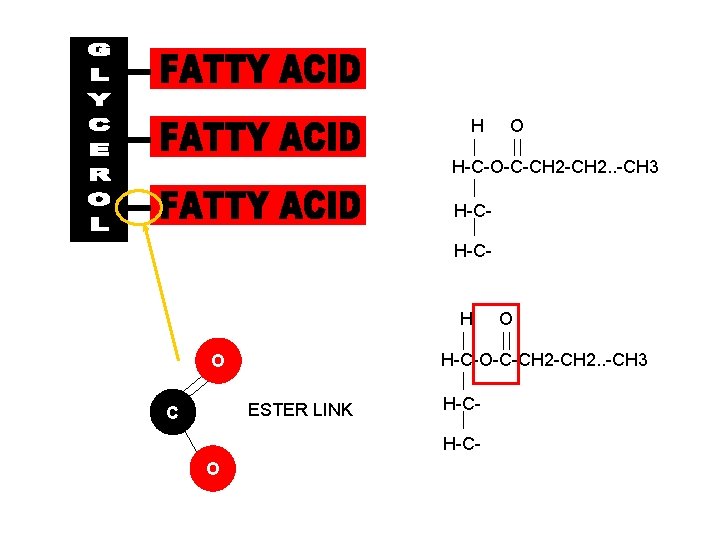
H O H-C-O-C-CH 2. . -CH 3 H-CH-C- H H-C-O-C-CH 2. . -CH 3 O ESTER LINK C H-CH-C- O O
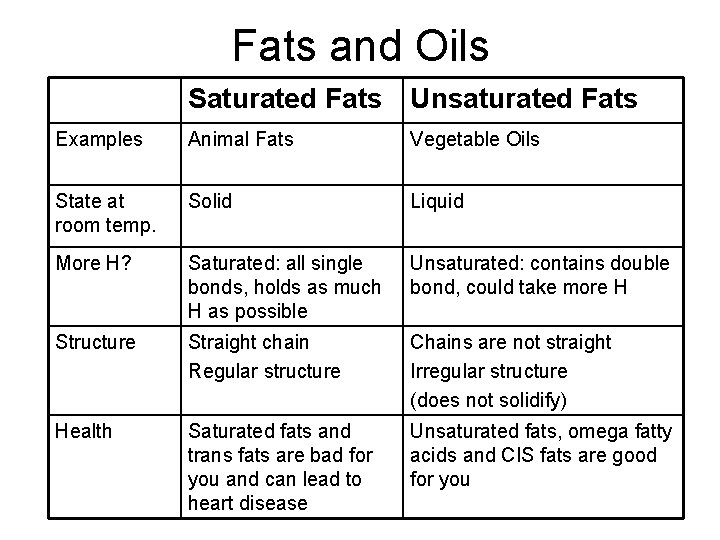
Fats and Oils Saturated Fats Unsaturated Fats Examples Animal Fats Vegetable Oils State at room temp. Solid Liquid More H? Saturated: all single bonds, holds as much H as possible Unsaturated: contains double bond, could take more H Structure Straight chain Regular structure Chains are not straight Irregular structure (does not solidify) Health Saturated fats and trans fats are bad for you and can lead to heart disease Unsaturated fats, omega fatty acids and CIS fats are good for you

MARGARINE: Nickel catalyst Vegetable oils Margarine Unsaturated Saturated ( Heart Disease) This is HYDROGENATION Hardening vegetable oils is cheaper than butter but it is just as harmful.
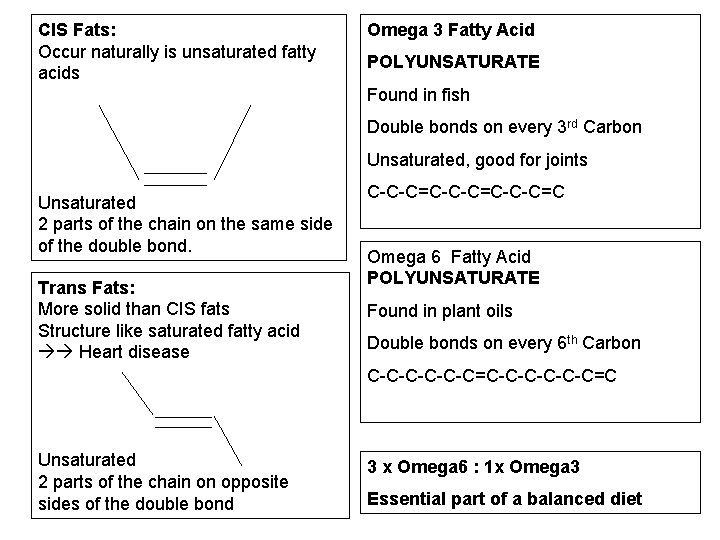
CIS Fats: Occur naturally is unsaturated fatty acids Omega 3 Fatty Acid POLYUNSATURATE Found in fish Double bonds on every 3 rd Carbon Unsaturated, good for joints Unsaturated 2 parts of the chain on the same side of the double bond. Trans Fats: More solid than CIS fats Structure like saturated fatty acid Heart disease C-C-C=C-C-C=C Omega 6 Fatty Acid POLYUNSATURATE Found in plant oils Double bonds on every 6 th Carbon C-C-C-C-C-C=C Unsaturated 2 parts of the chain on opposite sides of the double bond 3 x Omega 6 : 1 x Omega 3 Essential part of a balanced diet
- Slides: 23