Organic Chemistry Assign Seats by the Seating Chart

Organic Chemistry

Assign Seats by the Seating Chart

Distribute Syllabi

Read Syllabi

Begin Instruction

Organic Chemistry • The chemistry of carbon compounds • Sometimes called carbon chemistry

Organic Chemistry • The backbone element in the molecules of all living things is carbon

Carbon Chemistry • Carbon is one of the only elements that can form long chains • Carbon can form 4 covalent bonds

Organic Chemistry • Carbon forms covalent bonds with hydrogen, oxygen, nitrogen, phosphorus, sulfur, and the halogens

Carbon Bonding • Carbon has 4 valence electrons which can hybridize in many ways

Carbon Bonding • To determine the hybridization, draw the Lewis Dot Diagram of the carbon compound

Hybridization 3 • sp 4 -single bonds 2 • sp 2 -single bonds & 1 -double bond • sp 2 doubles or T + S

Multiple Bonds • To make multiple bonds, p-orbitals are required

Hybridization 3 • sp no p-orbitals 2 • sp 1 p-orbital • sp 2 p-orbitals

Single bonds & the first bond in multiple bonds involve end-to-end overlap: s

Multiple Bonds • After the bond, any others are p p bonds: side-to-side p-overlap st 1
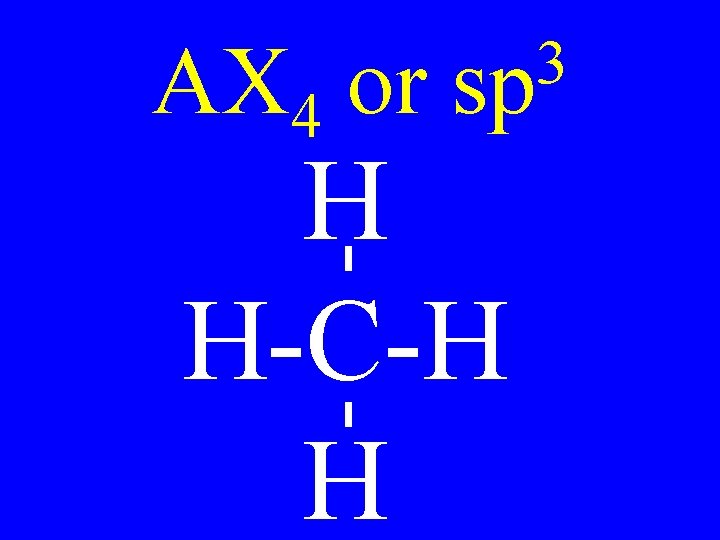
AX 4 or 3 sp H H-C-H H
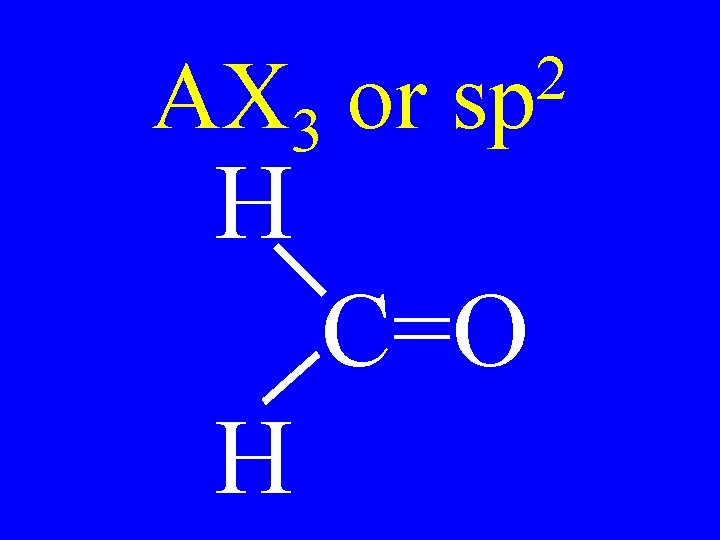
AX 3 or H 2 sp C=O H
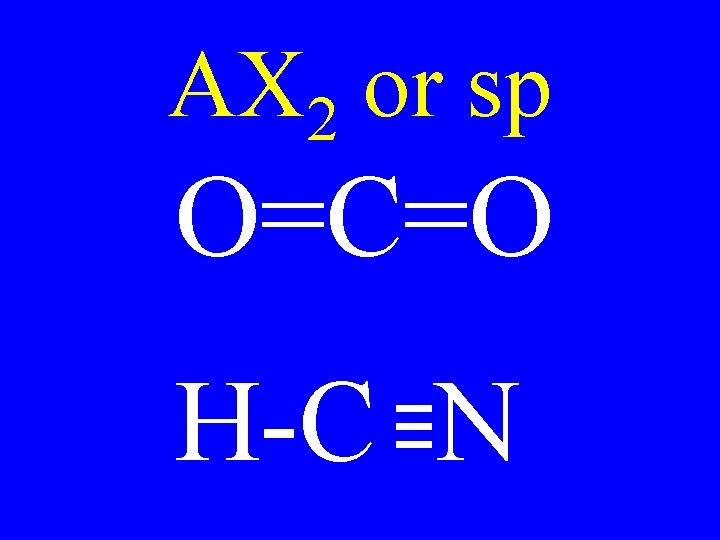
AX 2 or sp O=C=O H-C N

Carbon Bonds 4 s bonds 2 • sp 3 s & 1 p bonds • sp 2 s & 2 p bonds 3 • sp

Carbon • AX 4 • AX 3 E • AX 2 E 2 3 sp Shapes tetrahedral trigonal pyramidal v-shaped
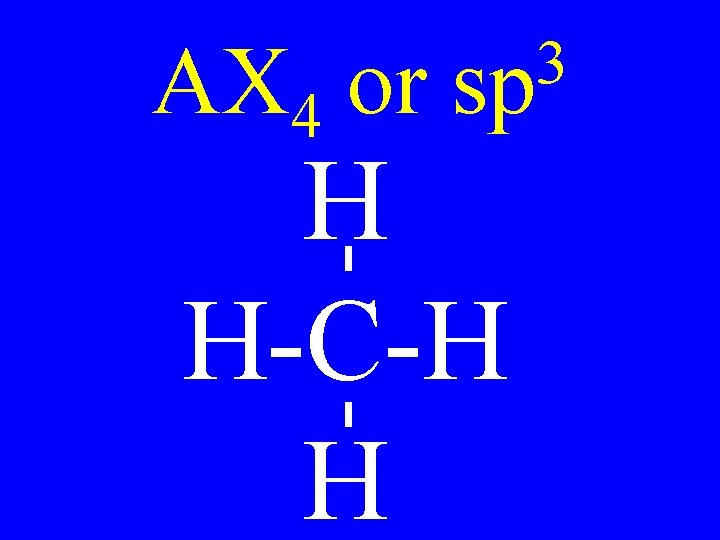
AX 4 or 3 sp H H-C-H H
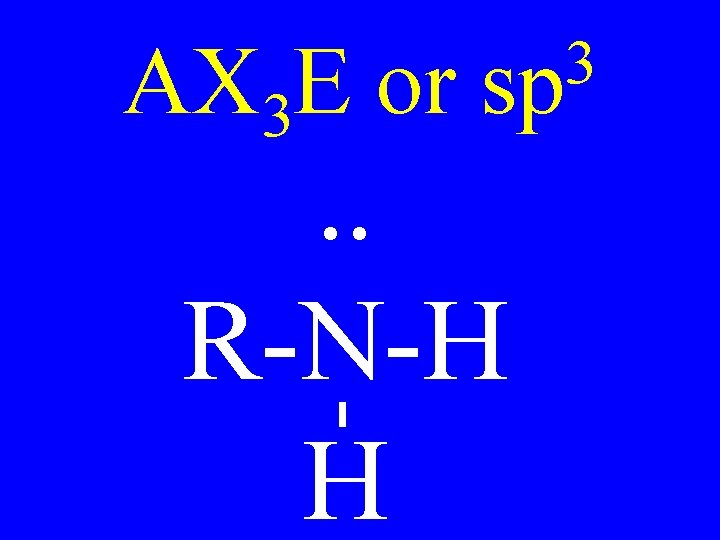
AX 3 E or 3 sp . . R-N-H H
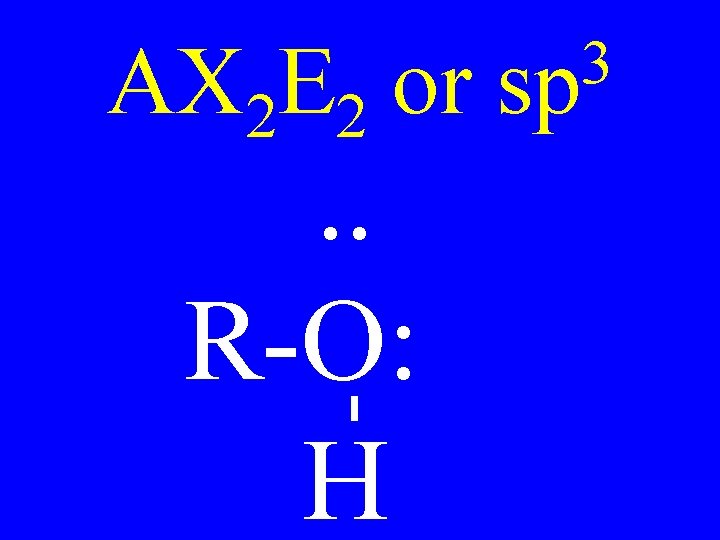
AX 2 E 2 or . . R-O: H 3 sp

Carbon • AX 3 • AX 2 E 2 sp Shapes trigonal planar v-shaped
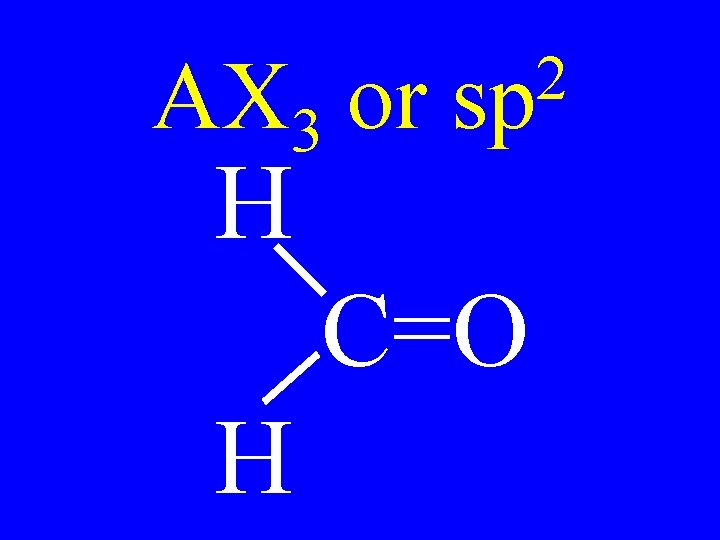
AX 3 or H 2 sp C=O H
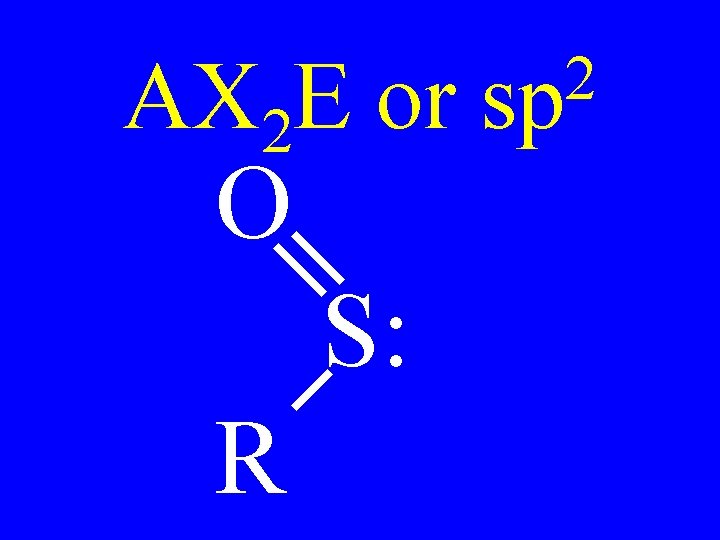
AX 2 E or O S: R 2 sp

Carbon sp Shape • AX 2 linear
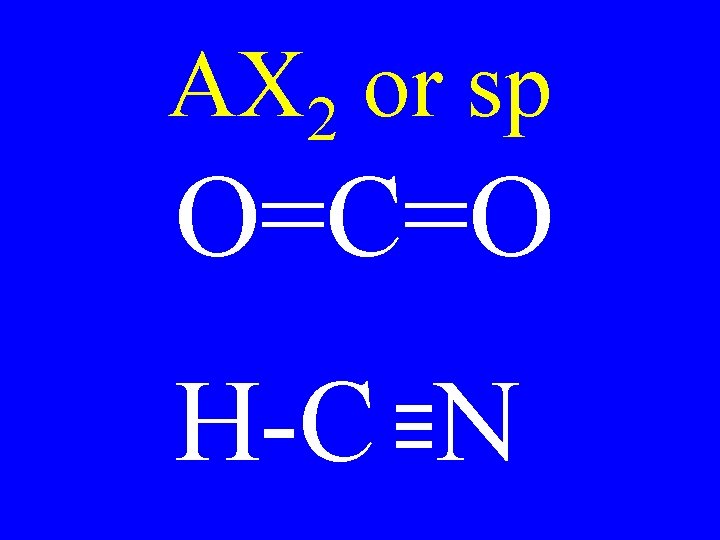
AX 2 or sp O=C=O H-C N
- Slides: 29