Organic chemistry B Chapter 11 Conjugation and Resonance

Organic chemistry B Chapter 11 Conjugation and Resonance By Prof. Dr. Adel M. Awadallah Islamic University of Gaza
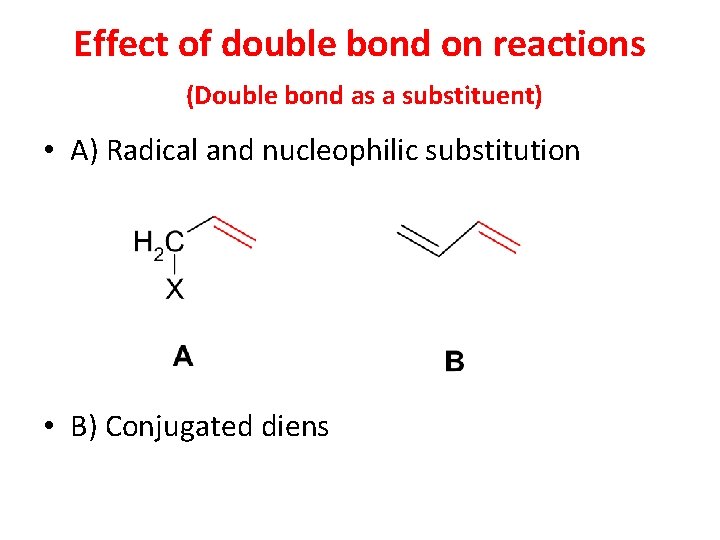
Effect of double bond on reactions (Double bond as a substituent) • A) Radical and nucleophilic substitution • B) Conjugated diens
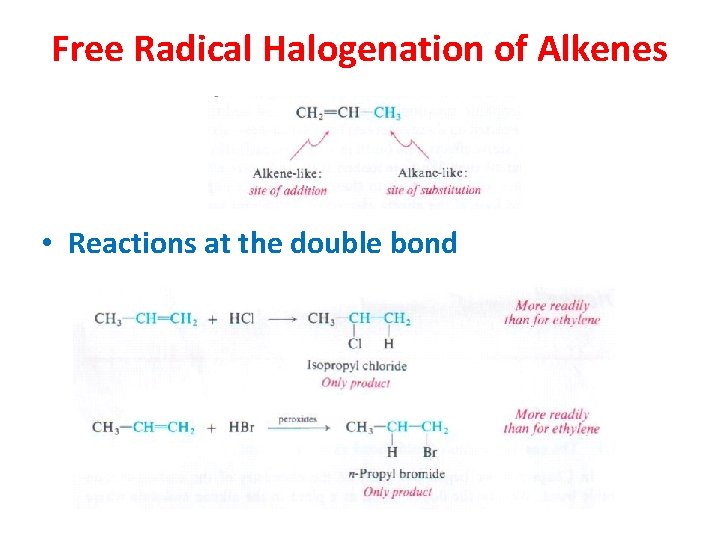
Free Radical Halogenation of Alkenes • Reactions at the double bond
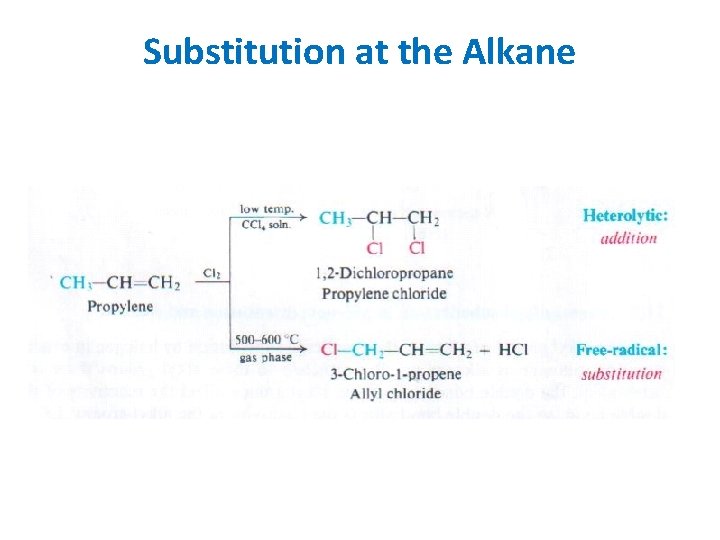
Substitution at the Alkane
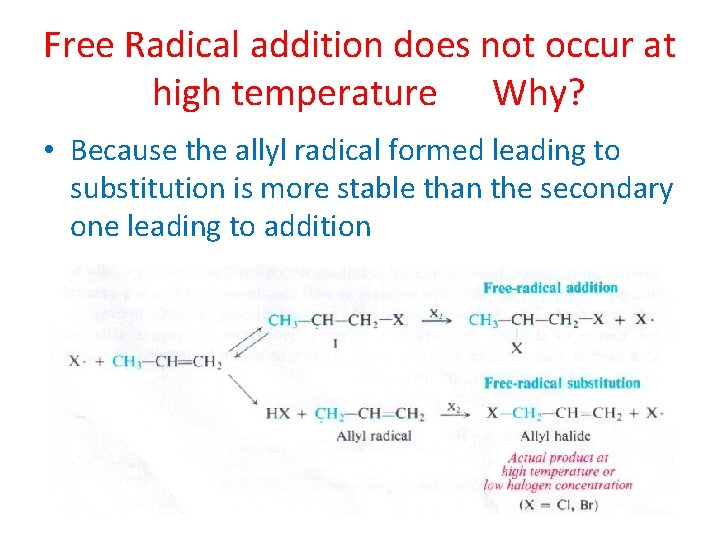
Free Radical addition does not occur at high temperature Why? • Because the allyl radical formed leading to substitution is more stable than the secondary one leading to addition
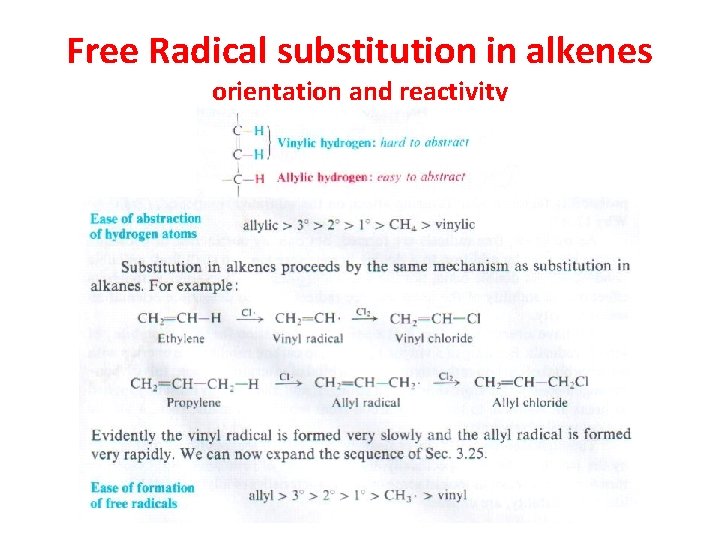
Free Radical substitution in alkenes orientation and reactivity
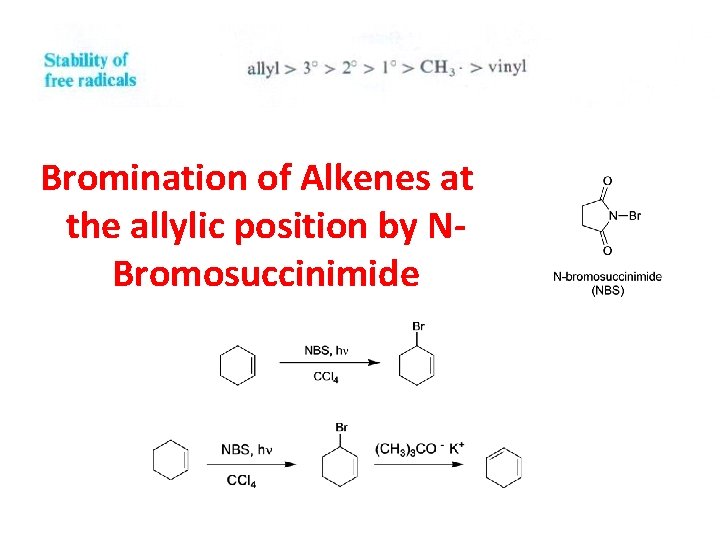
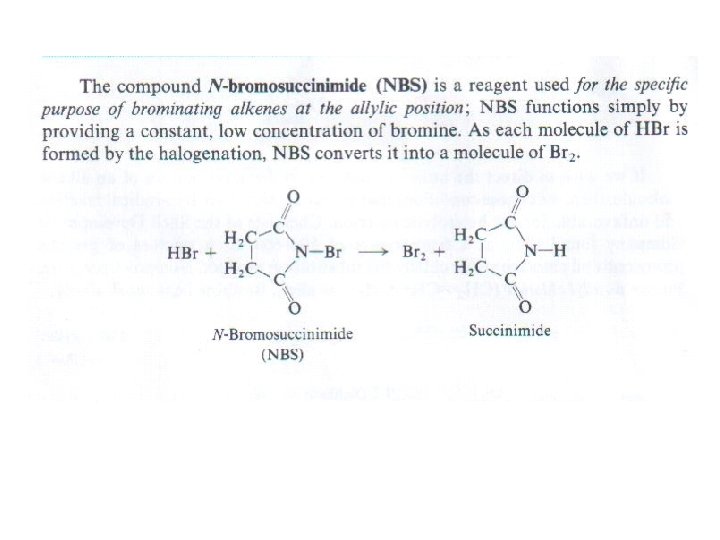
Bromination of Alkenes at the allylic position by NBromosuccinimide
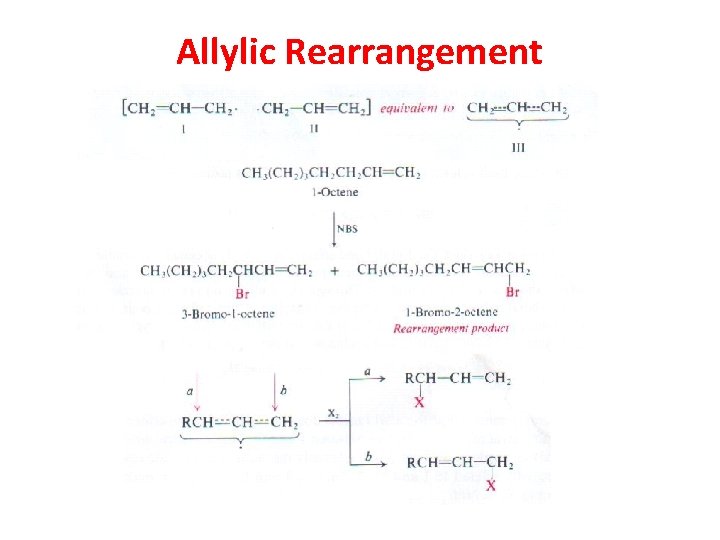
Allylic Rearrangement
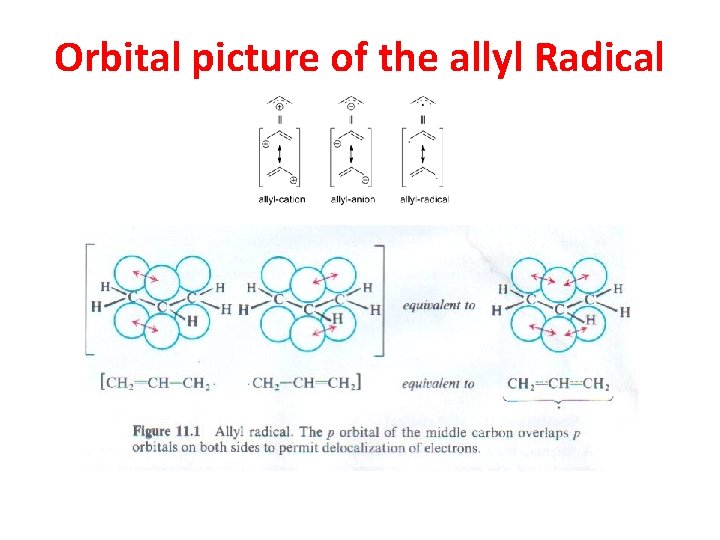
Orbital picture of the allyl Radical
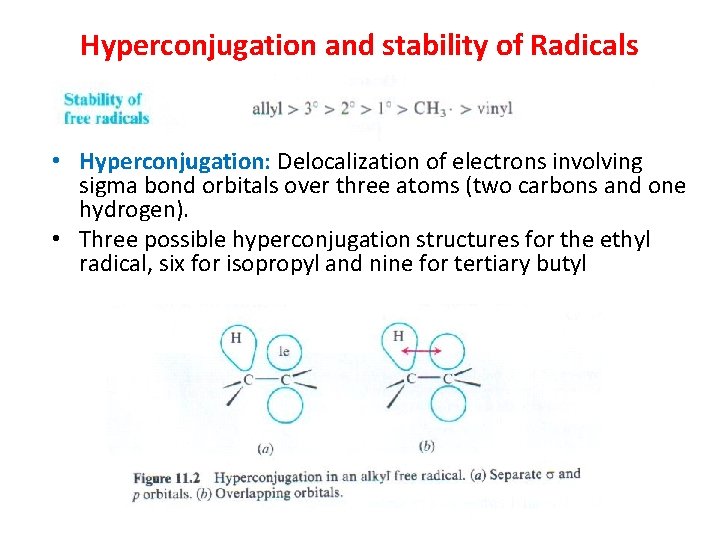
Hyperconjugation and stability of Radicals • Hyperconjugation: Delocalization of electrons involving sigma bond orbitals over three atoms (two carbons and one hydrogen). • Three possible hyperconjugation structures for the ethyl radical, six for isopropyl and nine for tertiary butyl
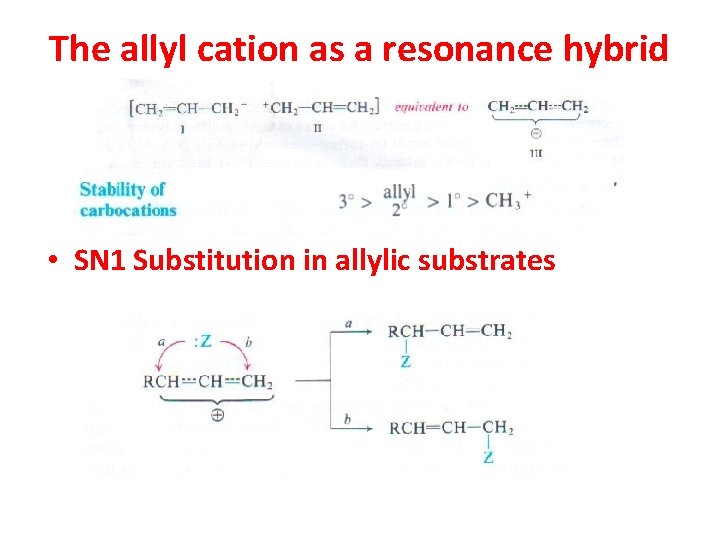
The allyl cation as a resonance hybrid • SN 1 Substitution in allylic substrates
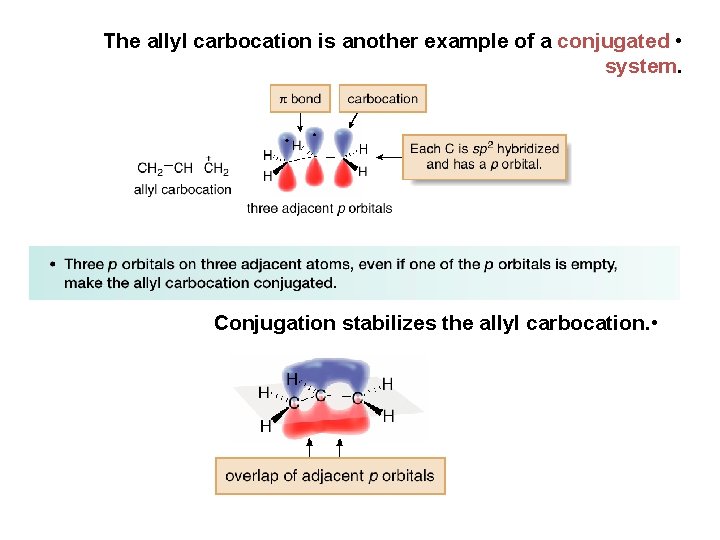
The allyl carbocation is another example of a conjugated • system. Conjugation stabilizes the allyl carbocation. •
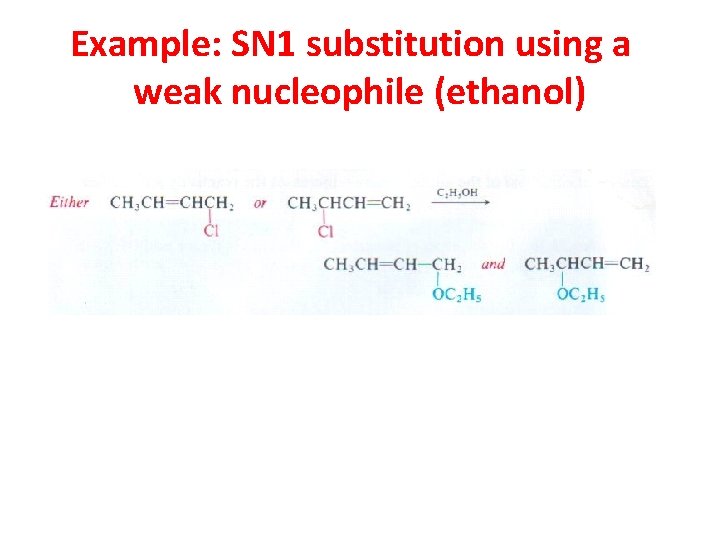
Example: SN 1 substitution using a weak nucleophile (ethanol)
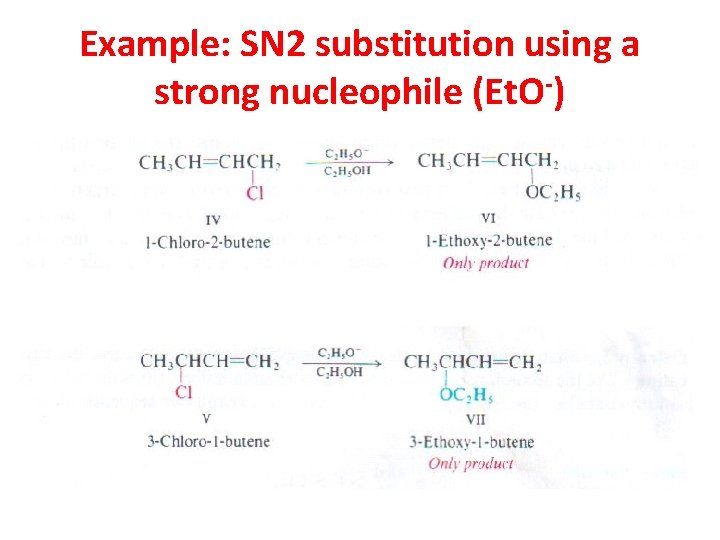
Example: SN 2 substitution using a strong nucleophile (Et. O-)
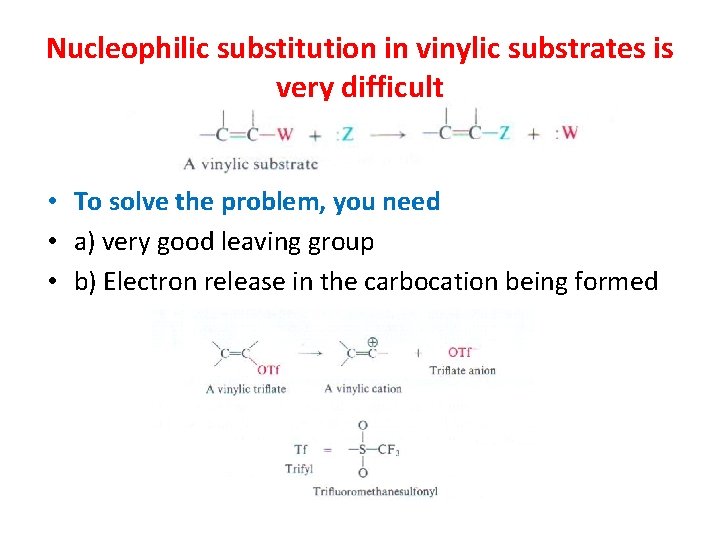
Nucleophilic substitution in vinylic substrates is very difficult • To solve the problem, you need • a) very good leaving group • b) Electron release in the carbocation being formed
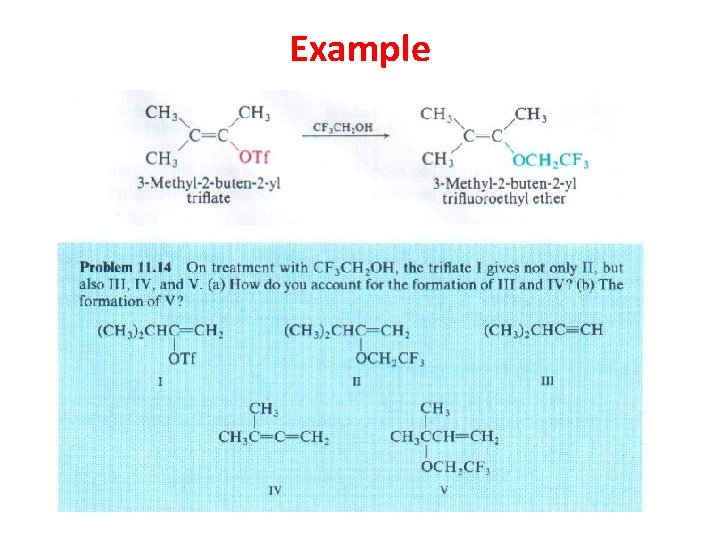
Example
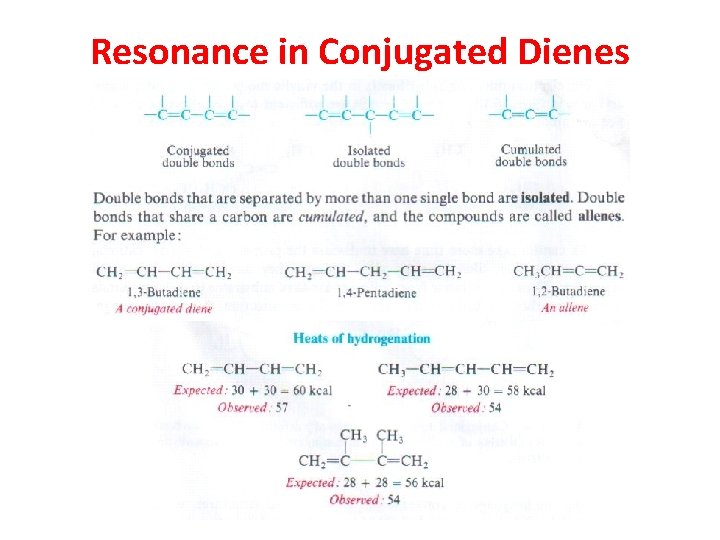
Resonance in Conjugated Dienes
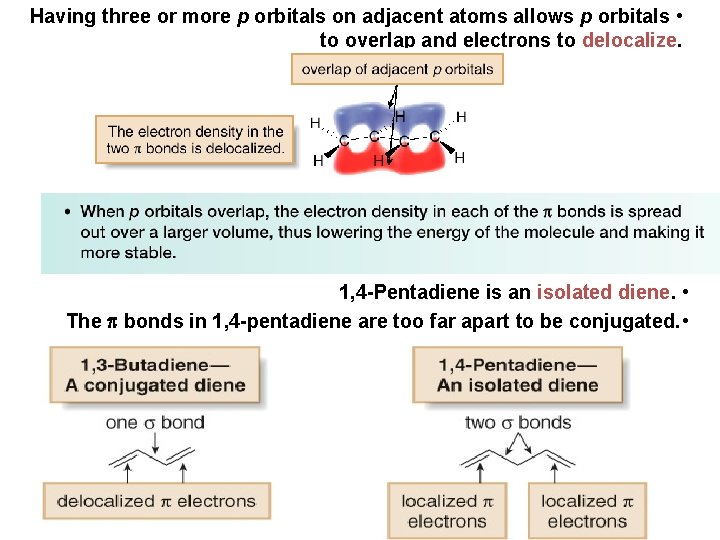
Having three or more p orbitals on adjacent atoms allows p orbitals • to overlap and electrons to delocalize. 1, 4 -Pentadiene is an isolated diene. • The bonds in 1, 4 -pentadiene are too far apart to be conjugated. •
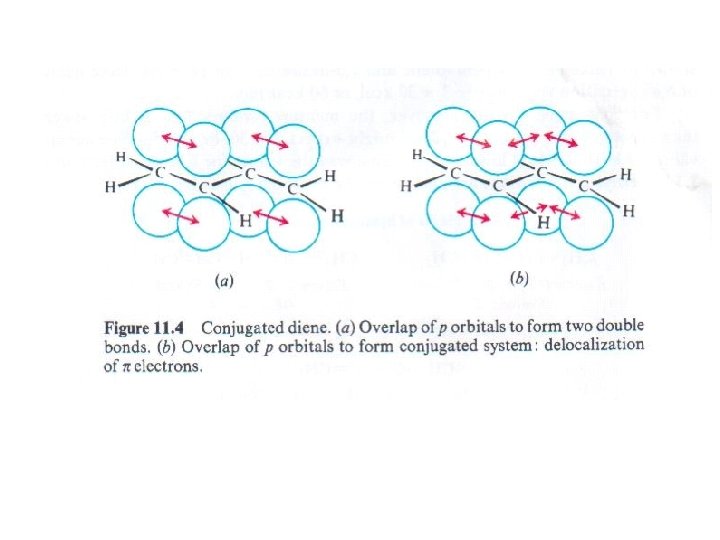
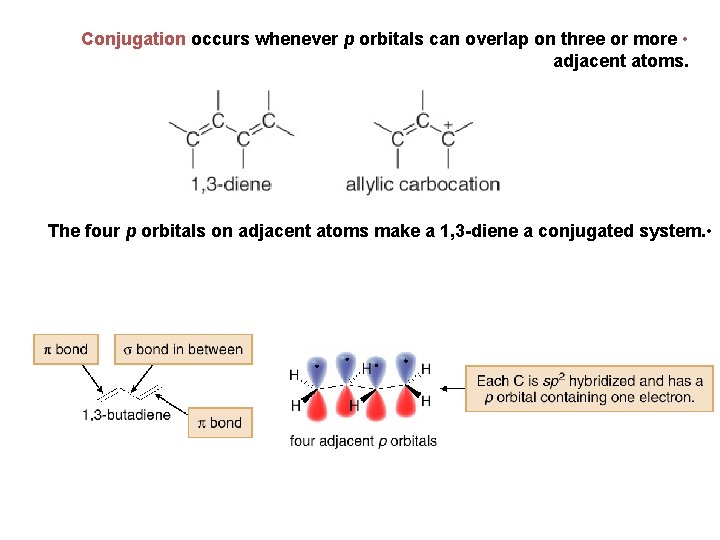
Conjugation occurs whenever p orbitals can overlap on three or more • adjacent atoms. The four p orbitals on adjacent atoms make a 1, 3 -diene a conjugated system. •
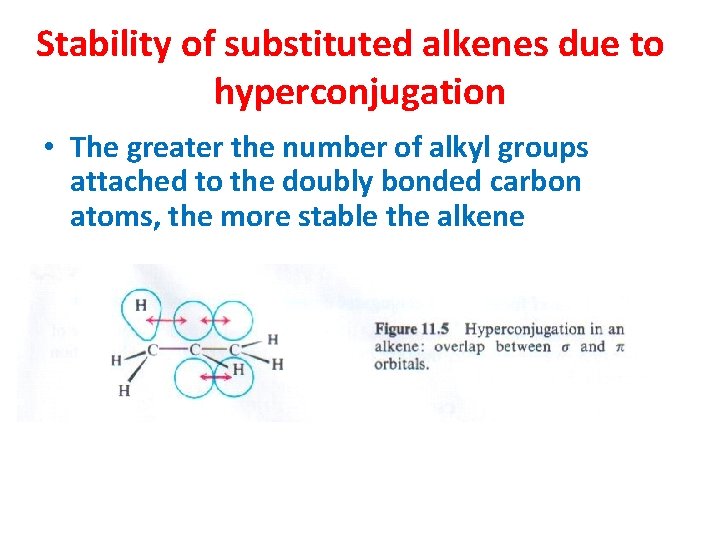
Stability of substituted alkenes due to hyperconjugation • The greater the number of alkyl groups attached to the doubly bonded carbon atoms, the more stable the alkene
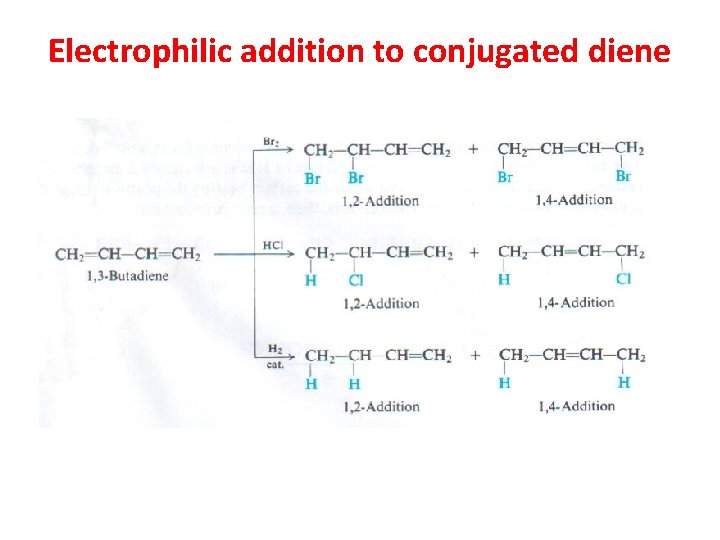
Electrophilic addition to conjugated diene
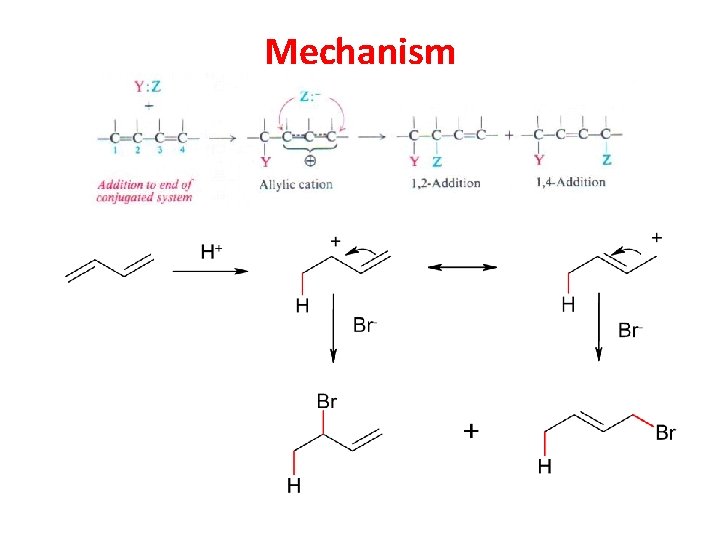
Mechanism
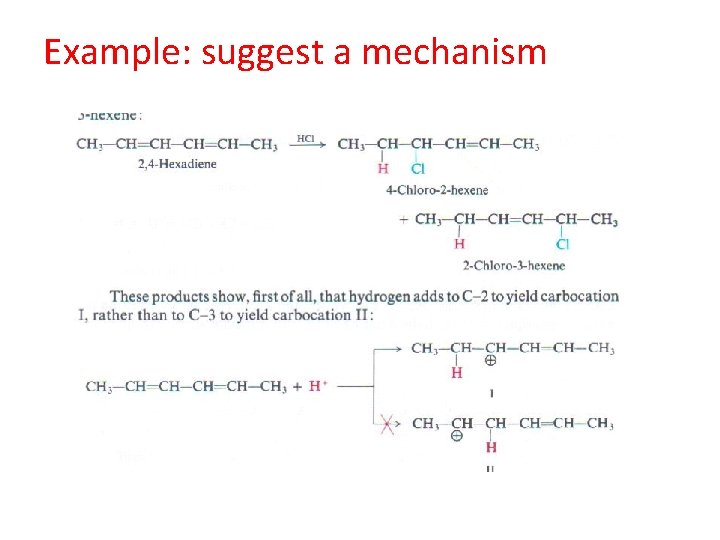
Example: suggest a mechanism
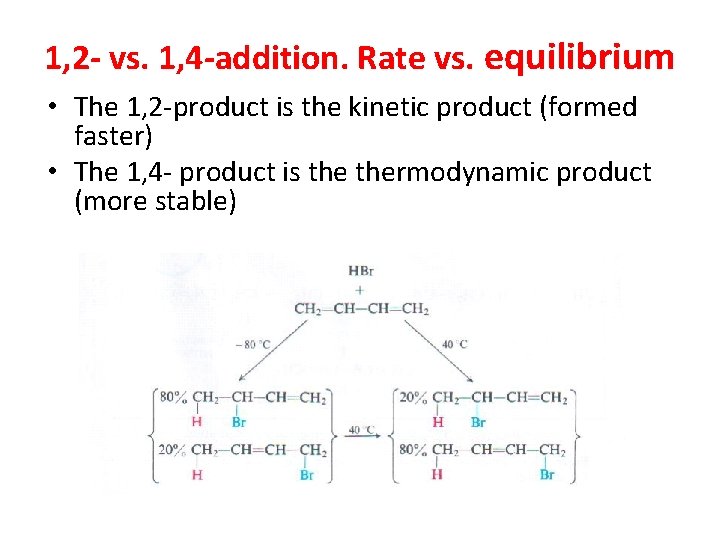
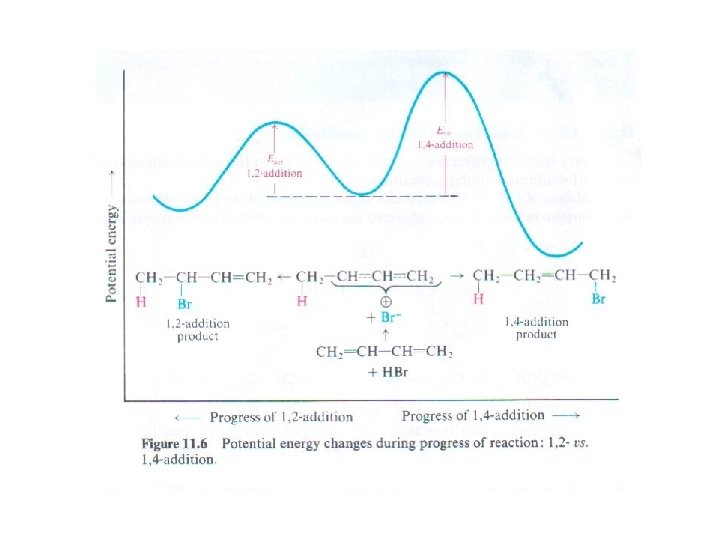
1, 2 - vs. 1, 4 -addition. Rate vs. equilibrium • The 1, 2 -product is the kinetic product (formed faster) • The 1, 4 - product is thermodynamic product (more stable)
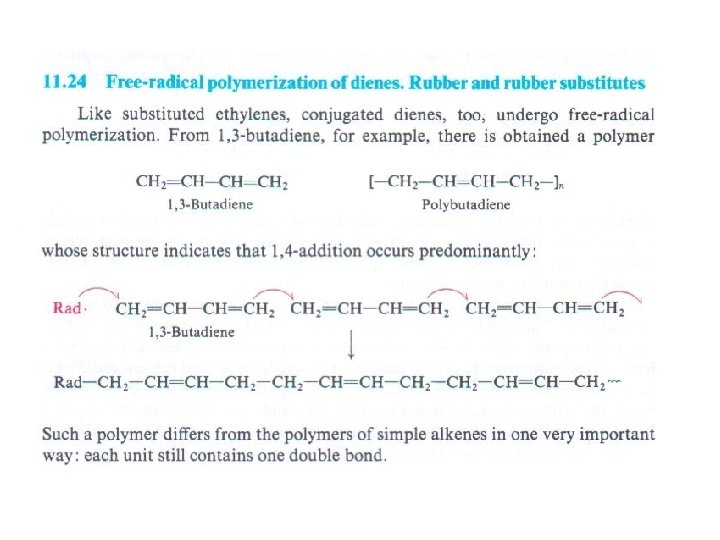
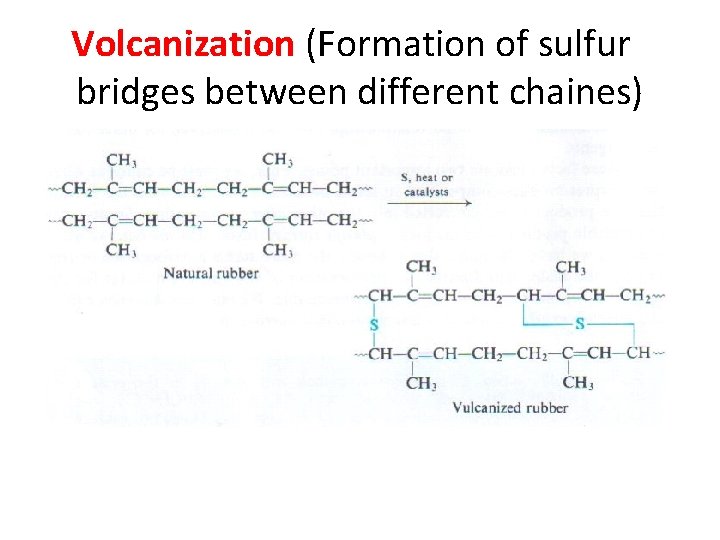
Volcanization (Formation of sulfur bridges between different chaines)
- Slides: 29