Organic Chemistry An Introduction to Hydrocarbons Alkanes and

Organic Chemistry: An Introduction to Hydrocarbons, Alkanes and Branched Alkanes Learning Goals: I will understand what a hydrocarbon is, and be able to name and draw straight-chained and branched alkanes Intro
Organic Chemistry - Introduction • Organic chemistry is the study of carbon compounds. • Animals, plants, and other forms of life consist of organic compounds. – Nucleic acids, proteins, fats, carbohydrates, enzymes, vitamins, and hormones are all organic compounds. • Biochemistry was developed later as the study of the chemical compounds and reactions in living cells. Intro

Bonding in Organic Compounds • Besides carbon, the most common elements in organic compounds are hydrogen, oxygen, nitrogen, sulfur, and the halogens. • All of the preceding elements are non-metals, therefore organic compounds have covalent bonding. Section 14. 1
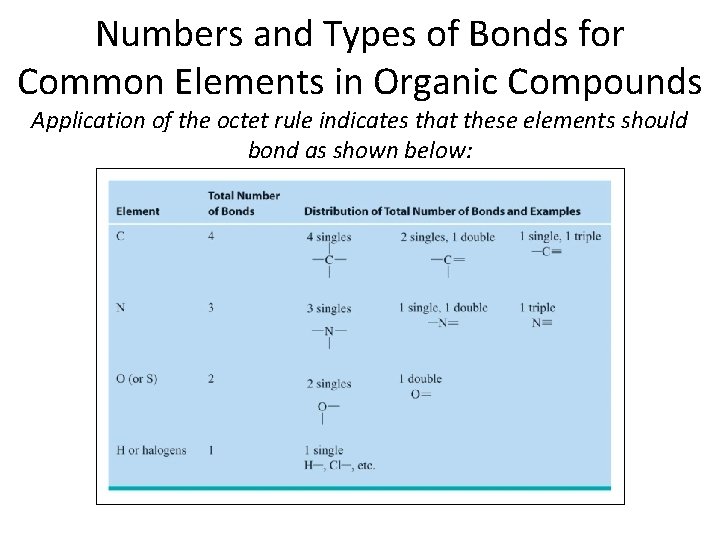
Numbers and Types of Bonds for Common Elements in Organic Compounds Application of the octet rule indicates that these elements should bond as shown below: Section 14. 1
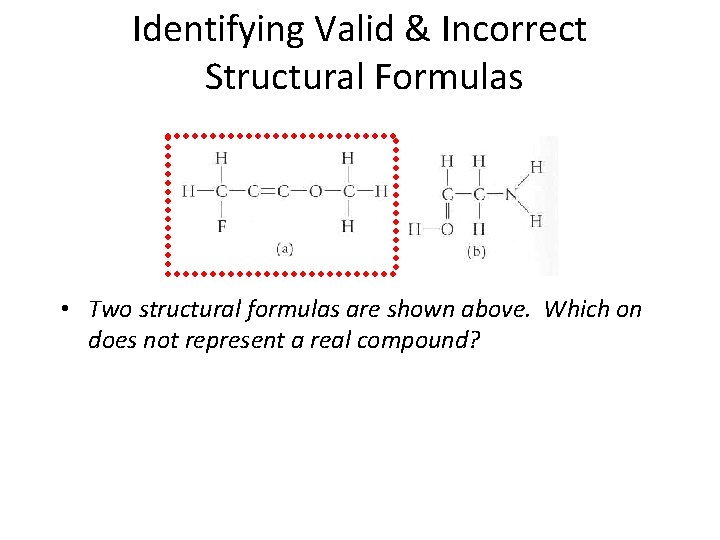
Identifying Valid & Incorrect Structural Formulas • Two structural formulas are shown above. Which on does not represent a real compound? Section 14. 1
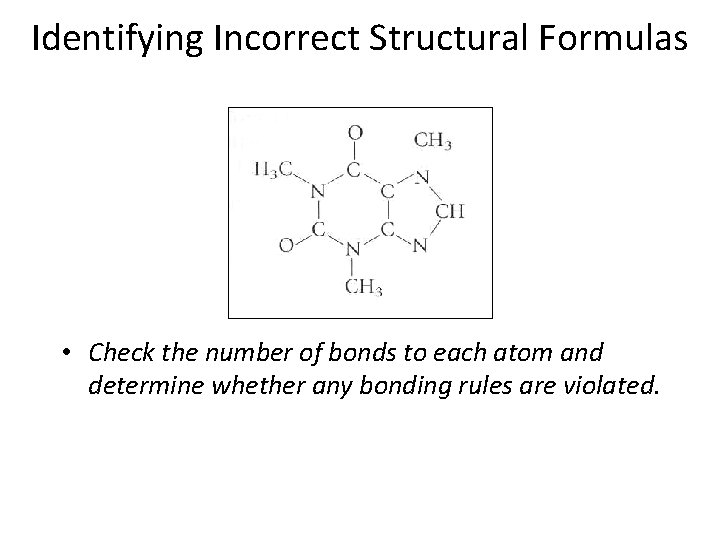
Identifying Incorrect Structural Formulas • Check the number of bonds to each atom and determine whether any bonding rules are violated. Section 14. 1

Hydrocarbons • Hydrocarbons are the most simple organic compounds. • Hydrocarbons contain only carbon (C) and hydrogen. (H) • For classification purposes, all other organic compounds (containing O, N, Cl, etc) are considered derivatives of hydrocarbons. • Hydrocarbons can be divided into aromatic and aliphatic hydrocarbons. Section 14. 2
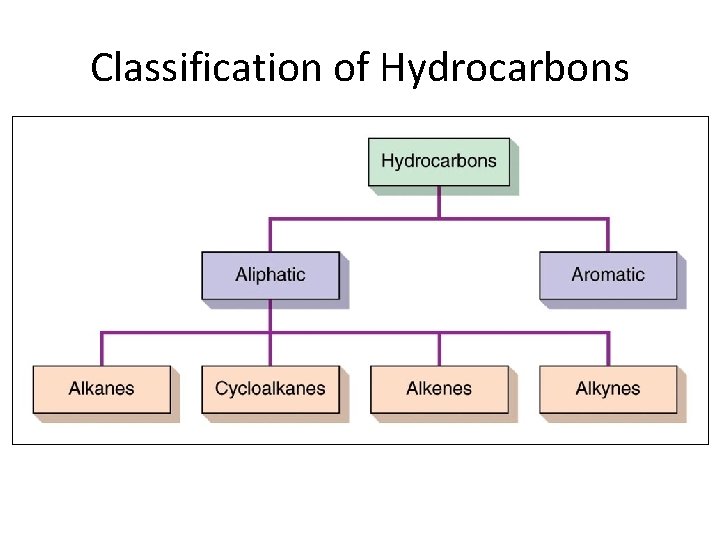
Classification of Hydrocarbons Section 14. 2

Aliphatic Hydrocarbons • Aliphatic hydrocarbons are hydrocarbons where C atoms form open chains with no aromatic rings • Aliphatic hydrocarbons can be divided into four major divisions: – Alkanes – Cycloalkanes – Alkenes – Alkynes Section 14. 3
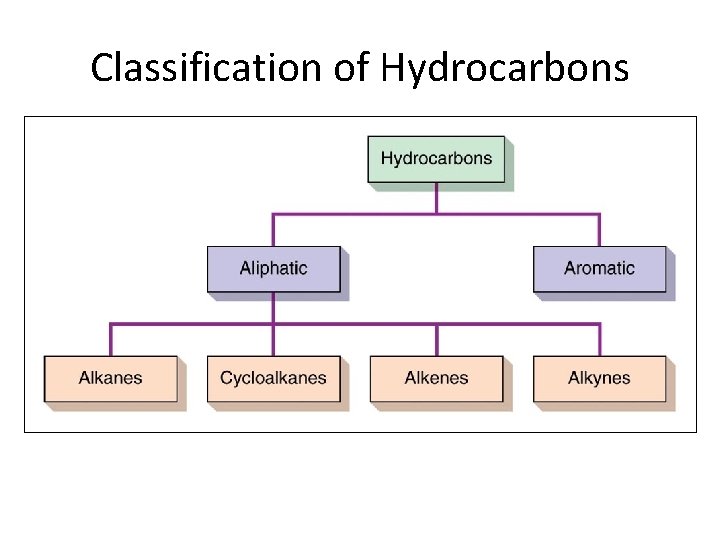
Classification of Hydrocarbons Section 14. 3

Alkanes • Alkanes are hydrocarbons that contain only single bonds. • Alkanes are said to be saturated hydrocarbons – Because their hydrogen content is at a maximum. • • • Alkane general formula Cn. H 2 n + 2 The names of alkanes all end in “-ane. ” Methane butane are gases Pentane C 17 H 36 are liquids C 18 H 38 and higher are solids Section 14. 3

Using Cn. H 2 n + 2 How many hydrogens would there be in a saturated alkane if there were 100 carbon atoms? Section 14. 3
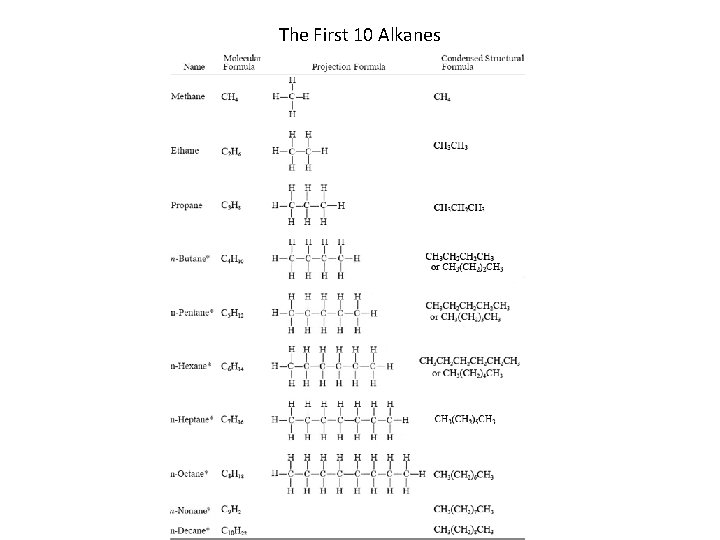
The First 10 Alkanes Section 14. 3
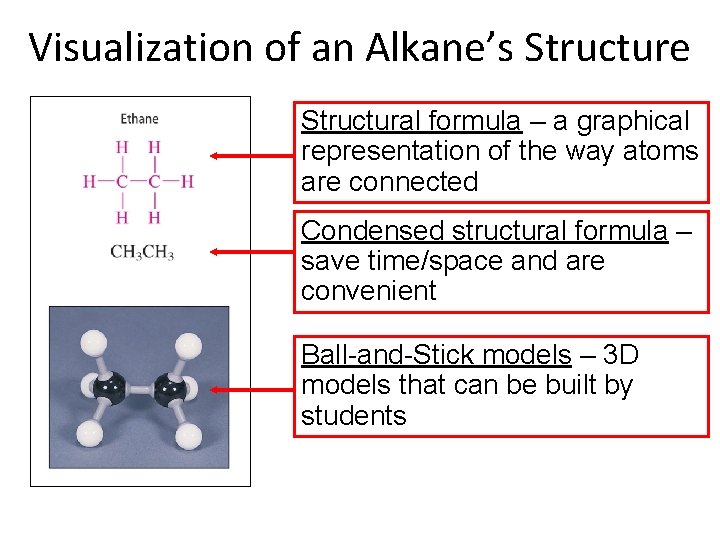
Visualization of an Alkane’s Structure Structural formula – a graphical representation of the way atoms are connected Condensed structural formula – save time/space and are convenient Ball-and-Stick models – 3 D models that can be built by students
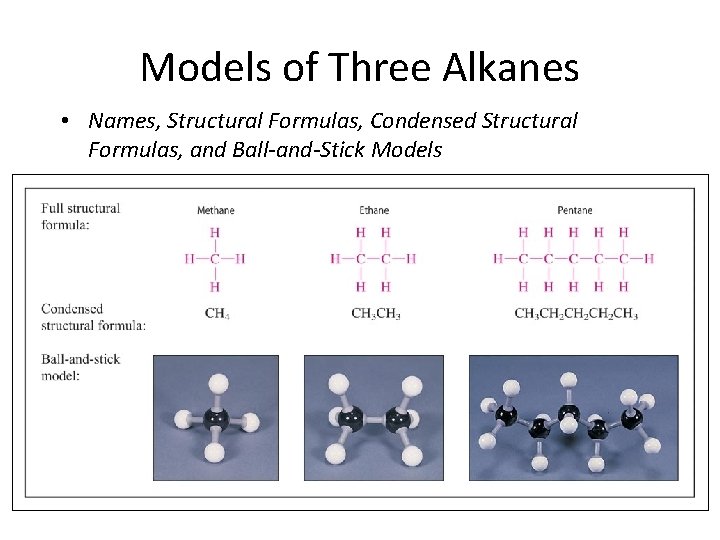
Models of Three Alkanes • Names, Structural Formulas, Condensed Structural Formulas, and Ball-and-Stick Models
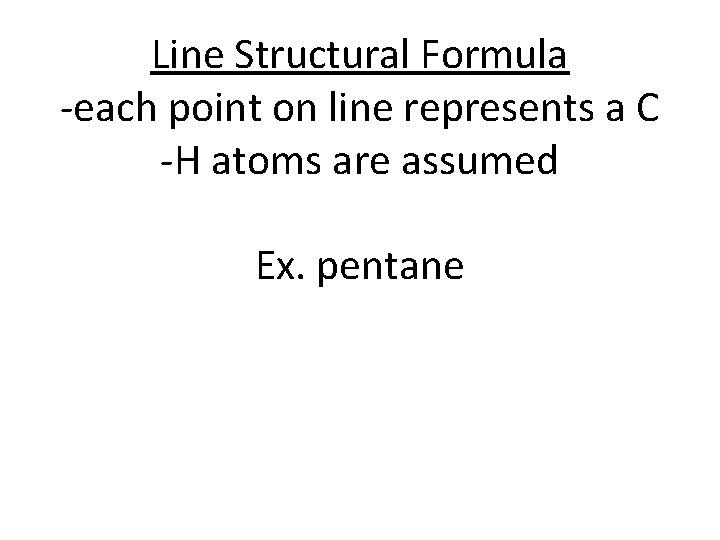
Line Structural Formula -each point on line represents a C -H atoms are assumed Ex. pentane
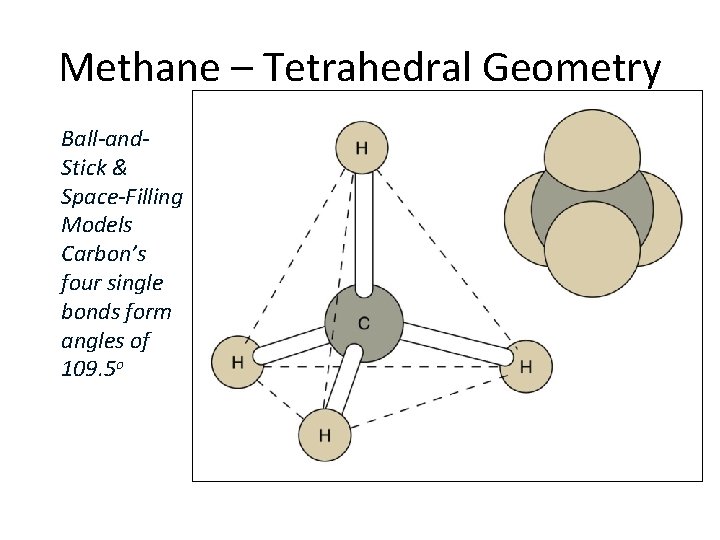
Methane – Tetrahedral Geometry Ball-and. Stick & Space-Filling Models Carbon’s four single bonds form angles of 109. 5 o Section 14. 3

3 -D Structural Formula: Methane *normal line lies in plane of paper *dashed line extends backwards *solid line extends forwards toward you

Alkanes – Uses • Methane = primary component of natural gas • Propane & Butane = primary component of gas in pressurized tanks • Gasoline = pentane to decane • Kerosene = alkanes with n = 10 to 16 • Alkanes with n > 16 diesel fuel, fuel oil, petroleum jelly, paraffin wax, lubricating oil, and asphalt Section 14. 3

Alkane Products • Alkanes are also found in paints, plastics, drugs, detergents, insecticides, and cosmetics. – Only 6% of the petroleum consumed goes into making these products. • The remaining 94% of the petroleum is burned as one of the various energy-related products. • Although alkanes are highly combustible, they are otherwise not very reactive. – Any reaction would require the breaking of the strong C —H and C—C bonds. Section 14. 3

Drawing Straight-Chained Alkanes: Example a) Draw the structural, condensed and line formula for propane Section 14. 3

Drawing Straight-Chained Alkanes: You Try a) Draw the structural, condensed and line formula for heptane Section 14. 3

Substituent Groups • Organic compounds can contain an atom or group of atoms in place of a hydrogen atom on the parent chain (called side group or substituent group) • When the side group is derived from an alkane, the side group is called and alkyl group Section 14. 3

Branched Alkanes Contain Alkyl Groups • Alkyl group contains one less hydrogen than the corresponding alkane. • In naming this group the “-ane” is dropped and “-yl” is added. • For example, methane becomes methyl. • Ethane becomes ethyl. Section 14. 3
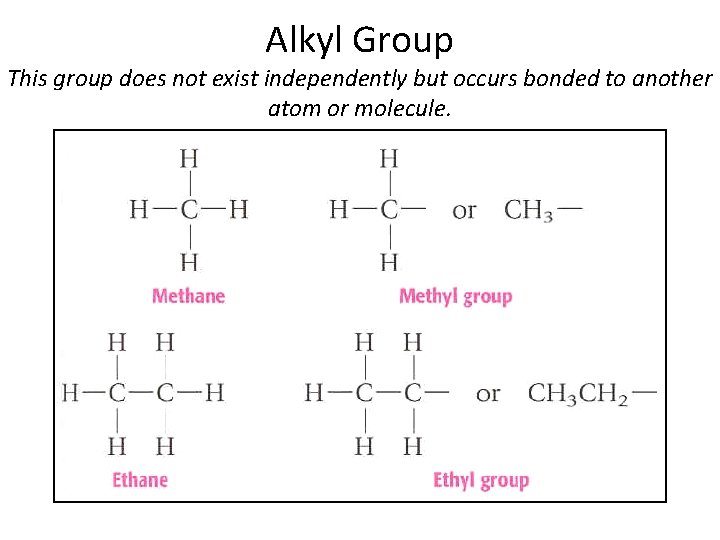
Alkyl Group This group does not exist independently but occurs bonded to another atom or molecule.

You Try: Draw a decane molecule with a propyl group attached at carbon 2 Section 14. 3

Isopropyl Group 2 -propyldecane vs. 2 -isopropyldectane Section 14. 3
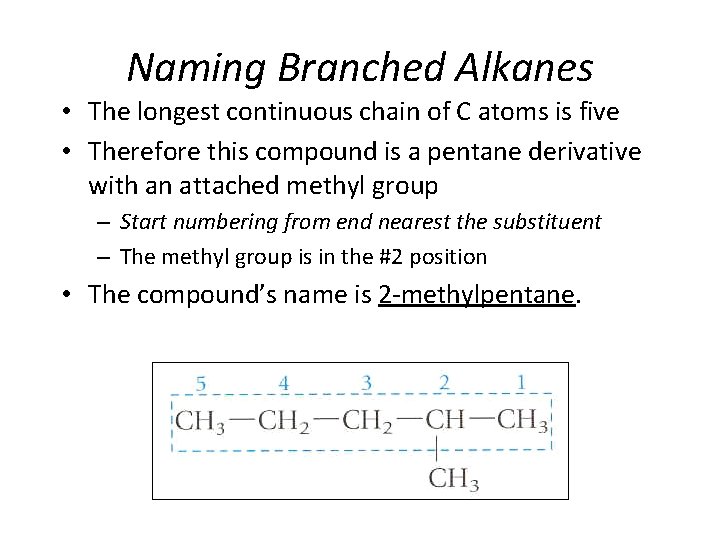
Naming Branched Alkanes • The longest continuous chain of C atoms is five • Therefore this compound is a pentane derivative with an attached methyl group – Start numbering from end nearest the substituent – The methyl group is in the #2 position • The compound’s name is 2 -methylpentane. Section 14. 3
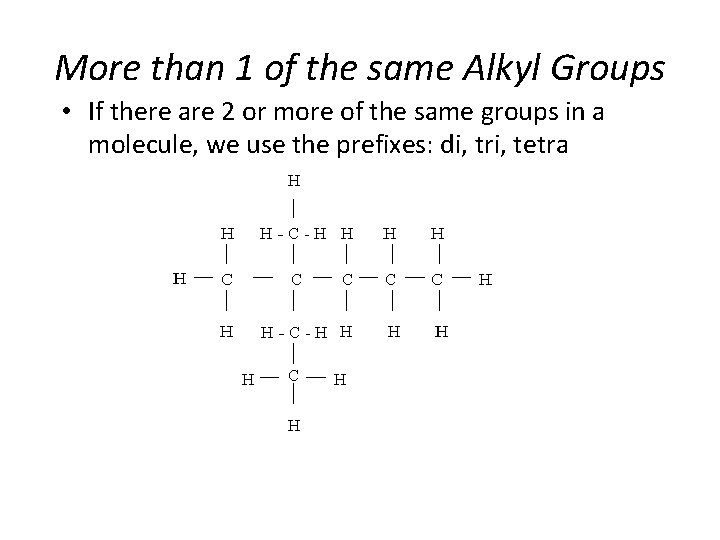
More than 1 of the same Alkyl Groups • If there are 2 or more of the same groups in a molecule, we use the prefixes: di, tri, tetra Section 14. 3
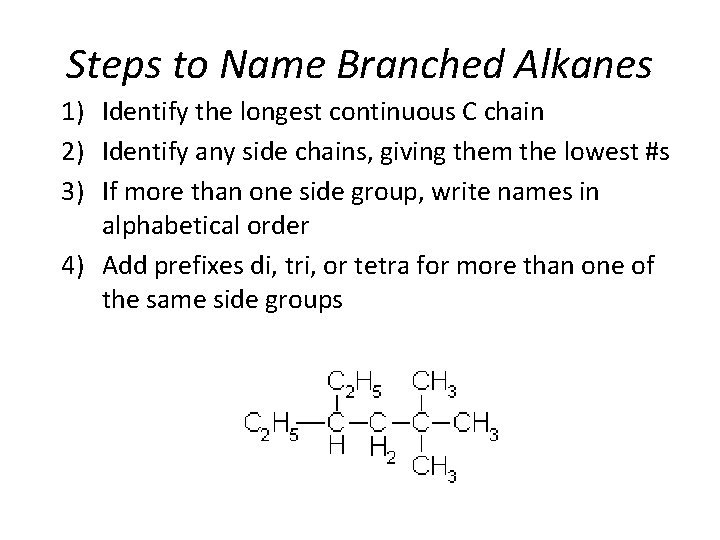
Steps to Name Branched Alkanes 1) Identify the longest continuous C chain 2) Identify any side chains, giving them the lowest #s 3) If more than one side group, write names in alphabetical order 4) Add prefixes di, tri, or tetra for more than one of the same side groups Section 14. 3
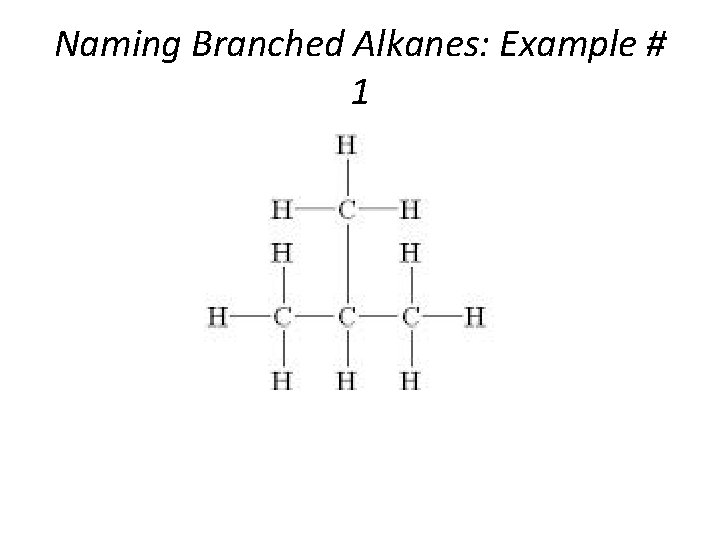
Naming Branched Alkanes: Example # 1 Section 14. 3
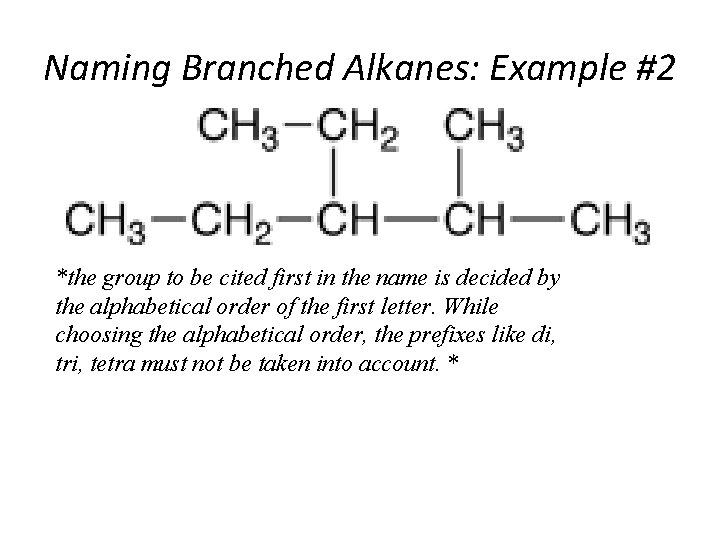
Naming Branched Alkanes: Example #2 *the group to be cited first in the name is decided by the alphabetical order of the first letter. While choosing the alphabetical order, the prefixes like di, tri, tetra must not be taken into account. * Section 14. 3
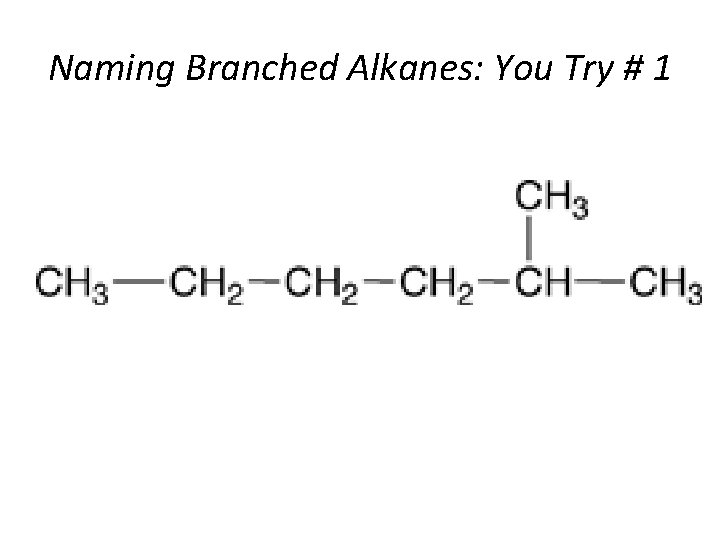
Naming Branched Alkanes: You Try # 1 Section 14. 3
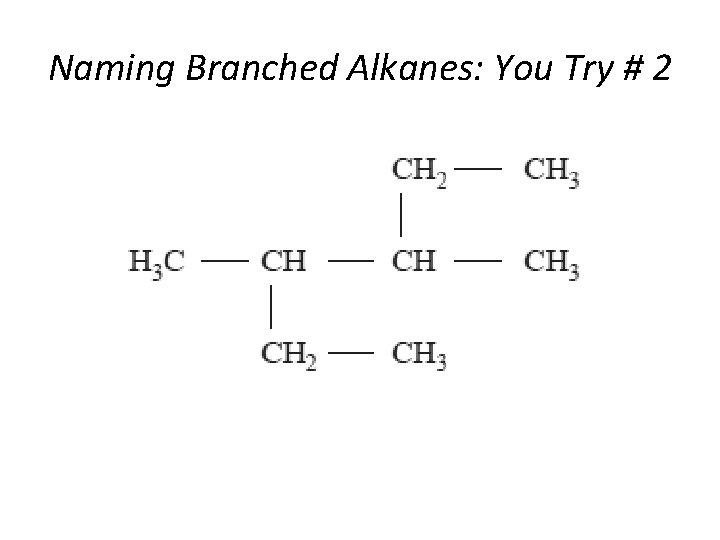
Naming Branched Alkanes: You Try # 2 Section 14. 3
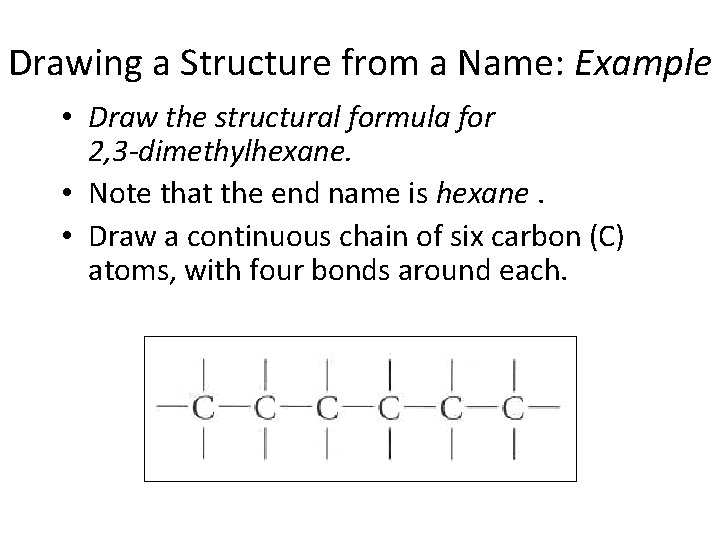
Drawing a Structure from a Name: Example • Draw the structural formula for 2, 3 -dimethylhexane. • Note that the end name is hexane. • Draw a continuous chain of six carbon (C) atoms, with four bonds around each. Section 14. 3
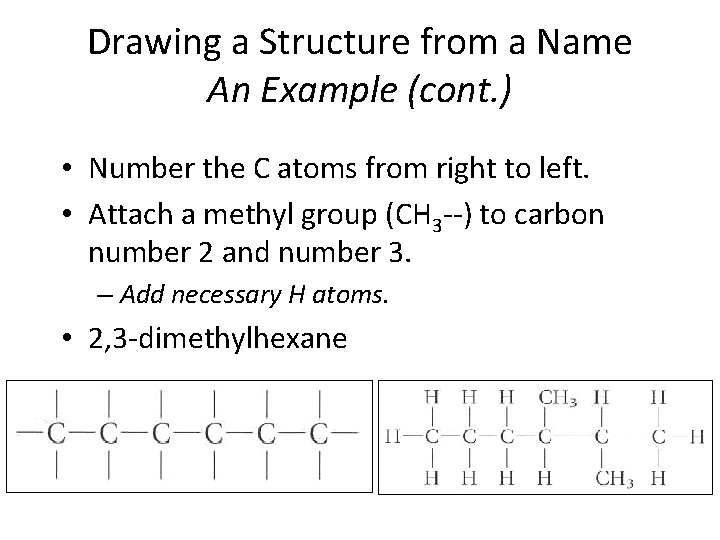
Drawing a Structure from a Name An Example (cont. ) • Number the C atoms from right to left. • Attach a methyl group (CH 3 --) to carbon number 2 and number 3. – Add necessary H atoms. • 2, 3 -dimethylhexane Section 14. 3
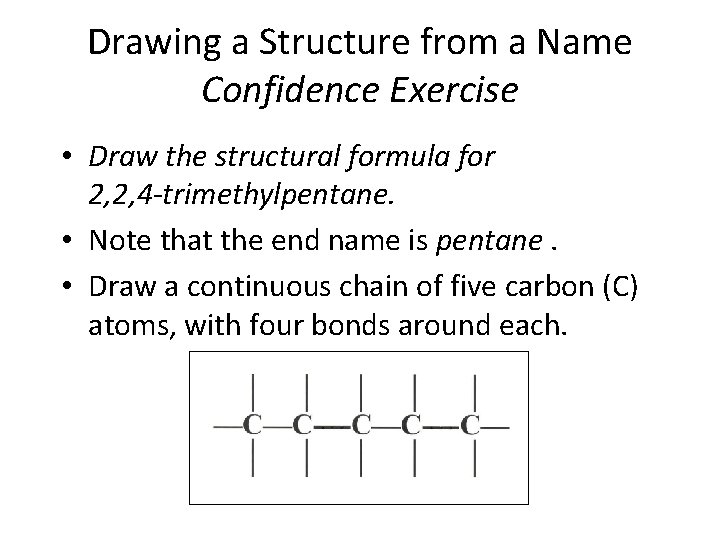
Drawing a Structure from a Name Confidence Exercise • Draw the structural formula for 2, 2, 4 -trimethylpentane. • Note that the end name is pentane. • Draw a continuous chain of five carbon (C) atoms, with four bonds around each. Section 14. 3
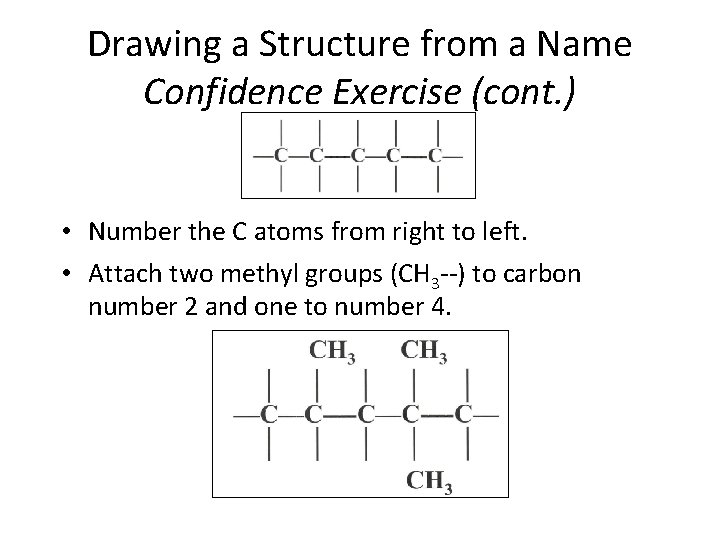
Drawing a Structure from a Name Confidence Exercise (cont. ) • Number the C atoms from right to left. • Attach two methyl groups (CH 3 --) to carbon number 2 and one to number 4. Section 14. 3
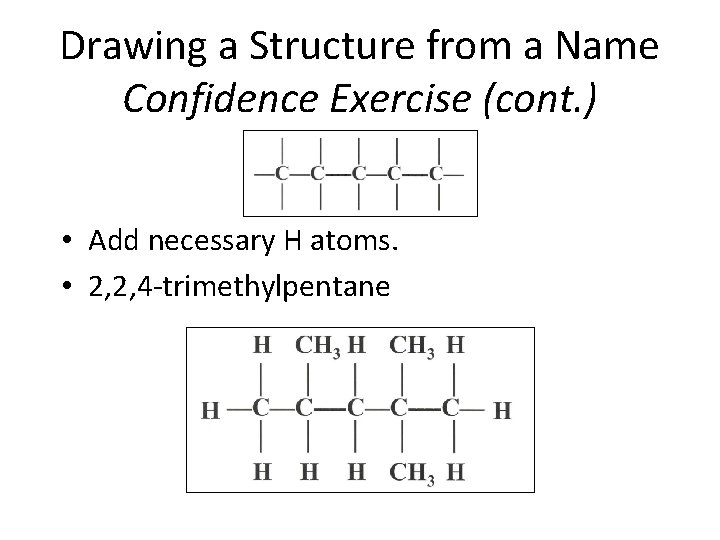
Drawing a Structure from a Name Confidence Exercise (cont. ) • Add necessary H atoms. • 2, 2, 4 -trimethylpentane Section 14. 3
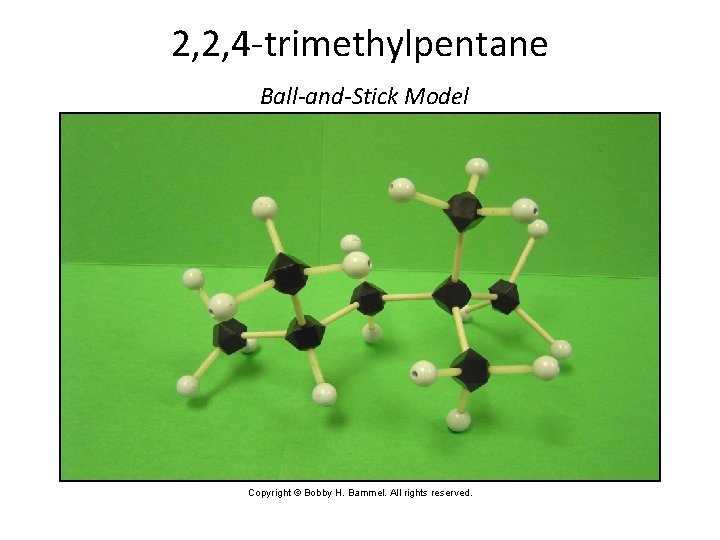
2, 2, 4 -trimethylpentane Ball-and-Stick Model Copyright © Bobby H. Bammel. All rights reserved. Section 14. 3

Drawing Branched Alkanes: Example a) Draw the structural formula for 4 -ethyl-2 -methyloctane b) Why would the name 2 -methyl-4 -ethyloctane be wrong? What about 5 -ethyl-7 -methyloctane? Section 14. 3

Drawing Branched Alkanes: You Try a) Draw the structural formula for 2, 3 -diethyl-4 -methylhexane b) Examine the molecule you drew. What is incorrect about how it was named? How should it be named? Section 14. 3

How Did We Do? Learning Goals: I will understand what a hydrocarbon is, and be able to name and draw straight-chained and branched alkanes Intro
- Slides: 43