ORGANIC CHEMISTRY BASICS General Chemistry Properties of Carbon

ORGANIC CHEMISTRY BASICS General Chemistry

Properties of Carbon � � Carbon is a non-metal Carbon has 4 valence electrons. Carbon can form up to 4 bonds. The Lewis Dot Structure for carbon shows how carbon can form these 4 bonds.

Prefixes for Carbon Compounds � � � � � Meth – 1 carbon Eth – 2 carbons Prop – 3 carbons But – 4 carbons Pent – 5 carbons Hex – 6 carbons Hept – 7 carbons Oct – 8 carbons Non – 9 carbons Dec – 10 carbons

Types of Organic Compounds � � Hydrocarbons – contains the elements, hydrogen and carbon Carbohydrates – contains the elements, hydrogen, carbon, and oxygen Complex polymers Biological molecules

Hydrocarbons � Three basic hydrocarbons � Alkanes – single bonds between carbon atoms � Alkenes – double bonds between carbons atoms � Alkynes – triple bonds between carbon atoms � Examples: C-C is ethane C=C is ethene C= C is ethyne

Petrochemicals � Petrochemicals contain hydrocarbons. � Propane, butane, and octane are some of the most common. � Propane is a single chained carbon molecule with 3 carbon atoms � Butane is a single chained carbon molecule with 4 carbon atoms. � Octane is a single chained carbon molecule with 8 carbon atoms

Identification of Carbon Chains � � Draw a Lewis Dot of the molecule. Identify the number of hydrogen atoms and attach as appropriate. Spread evenly. Keep functional groups and carbon/hydrogen groupings together. Name the compound based on Functional Groups.
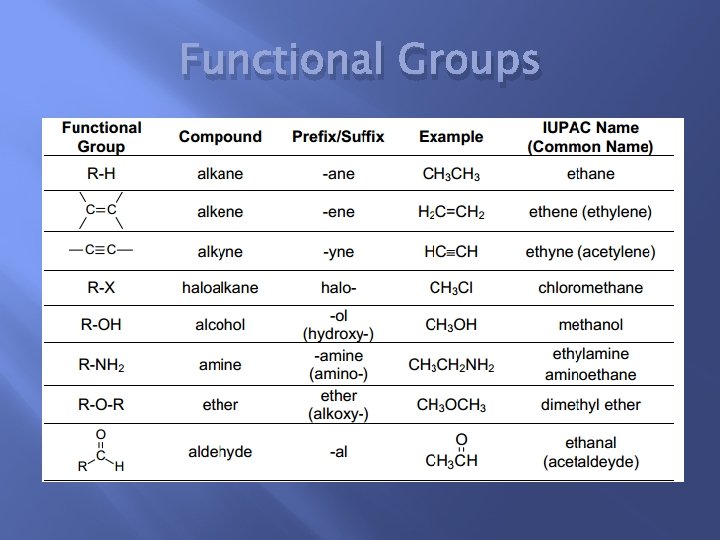
Functional Groups

Functional Groups Continued
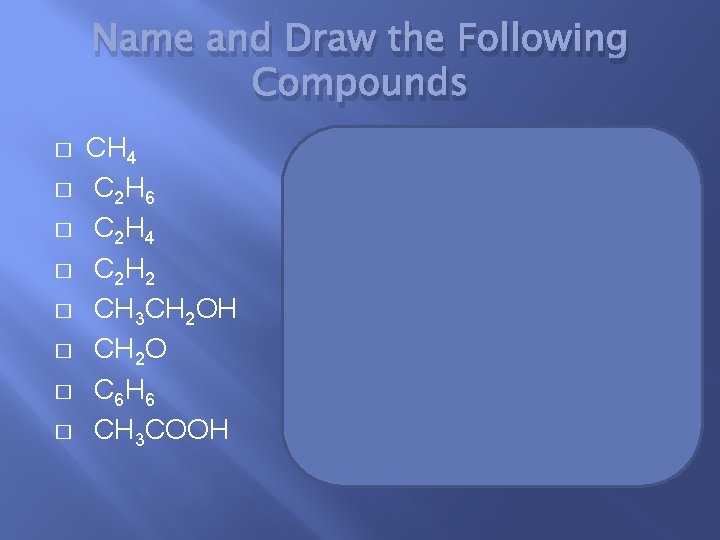
Name and Draw the Following Compounds � � � � CH 4 C 2 H 6 C 2 H 4 C 2 H 2 CH 3 CH 2 OH CH 2 O C 6 H 6 CH 3 COOH methane ethene ethyne ethanol formaldehyde benzene acetic acid (ethanoic acid)
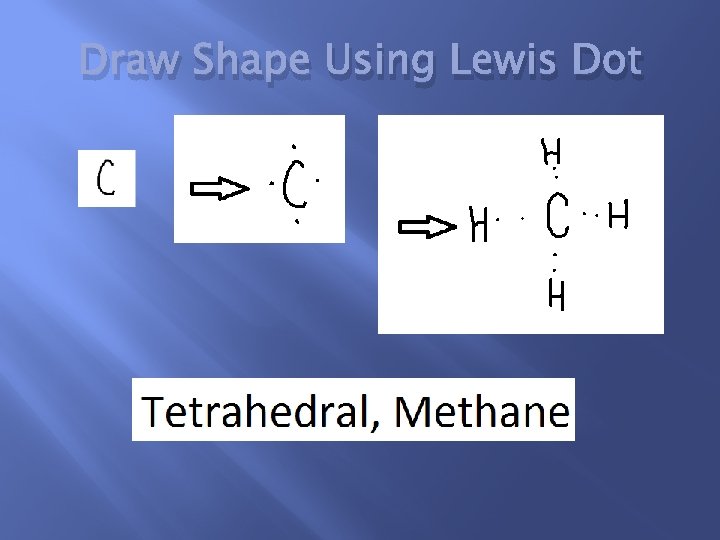
Draw Shape Using Lewis Dot

Saturated vs. Unsaturated Hydrocarbons Saturated – a molecule whose carbon atoms bond to the maximum number of hydrogen atoms Unsaturated – a molecule that contains a carbon multiple bond, to which more hydrogen atoms can be added Alkanes are typically saturated. Alkenes and alkynes are unsaturated due to their multiple bonds.

Carbohydrates � � Composed of carbon, hydrogen and oxygen. Examples: � Sucrose � Glucose
Polymers � � � Polymers are created when small molecules link together in repetitive subunits. Polymers can be natural or synthetic (humanmade) Natural polymers: proteins and nucleic acids Synthetic polymers: polythene, nylon, and Kevlar Common pharmaceuticals such as aspirin, vitamins, and insulin are organic.
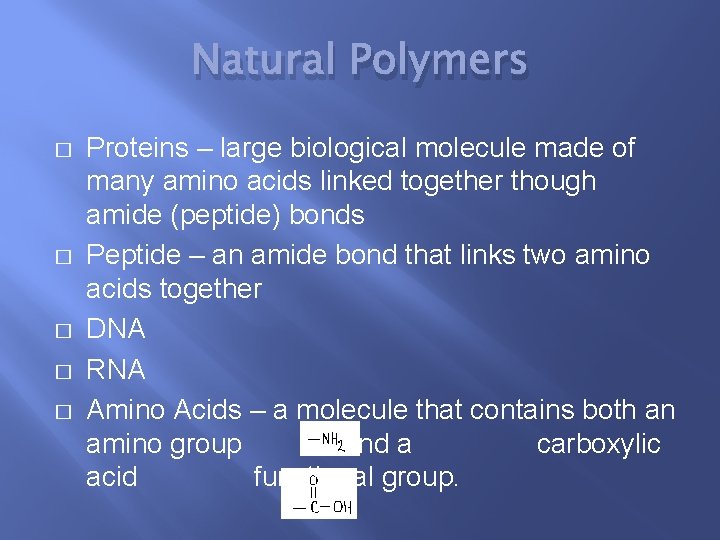
Natural Polymers � � � Proteins – large biological molecule made of many amino acids linked together though amide (peptide) bonds Peptide – an amide bond that links two amino acids together DNA RNA Amino Acids – a molecule that contains both an amino group and a carboxylic acid functional group.

Synthetic Polymers � � � Nylon– a large molecule that is made of repeating units containing polyamide (nitrogen containing functional group) Kevlar – see reading Plastics – compounds formed from petrochemicals which consist of long chains. The stronger the plastic the more layering of these long chains. � Why are bottles for soda, much stronger than those of water?
- Slides: 16