Organic chemistry Alcohols Ethers and Phenols Organic chemistry







![有机化学 Organic chemistry B. Oxidation of secondary alcohols Secondary alcohols [O] C. Oxidation of 有机化学 Organic chemistry B. Oxidation of secondary alcohols Secondary alcohols [O] C. Oxidation of](https://slidetodoc.com/presentation_image_h/d726f14f1993189112639f843537c624/image-34.jpg)




- Slides: 88

有机化学 Organic chemistry 第九章 醇、酚、醚 Alcohols, Ethers and Phenols 主讲老师:刘玉霞

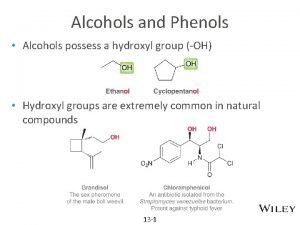
有机化学 Organic chemistry ROH Alcohols 醇 Ar. OH Phenols 酚 R-O-R’ Ether 醚 Compounds that have hydroxyl group bonded to a saturated, sp 3 -C atom. Compounds that have hydroxyl group bonded to a aromatic ring. Compounds that have a oxygen atom bonded to two carbon atom

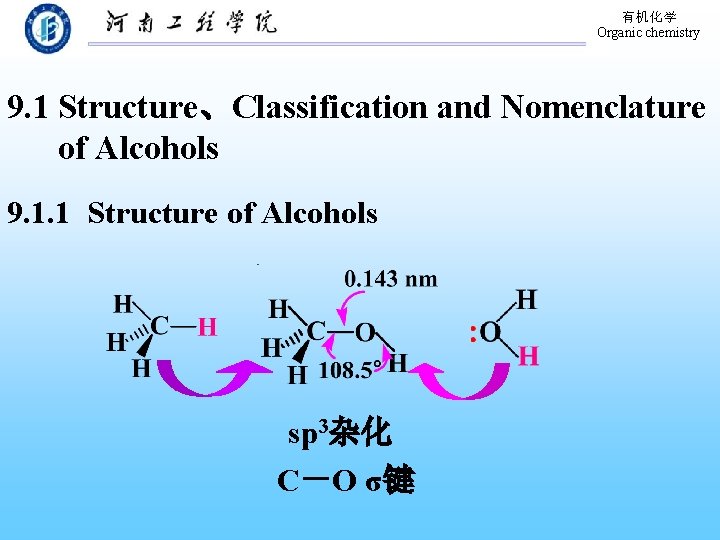
有机化学 Organic chemistry 9. 1 Structure、Classification and Nomenclature of Alcohols 9. 1. 1 Structure of Alcohols sp 3杂化 C-O σ键

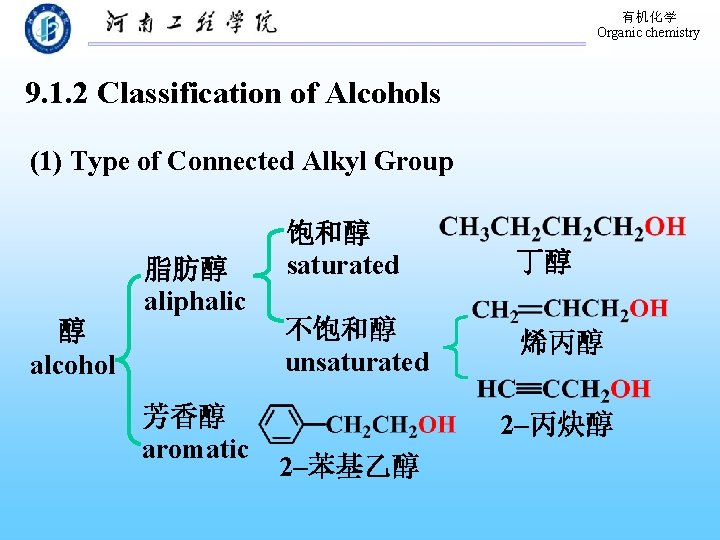
有机化学 Organic chemistry 9. 1. 2 Classification of Alcohols (1) Type of Connected Alkyl Group 醇 alcohol 脂肪醇 aliphalic 芳香醇 aromatic 饱和醇 saturated 丁醇 不饱和醇 unsaturated 烯丙醇 2–丙炔醇 2–苯基乙醇

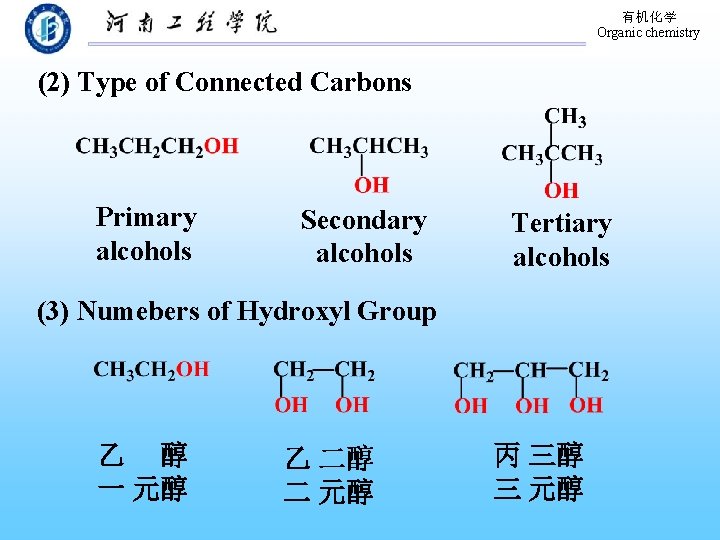
有机化学 Organic chemistry (2) Type of Connected Carbons Primary alcohols Secondary alcohols Tertiary alcohols (3) Numebers of Hydroxyl Group 乙 醇 一 元醇 乙 二醇 二 元醇 丙 三醇 三 元醇

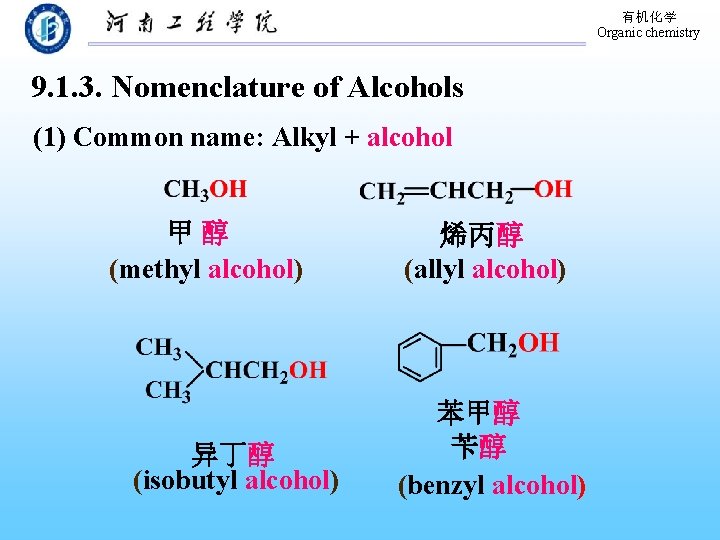
有机化学 Organic chemistry 9. 1. 3. Nomenclature of Alcohols (1) Common name: Alkyl + alcohol 甲醇 (methyl alcohol) 异丁醇 (isobutyl alcohol) 烯丙醇 (allyl alcohol) 苯甲醇 苄醇 (benzyl alcohol)

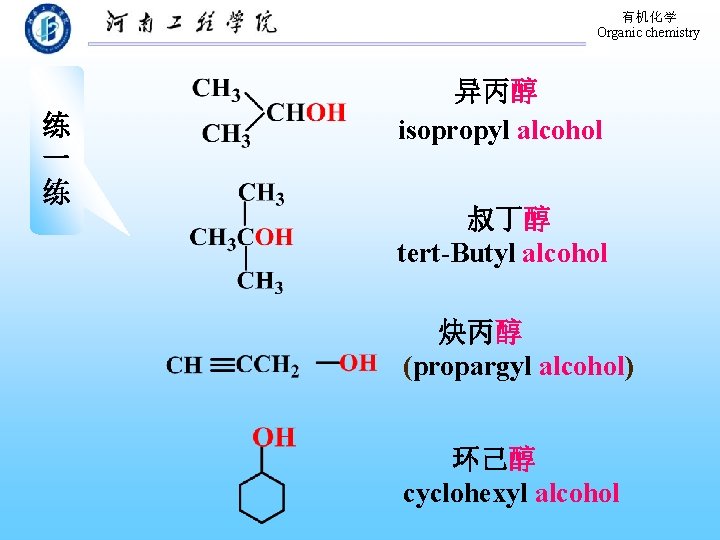
有机化学 Organic chemistry 练 一 练 异丙醇 isopropyl alcohol 叔丁醇 tert-Butyl alcohol 炔丙醇 (propargyl alcohol) 环己醇 cyclohexyl alcohol

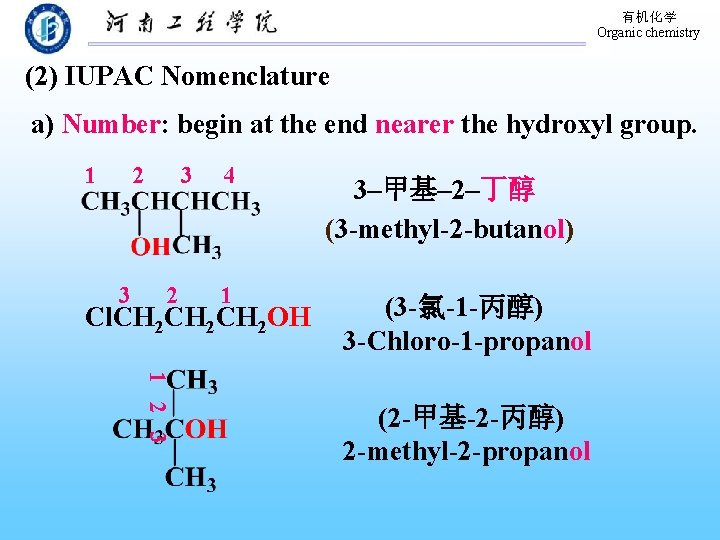
有机化学 Organic chemistry (2) IUPAC Nomenclature a) Number: begin at the end nearer the hydroxyl group. 1 2 3 3 2 4 1 Cl. CH 2 CH 2 OH 3–甲基– 2–丁醇 (3 -methyl-2 -butanol) (3 -氯-1 -丙醇) 3 -Chloro-1 -propanol 1 2 3 (2 -甲基-2 -丙醇) 2 -methyl-2 -propanol











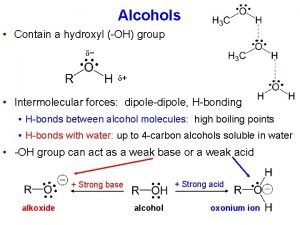
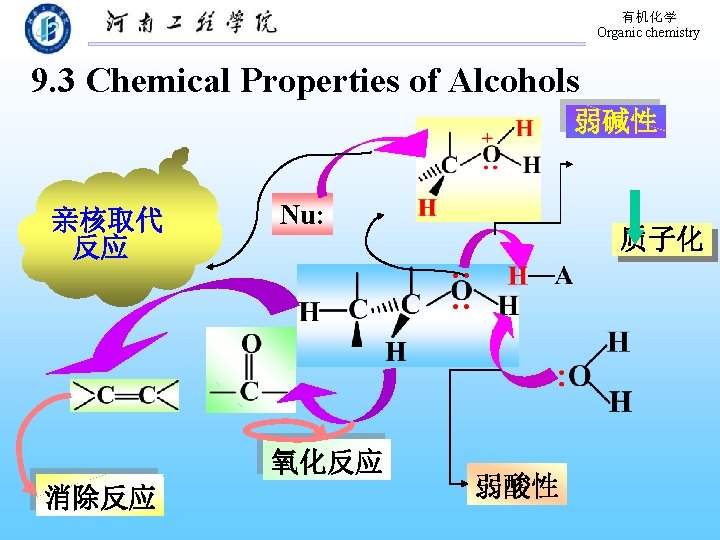
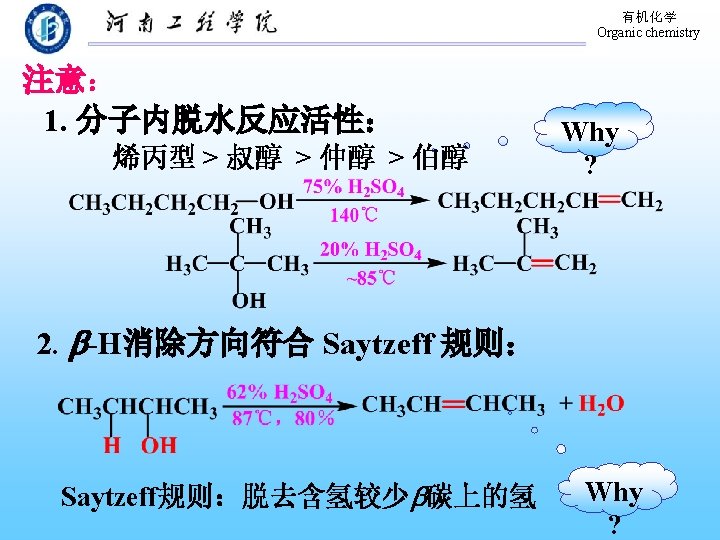
有机化学 Organic chemistry 9. 3 Chemical Properties of Alcohols 弱碱性 亲核取代 反应 Nu: 氧化反应 消除反应 质子化 弱酸性

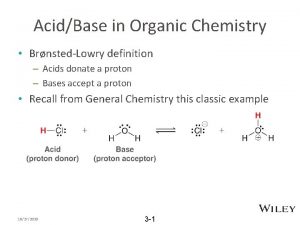
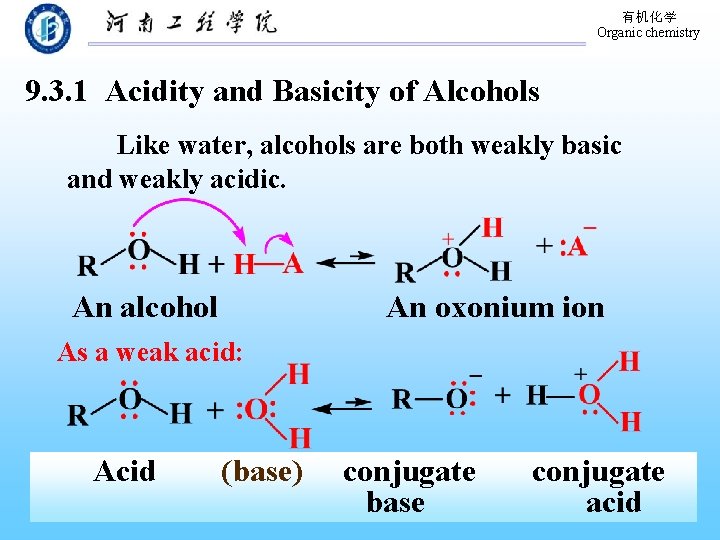
有机化学 Organic chemistry 9. 3. 1 Acidity and Basicity of Alcohols Like water, alcohols are both weakly basic and weakly acidic. An alcohol An oxonium ion As a weak acid: Acid (base) conjugate base conjugate acid

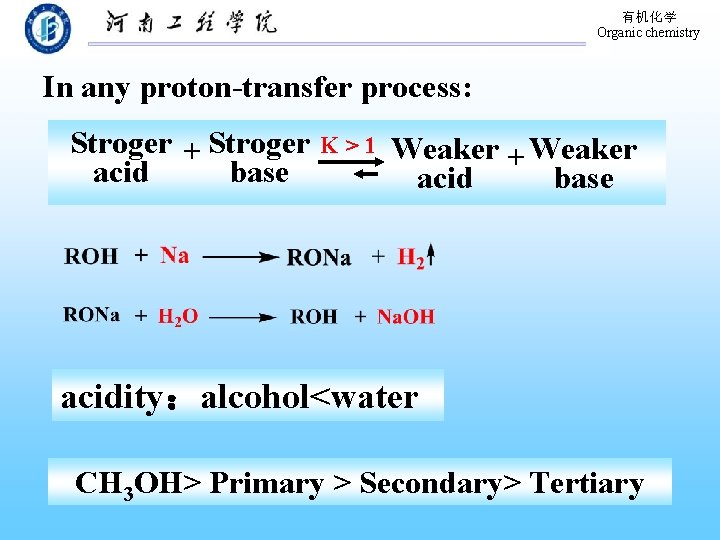
有机化学 Organic chemistry In any proton-transfer process: Stroger + Stroger acid base K>1 Weaker + Weaker acid base acidity:alcohol<water CH 3 OH> Primary > Secondary> Tertiary

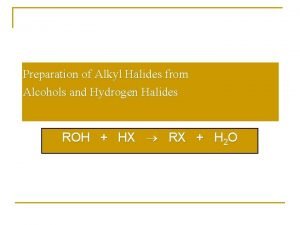
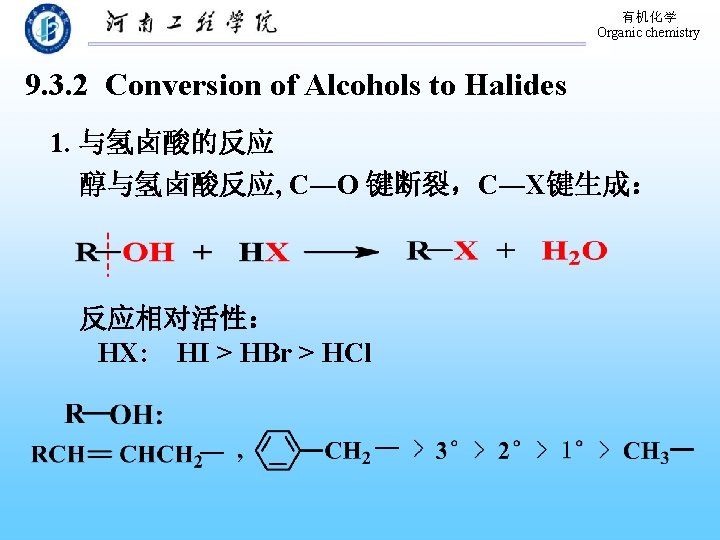
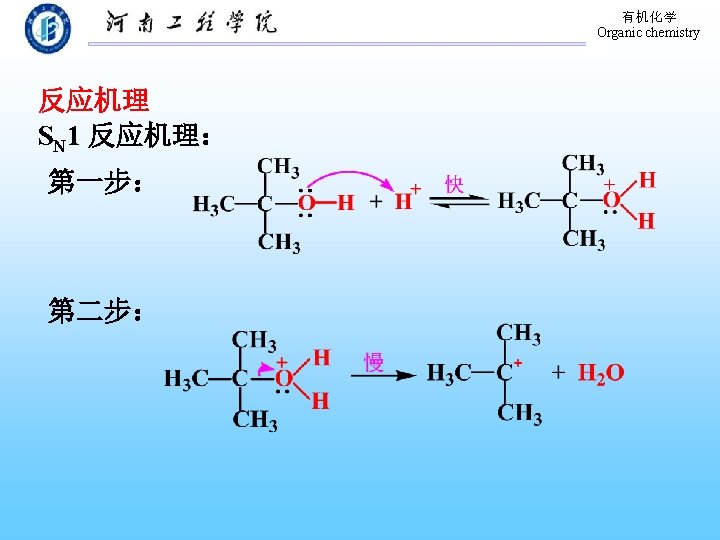
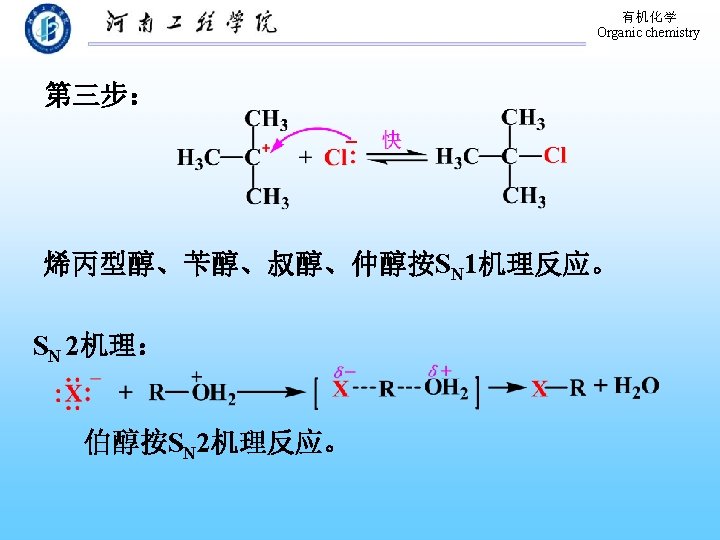
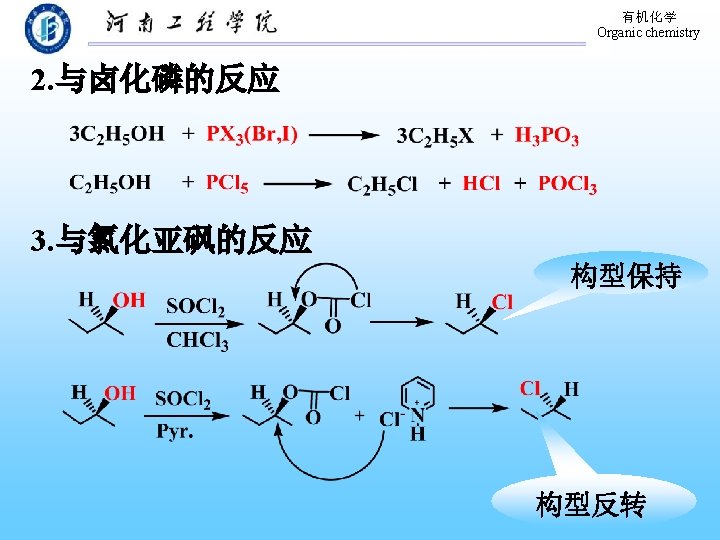
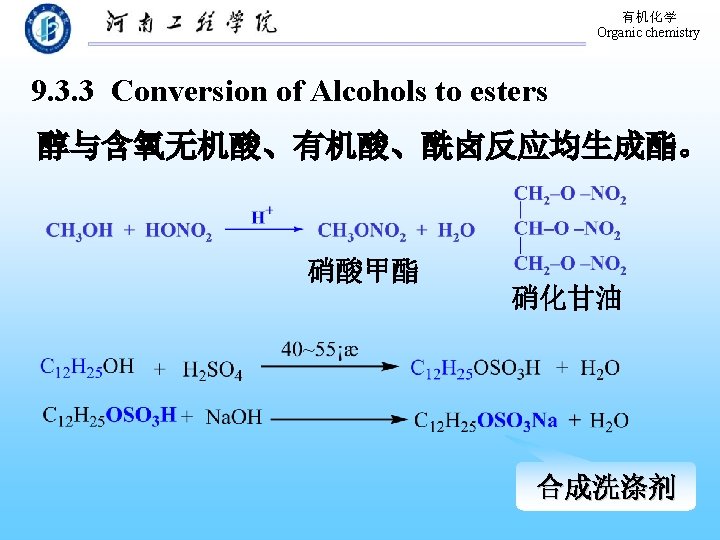
有机化学 Organic chemistry 9. 3. 2 Conversion of Alcohols to Halides 1. 与氢卤酸的反应 醇与氢卤酸反应, C―O 键断裂,C―X键生成: 反应相对活性: HX: HI > HBr > HCl










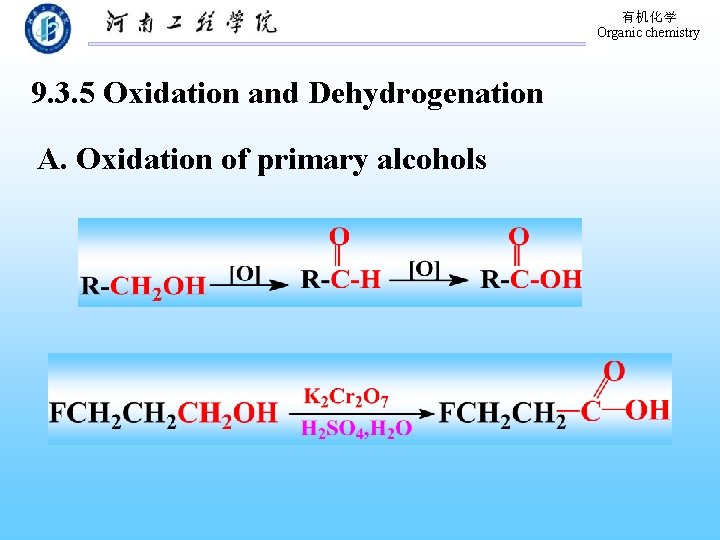
有机化学 Organic chemistry 9. 3. 5 Oxidation and Dehydrogenation A. Oxidation of primary alcohols

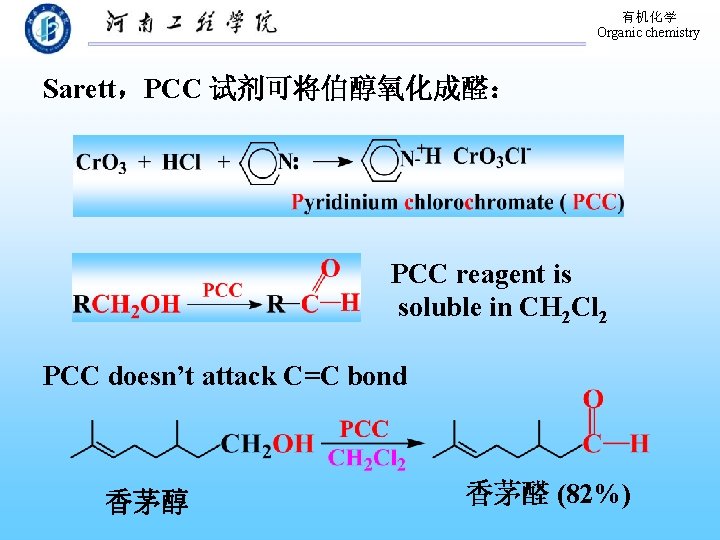
有机化学 Organic chemistry Sarett,PCC 试剂可将伯醇氧化成醛: PCC reagent is soluble in CH 2 Cl 2 PCC doesn’t attack C=C bond 香茅醇 香茅醛 (82%)
![有机化学 Organic chemistry B Oxidation of secondary alcohols Secondary alcohols O C Oxidation of 有机化学 Organic chemistry B. Oxidation of secondary alcohols Secondary alcohols [O] C. Oxidation of](https://slidetodoc.com/presentation_image_h/d726f14f1993189112639f843537c624/image-34.jpg)
有机化学 Organic chemistry B. Oxidation of secondary alcohols Secondary alcohols [O] C. Oxidation of Tertiary alcohols ketones


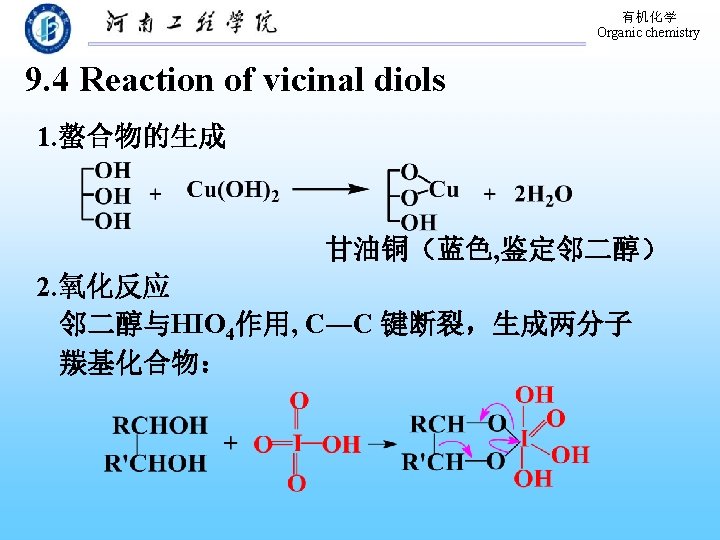
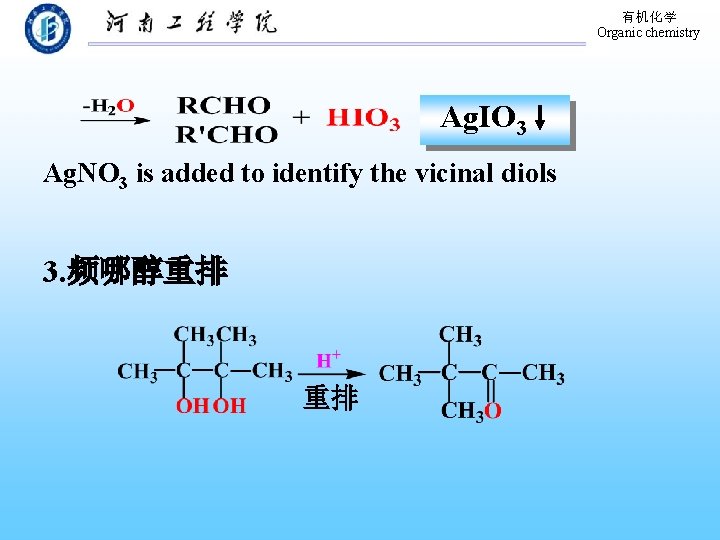
有机化学 Organic chemistry Ag. IO 3 Ag. NO 3 is added to identify the vicinal diols 3. 频哪醇重排 重排

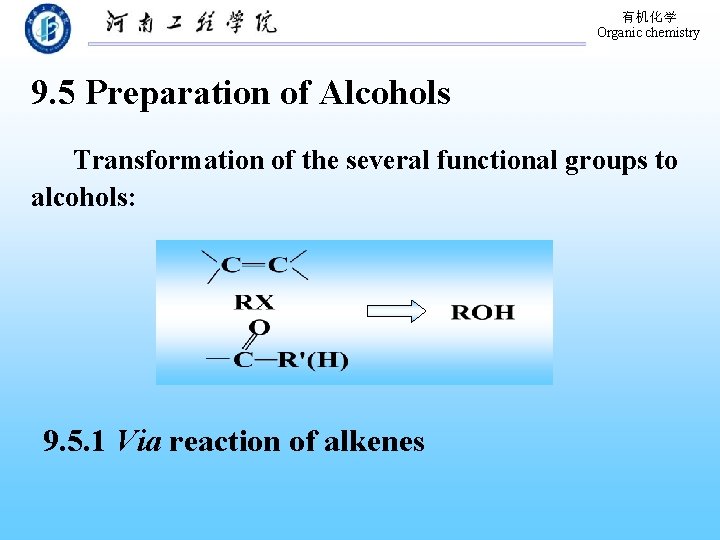
有机化学 Organic chemistry 9. 5 Preparation of Alcohols Transformation of the several functional groups to alcohols: 9. 5. 1 Via reaction of alkenes

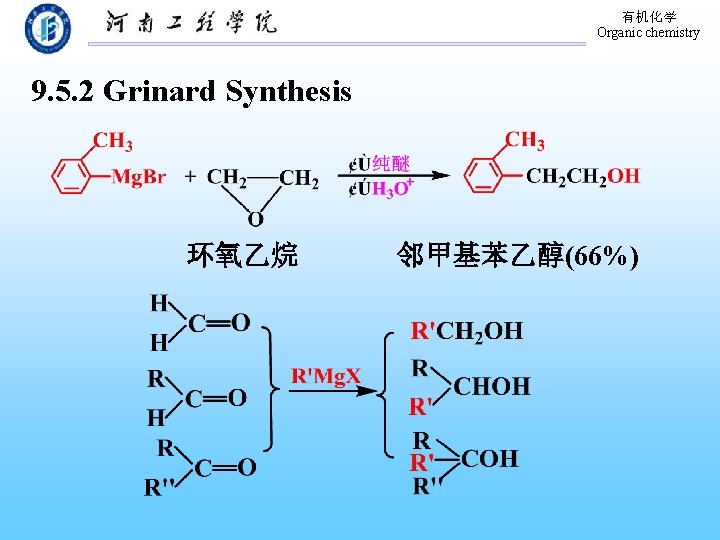
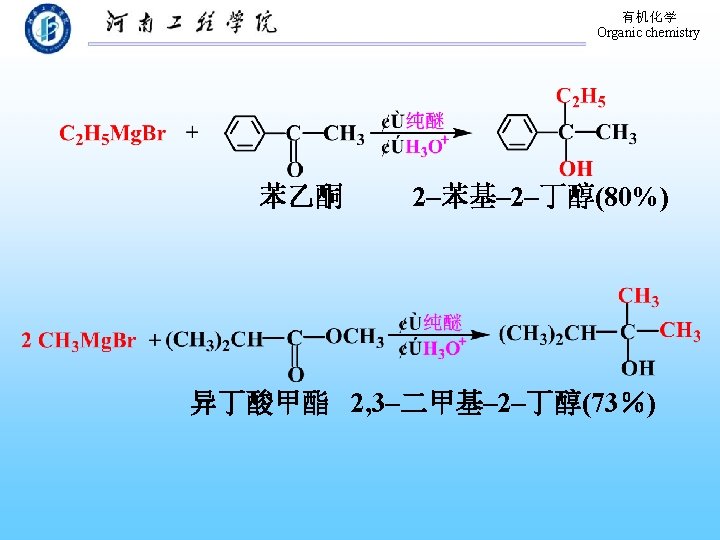
有机化学 Organic chemistry 9. 5. 2 Grinard Synthesis 环氧乙烷 邻甲基苯乙醇(66%)


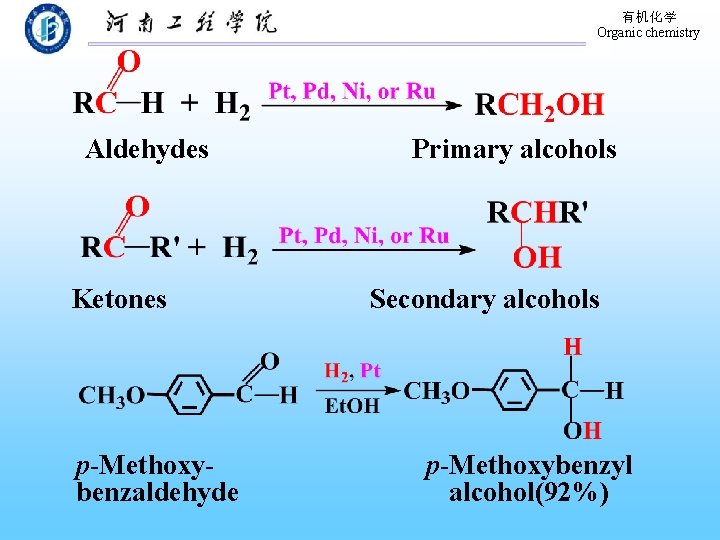
有机化学 Organic chemistry 9. 5. 3 Via Hydrolysis of Alkyl Halides It is rarely used for the synthesis of alcohol, since alkyl halides are normally prepared from alcohols. 9. 5. 4 Via Reduction of Carbonyl Compounds or Derivatives of Carboxylic Compounds (1) Hydrogenation of aldehydes and ketones by Catalysis of metals

有机化学 Organic chemistry Aldehydes Ketones p-Methoxybenzaldehyde Primary alcohols Secondary alcohols p-Methoxybenzyl alcohol(92%)

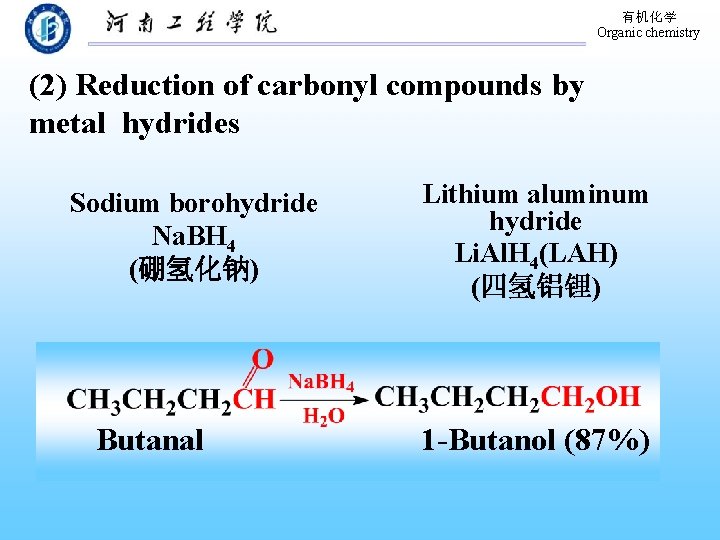
有机化学 Organic chemistry (2) Reduction of carbonyl compounds by metal hydrides Sodium borohydride Na. BH 4 (硼氢化钠) Butanal Lithium aluminum hydride Li. Al. H 4(LAH) (四氢铝锂) 1 -Butanol (87%)

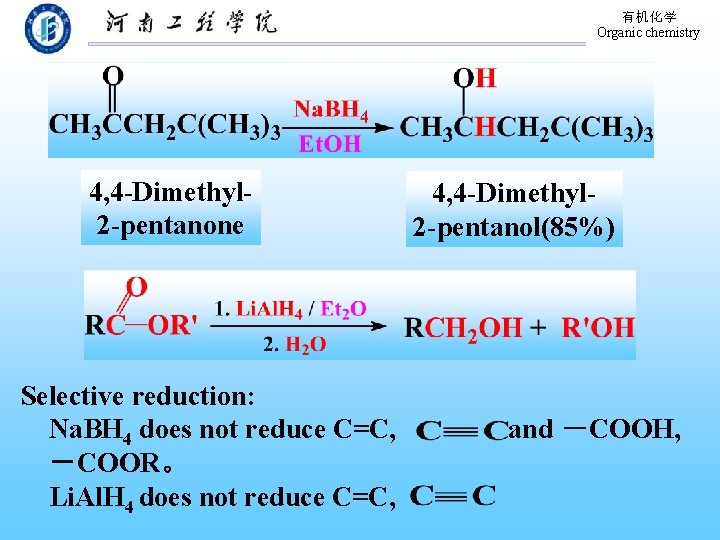
有机化学 Organic chemistry 4, 4 -Dimethyl 2 -pentanone Selective reduction: Na. BH 4 does not reduce C=C, -COOR。 Li. Al. H 4 does not reduce C=C, 4, 4 -Dimethyl 2 -pentanol(85%) and -COOH,

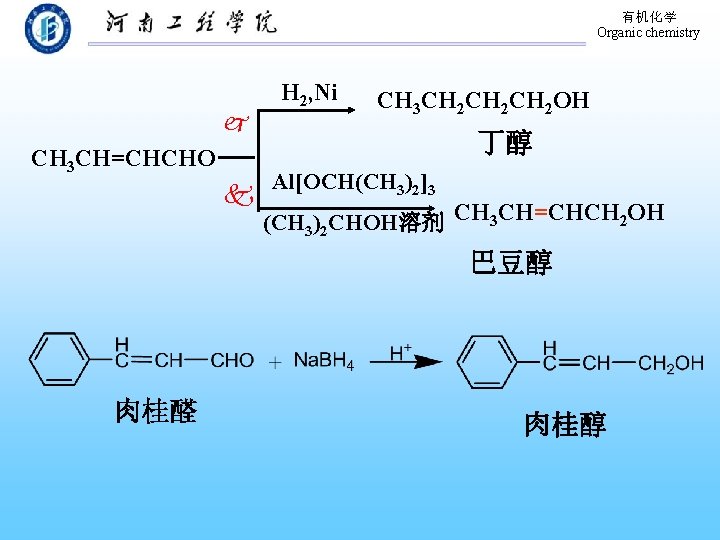
有机化学 Organic chemistry CH 3 CH=CHCHO H 2, Ni CH 3 CH 2 CH 2 OH 丁醇 Al[OCH(CH 3)2]3 (CH 3)2 CHOH溶剂 CH 3 CH=CHCH 2 OH 巴豆醇 肉桂醛 肉桂醇


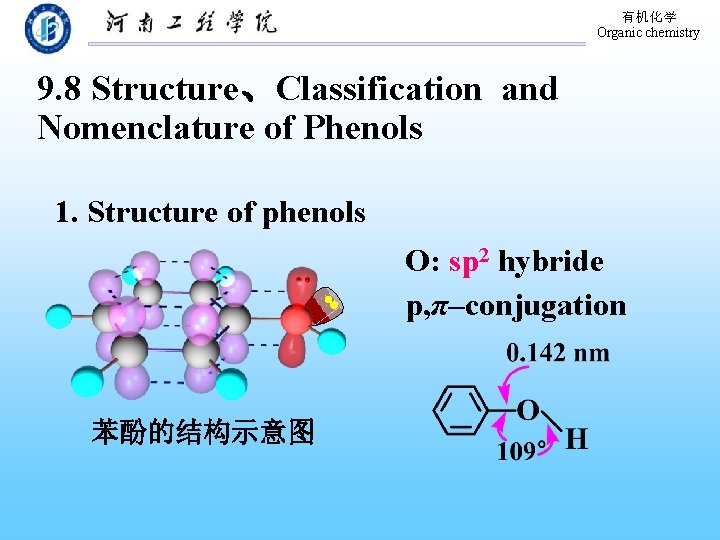
有机化学 Organic chemistry 9. 8 Structure、Classification and Nomenclature of Phenols 1. Structure of phenols O: sp 2 hybride p, π–conjugation 苯酚的结构示意图

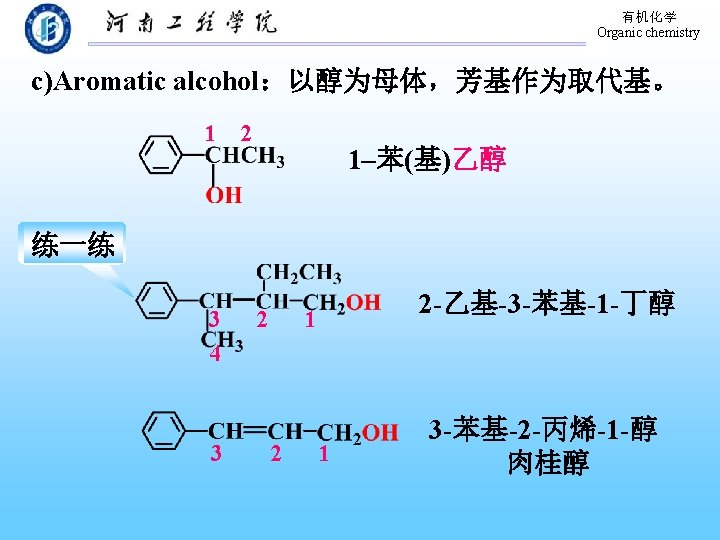
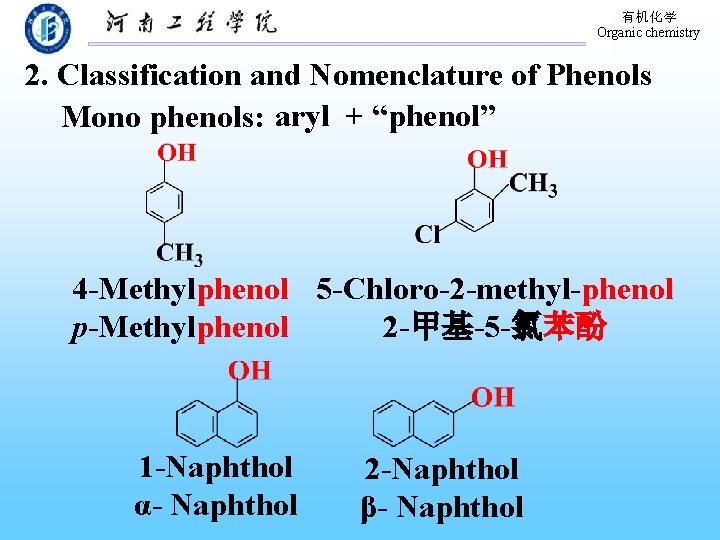
有机化学 Organic chemistry 2. Classification and Nomenclature of Phenols Mono phenols: aryl + “phenol” 4 -Methylphenol 5 -Chloro-2 -methyl-phenol p-Methylphenol 2 -甲基-5 -氯苯酚 1 -Naphthol α- Naphthol 2 -Naphthol β- Naphthol


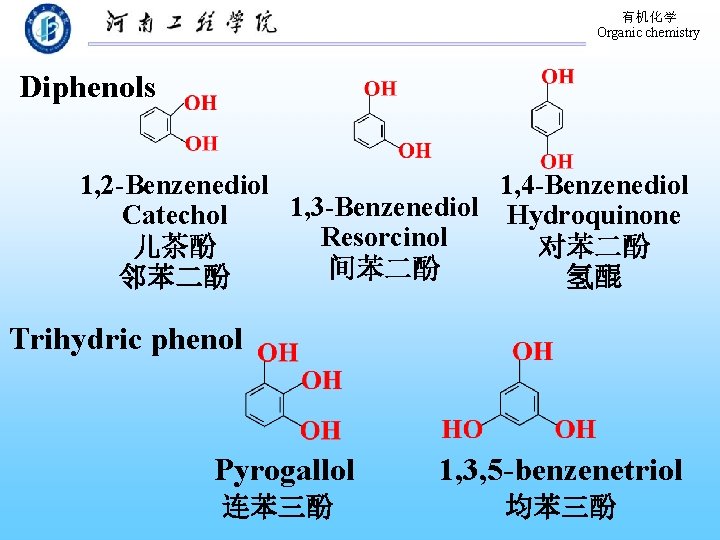
有机化学 Organic chemistry Diphenols 1, 2 -Benzenediol 1, 4 -Benzenediol 1, 3 -Benzenediol Hydroquinone Catechol Resorcinol 儿茶酚 对苯二酚 间苯二酚 邻苯二酚 氢醌 Trihydric phenol Pyrogallol 1, 3, 5 -benzenetriol 连苯三酚 均苯三酚

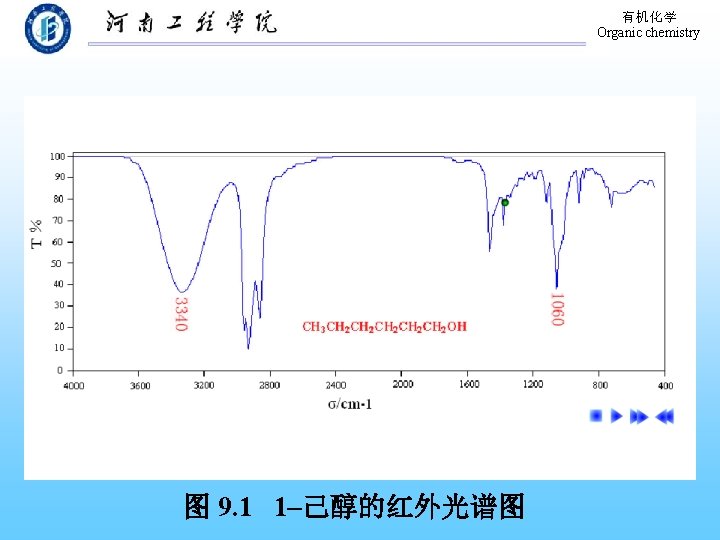
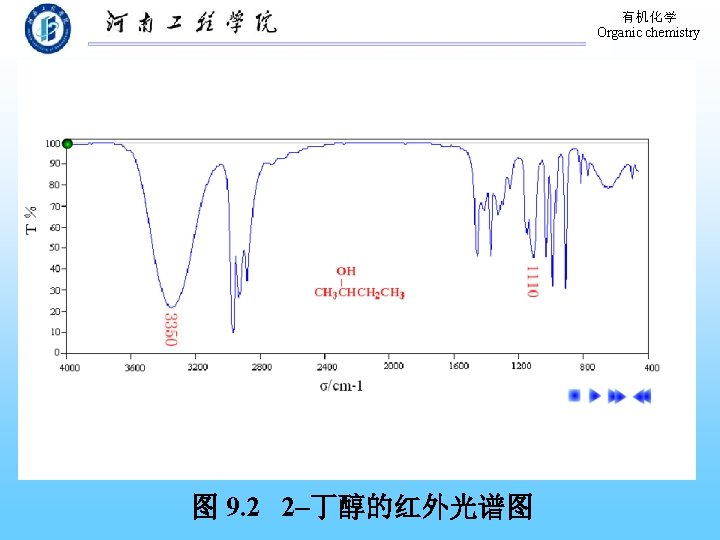
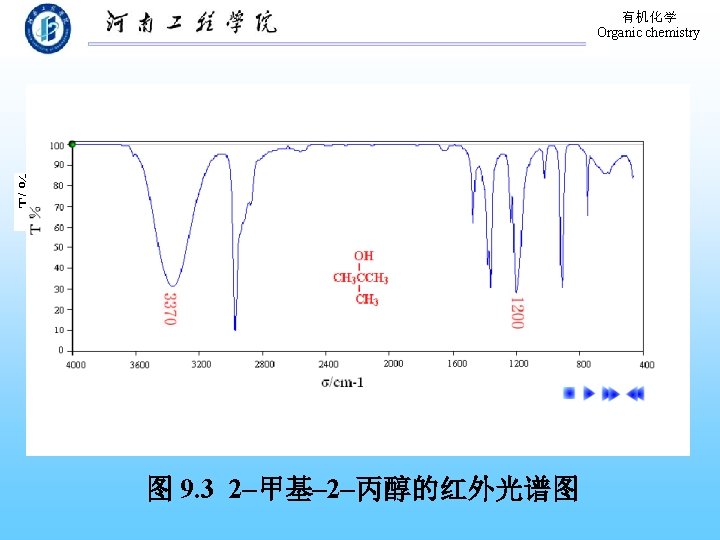
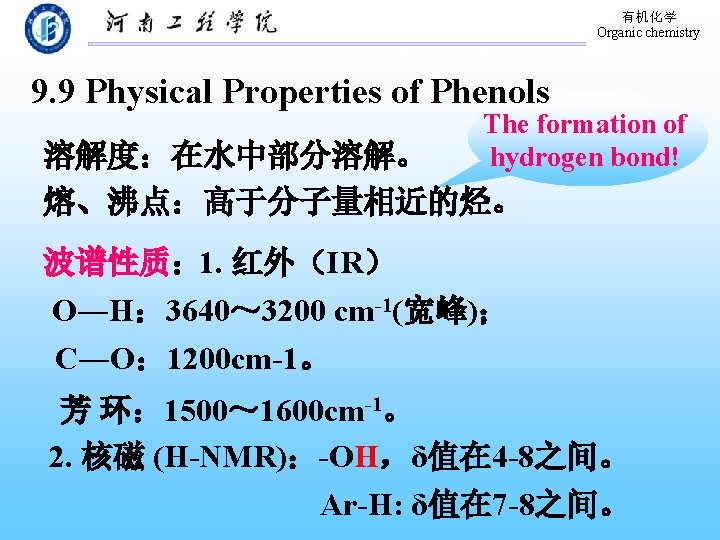
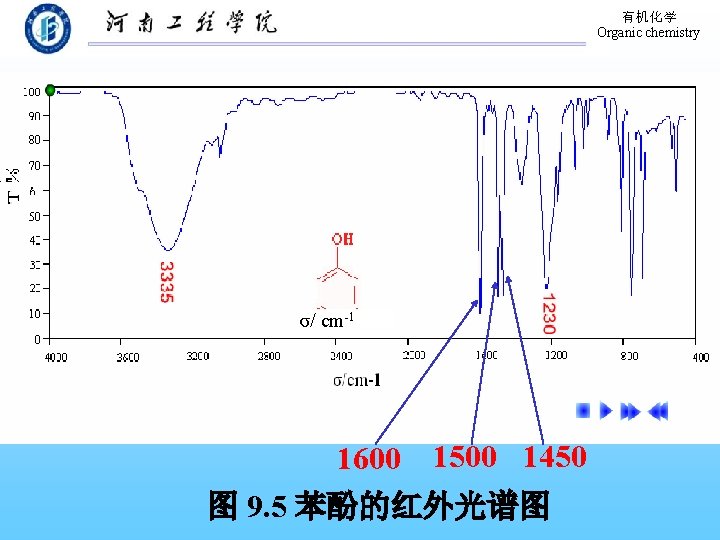
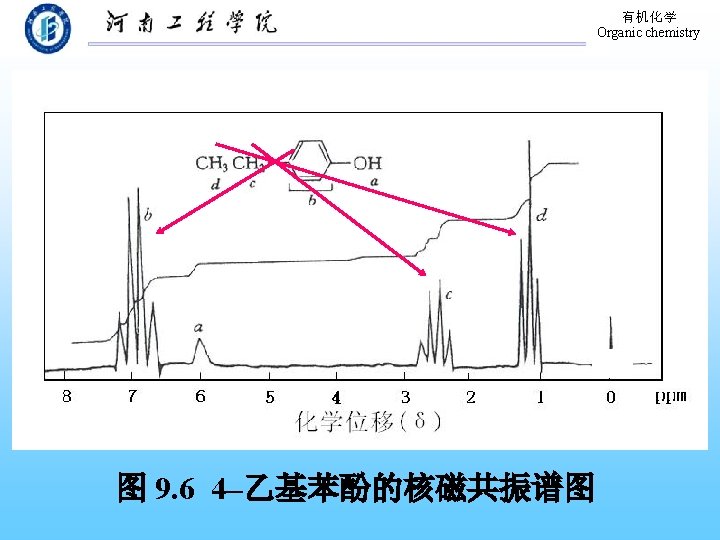
有机化学 Organic chemistry 9. 9 Physical Properties of Phenols The formation of hydrogen bond! 溶解度:在水中部分溶解。 熔、沸点:高于分子量相近的烃。 波谱性质: 1. 红外(IR) O―H: 3640~ 3200 cm-1(宽峰); C―O: 1200 cm-1。 芳 环: 1500~ 1600 cm-1。 2. 核磁 (H-NMR):-OH,δ值在 4 -8之间。 Ar-H: δ值在 7 -8之间。

有机化学 Organic chemistry σ/ cm-1 1600 1500 1450 图 9. 5 苯酚的红外光谱图


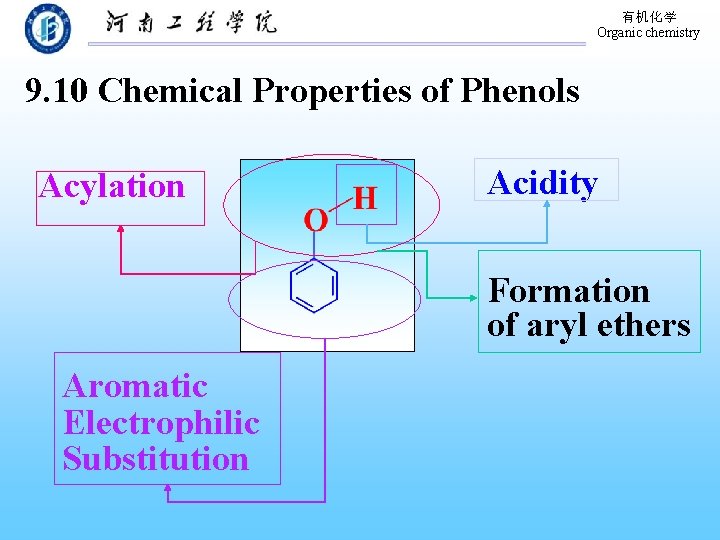
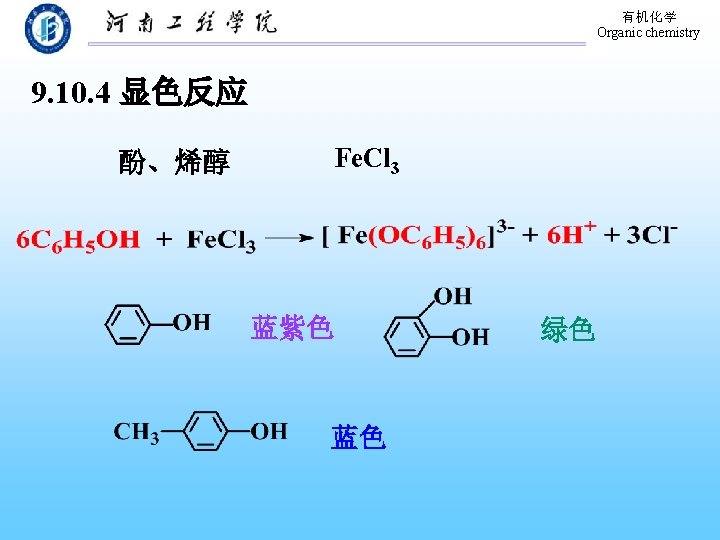
有机化学 Organic chemistry 9. 10 Chemical Properties of Phenols Acylation Acidity Formation of aryl ethers Aromatic Electrophilic Substitution

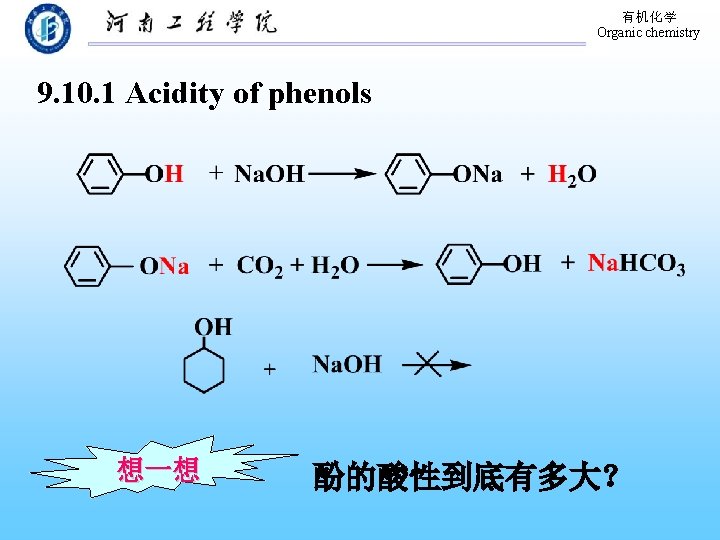
有机化学 Organic chemistry 9. 10. 1 Acidity of phenols 想一想 酚的酸性到底有多大?

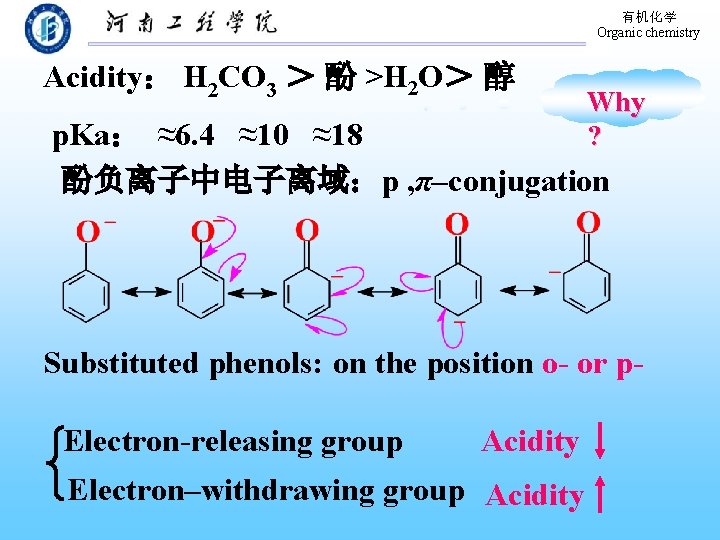
有机化学 Organic chemistry Acidity: H 2 CO 3 > 酚 >H 2 O> 醇 Why ? p. Ka: ≈6. 4 ≈10 ≈18 酚负离子中电子离域:p , π–conjugation Substituted phenols: on the position o- or p. Electron-releasing group Acidity Electron–withdrawing group Acidity

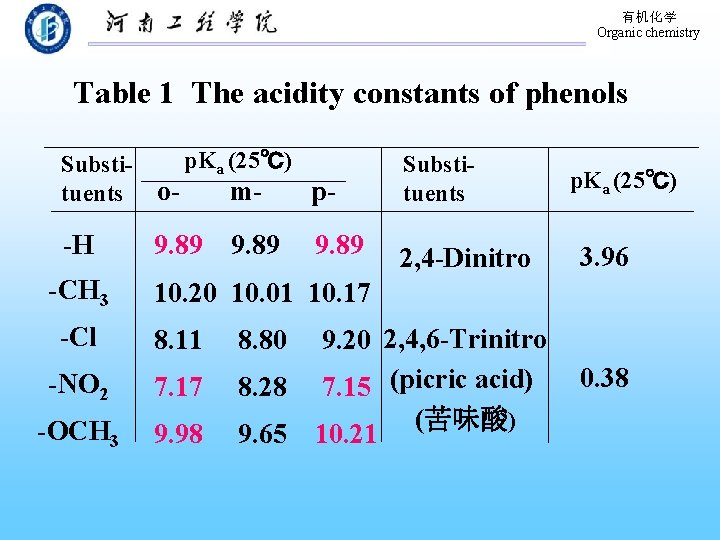
有机化学 Organic chemistry Table 1 The acidity constants of phenols p. Ka (25℃) Substimptuents o- -H -CH 3 9. 89 Substituents 2, 4 -Dinitro p. Ka (25℃) 3. 96 10. 20 10. 01 10. 17 -Cl 8. 11 -NO 2 7. 17 -OCH 3 9. 98 9. 20 2, 4, 6 -Trinitro 8. 28 7. 15 (picric acid) (苦味酸) 9. 65 10. 21 8. 80 0. 38

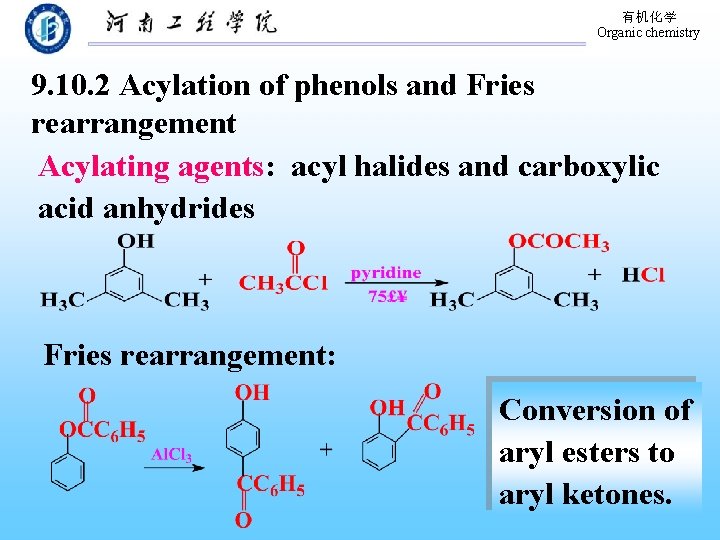
有机化学 Organic chemistry 9. 10. 2 Acylation of phenols and Fries rearrangement Acylating agents: acyl halides and carboxylic acid anhydrides Fries rearrangement: Conversion of aryl esters to aryl ketones.

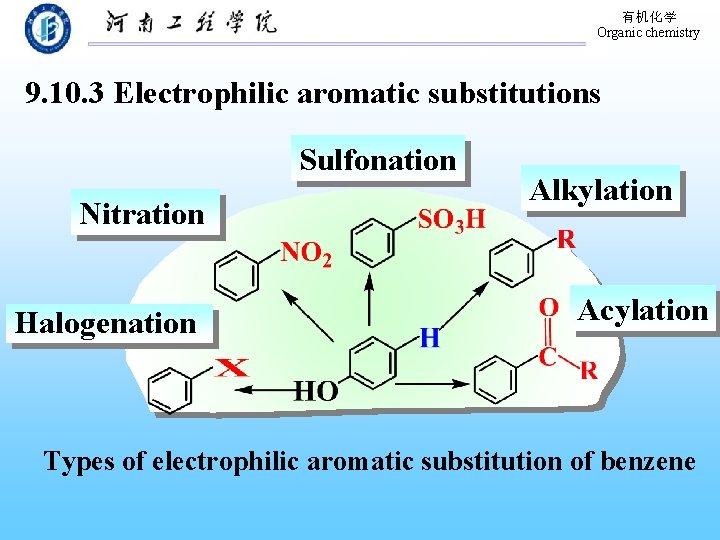
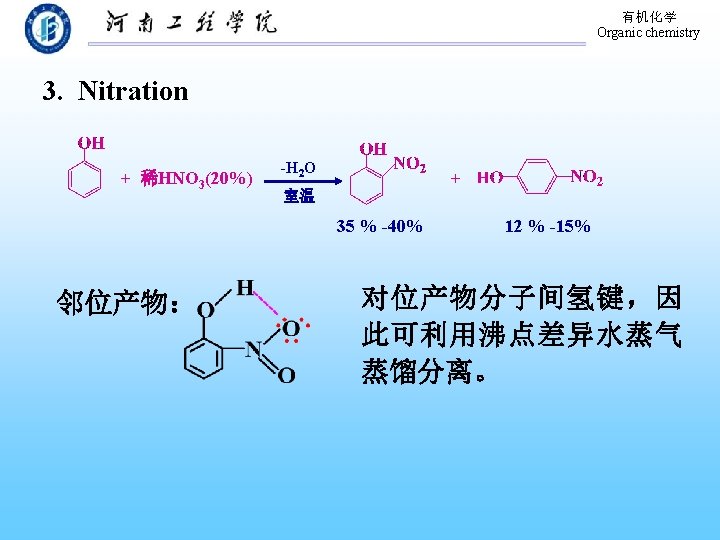
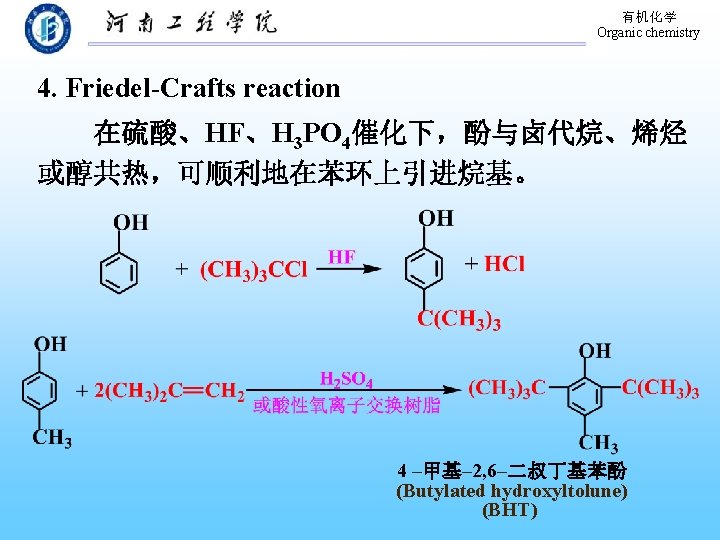
有机化学 Organic chemistry 9. 10. 3 Electrophilic aromatic substitutions Sulfonation Nitration Halogenation Alkylation Acylation Types of electrophilic aromatic substitution of benzene

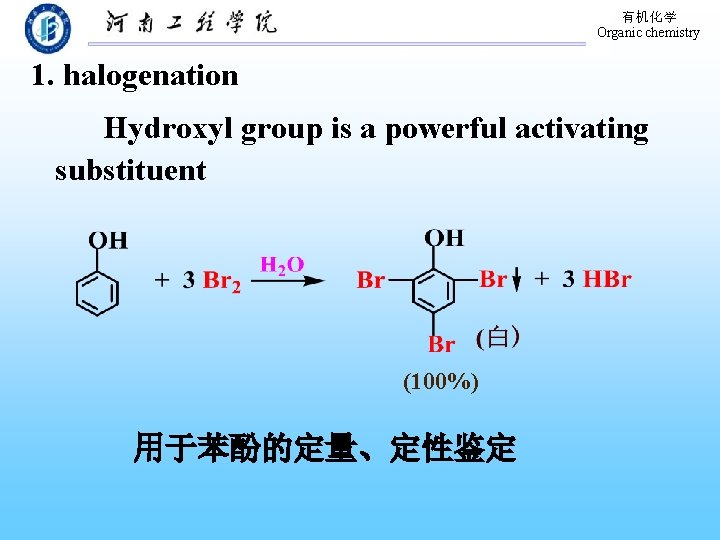
有机化学 Organic chemistry 1. halogenation Hydroxyl group is a powerful activating substituent (100%) 用于苯酚的定量、定性鉴定

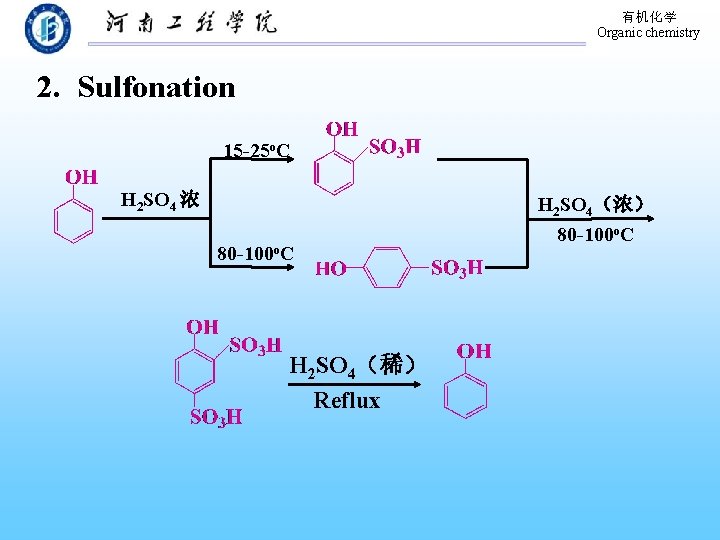
有机化学 Organic chemistry 2. Sulfonation 15 -25 o. C H 2 SO 4 浓 80 -100 o. C H 2 SO 4(稀) Reflux H 2 SO 4(浓) 80 -100 o. C

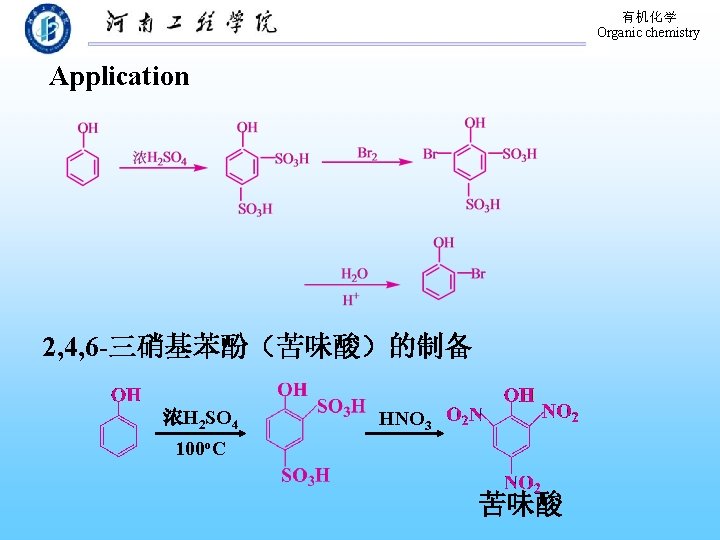
有机化学 Organic chemistry Application 2, 4, 6 -三硝基苯酚(苦味酸)的制备 浓H 2 SO 4 HNO 3 100 o. C 苦味酸



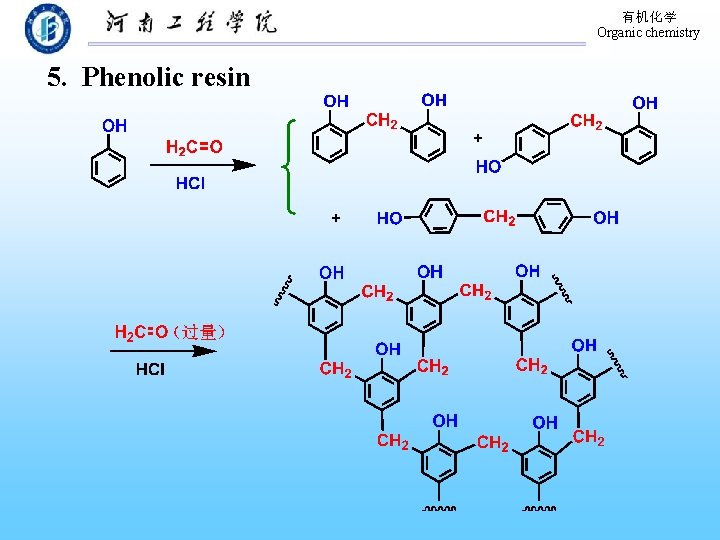
有机化学 Organic chemistry 5. Phenolic resin


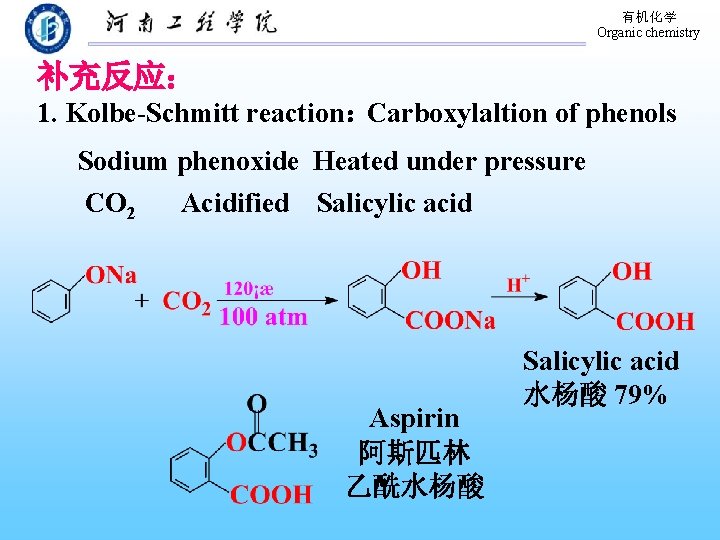
有机化学 Organic chemistry 补充反应: 1. Kolbe-Schmitt reaction:Carboxylaltion of phenols Sodium phenoxide Heated under pressure CO 2 Acidified Salicylic acid Aspirin 阿斯匹林 乙酰水杨酸 Salicylic acid 水杨酸 79%

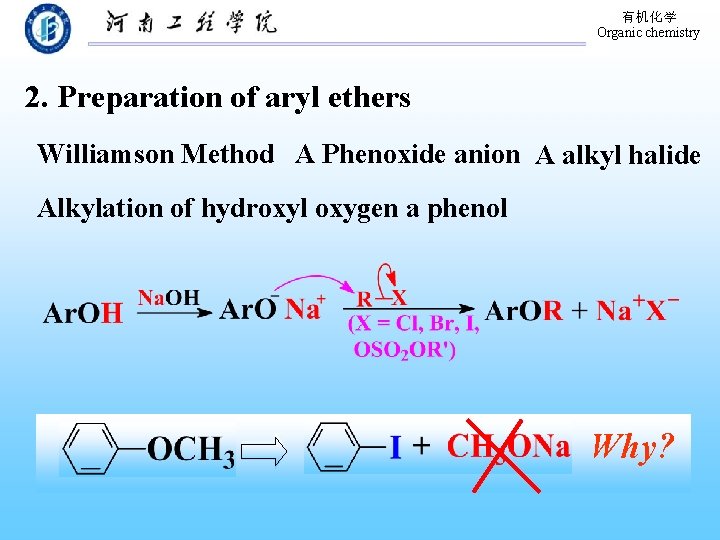
有机化学 Organic chemistry 2. Preparation of aryl ethers Williamson Method A Phenoxide anion A alkyl halide Alkylation of hydroxyl oxygen a phenol Why?

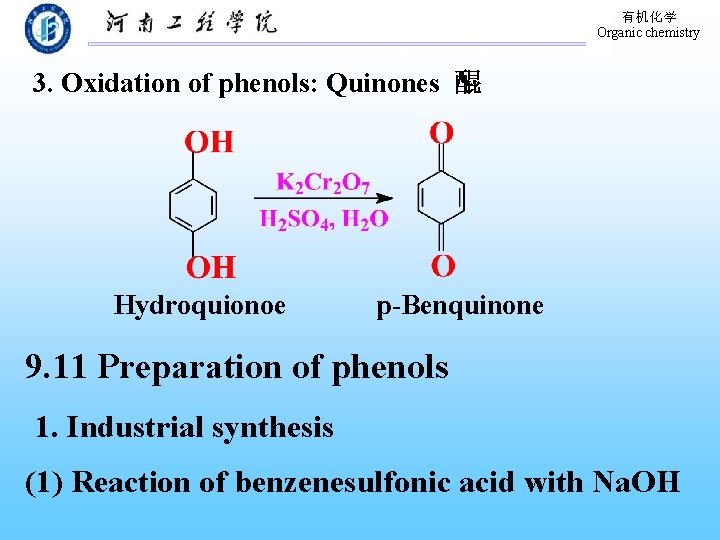
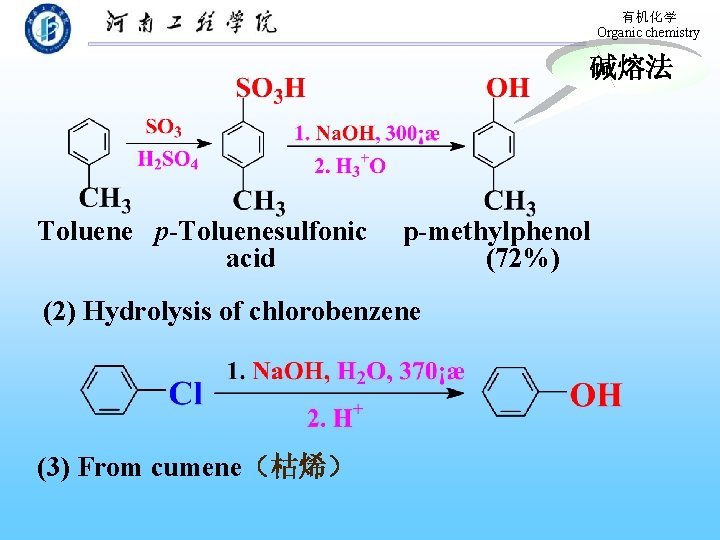
有机化学 Organic chemistry 3. Oxidation of phenols: Quinones 醌 Hydroquionoe p-Benquinone 9. 11 Preparation of phenols 1. Industrial synthesis (1) Reaction of benzenesulfonic acid with Na. OH

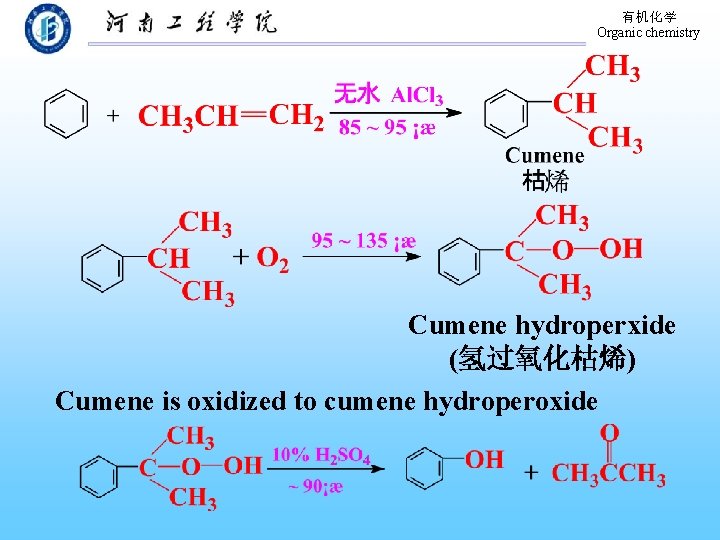
有机化学 Organic chemistry 碱熔法 Toluene p-Toluenesulfonic acid p-methylphenol (72%) (2) Hydrolysis of chlorobenzene (3) From cumene(枯烯)

有机化学 Organic chemistry Cumene hydroperxide (氢过氧化枯烯) Cumene is oxidized to cumene hydroperoxide

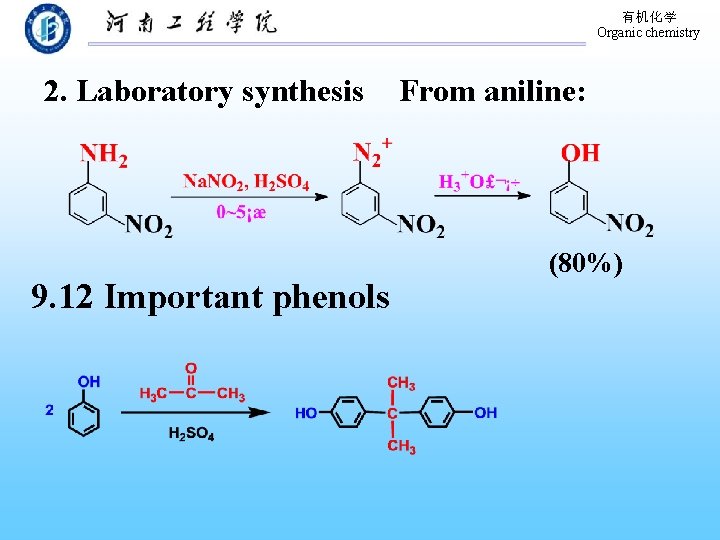
有机化学 Organic chemistry 2. Laboratory synthesis 9. 12 Important phenols From aniline: (80%)

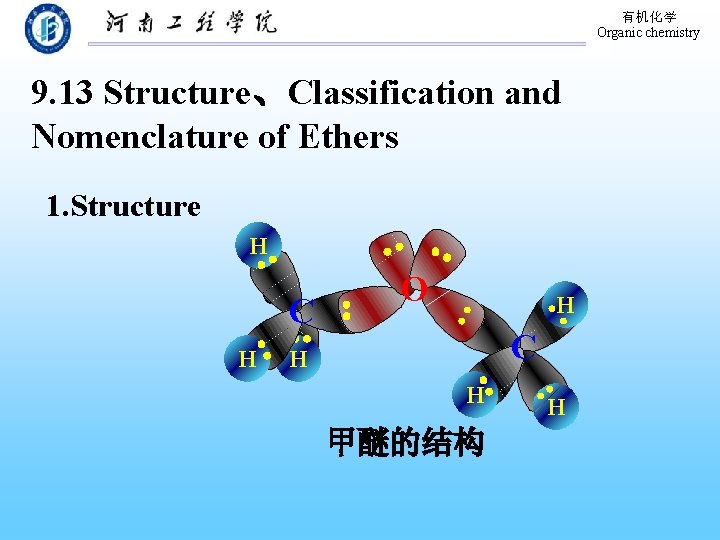
有机化学 Organic chemistry 9. 13 Structure、Classification and Nomenclature of Ethers 1. Structure H CC H O H C H H 甲醚的结构 H

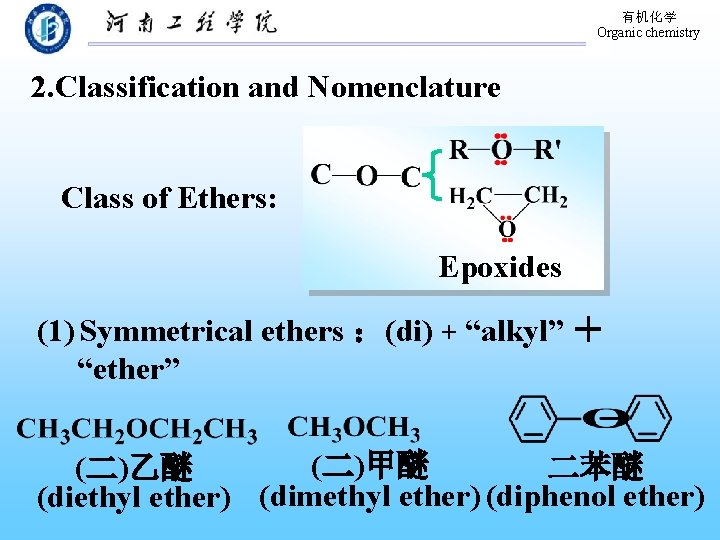
有机化学 Organic chemistry 2. Classification and Nomenclature Class of Ethers: Epoxides (1) Symmetrical ethers :(di) + “alkyl” + “ether” (二)甲醚 二苯醚 (二)乙醚 (diethyl ether) (dimethyl ether) (diphenol ether)

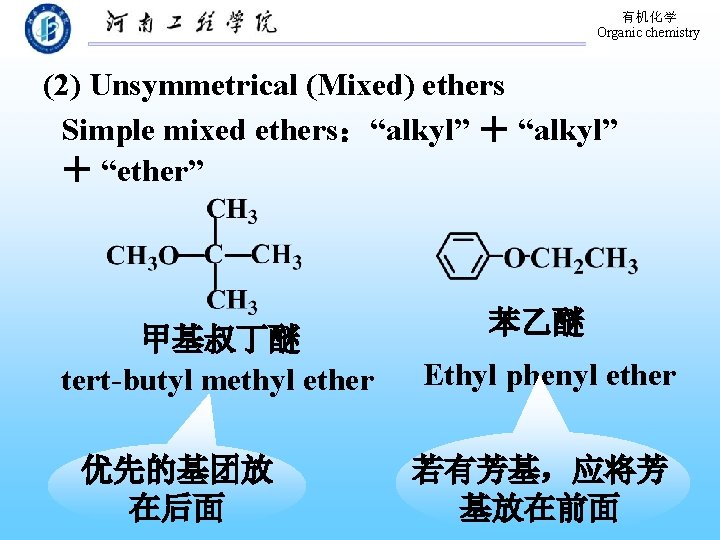
有机化学 Organic chemistry (2) Unsymmetrical (Mixed) ethers Simple mixed ethers:“alkyl” + “ether” 甲基叔丁醚 tert-butyl methyl ether 优先的基团放 在后面 苯乙醚 Ethyl phenyl ether 若有芳基,应将芳 基放在前面

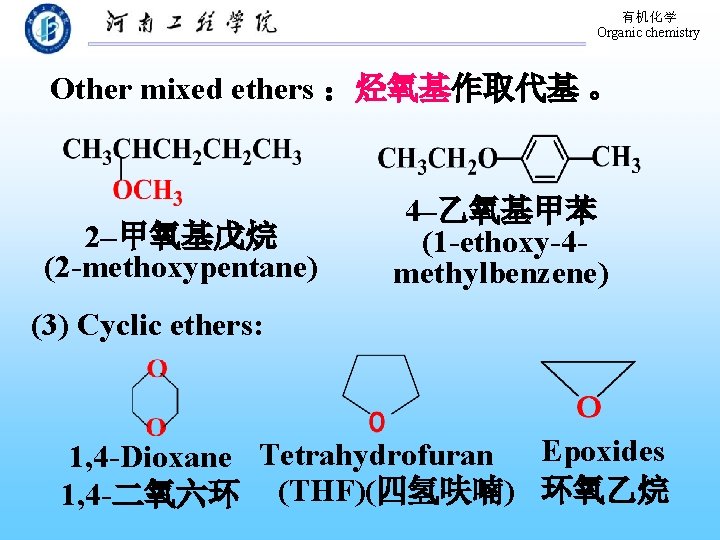
有机化学 Organic chemistry Other mixed ethers :烃氧基作取代基 。 2–甲氧基戊烷 (2 -methoxypentane) 4–乙氧基甲苯 (1 -ethoxy-4 methylbenzene) (3) Cyclic ethers: 1, 4 -Dioxane Tetrahydrofuran Epoxides 1, 4 -二氧六环 (THF)(四氢呋喃) 环氧乙烷

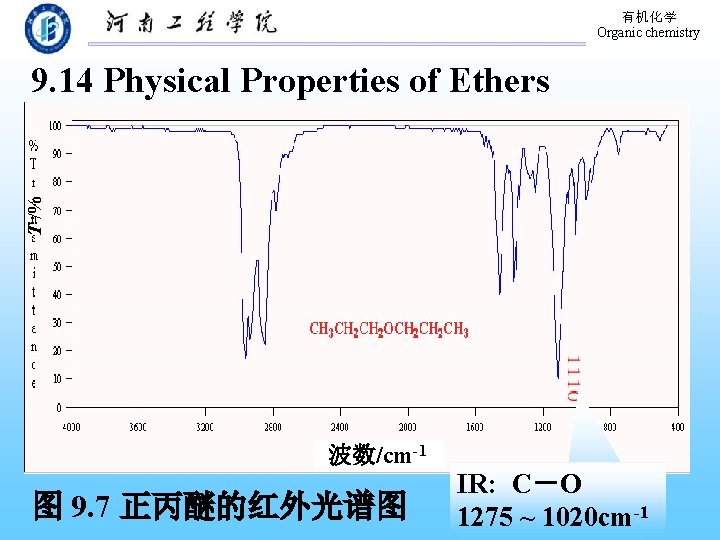
有机化学 Organic chemistry T /% 9. 14 Physical Properties of Ethers 波数/cm-1 图 9. 7 正丙醚的红外光谱图 IR: C―O 1275 ~ 1020 cm-1

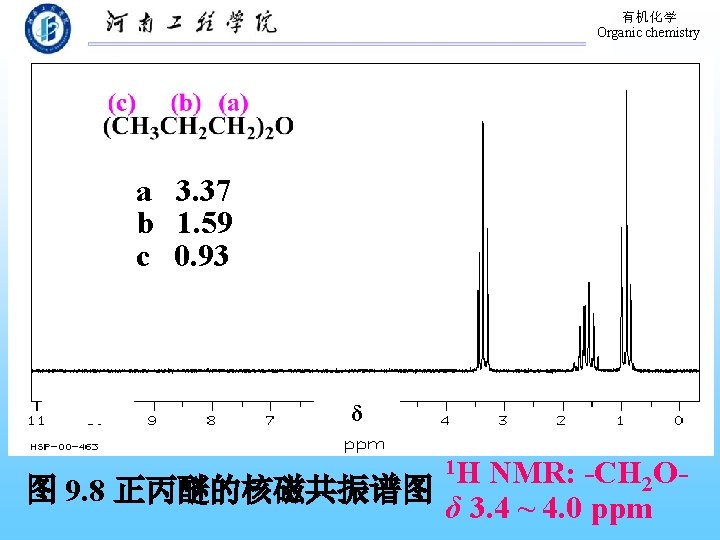
有机化学 Organic chemistry a 3. 37 b 1. 59 c 0. 93 δ 1 H NMR: -CH 2 O图 9. 8 正丙醚的核磁共振谱图 δ 3. 4 ~ 4. 0 ppm

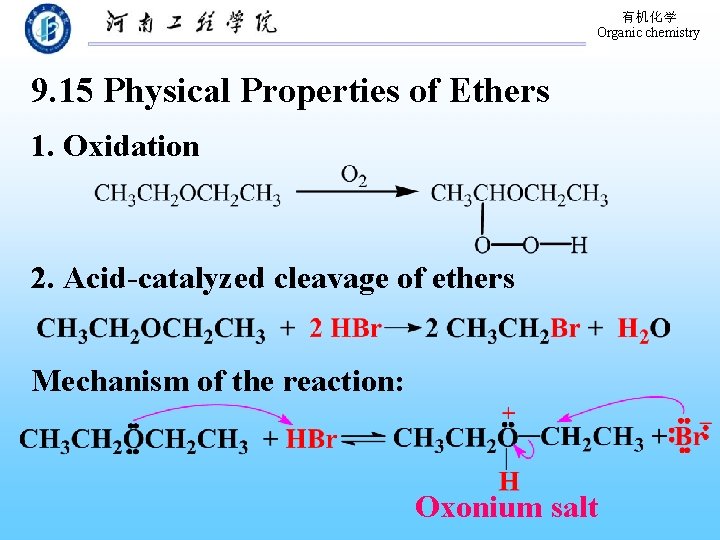
有机化学 Organic chemistry 9. 15 Physical Properties of Ethers 1. Oxidation 2. Acid-catalyzed cleavage of ethers Mechanism of the reaction: Oxonium salt

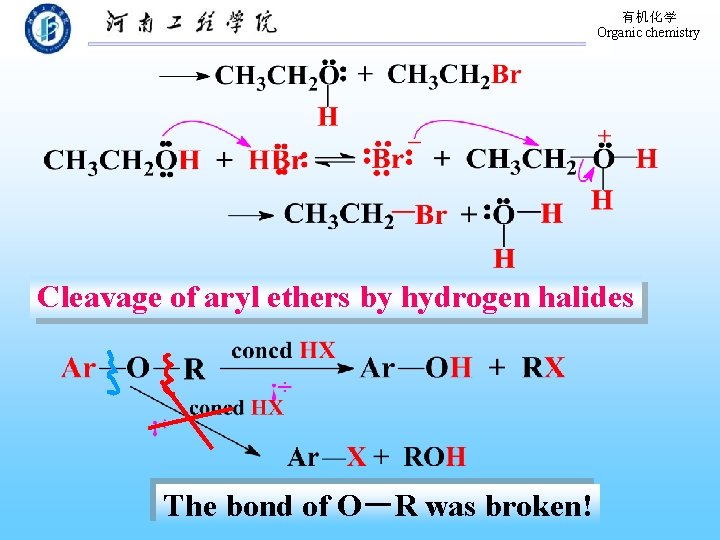
有机化学 Organic chemistry Cleavage of aryl ethers by hydrogen halides The bond of O-R was broken!

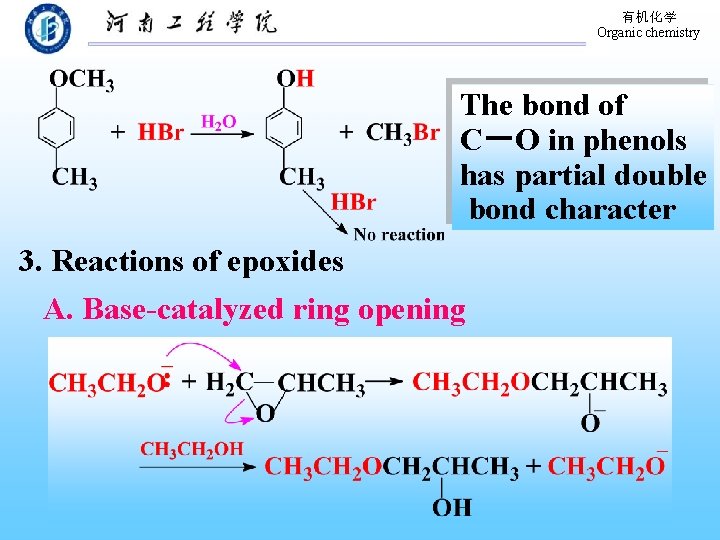
有机化学 Organic chemistry The bond of C-O in phenols has partial double bond character 3. Reactions of epoxides A. Base-catalyzed ring opening

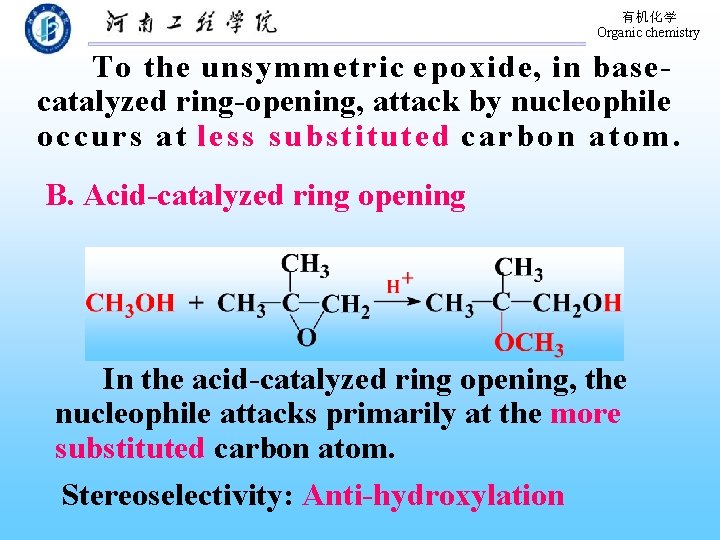
有机化学 Organic chemistry To the unsymmetric epoxide, in basecatalyzed ring-opening, attack by nucleophile oc c ur s at less substituted c a r b o n a t o m. B. Acid-catalyzed ring opening In the acid-catalyzed ring opening, the nucleophile attacks primarily at the more substituted carbon atom. Stereoselectivity: Anti-hydroxylation

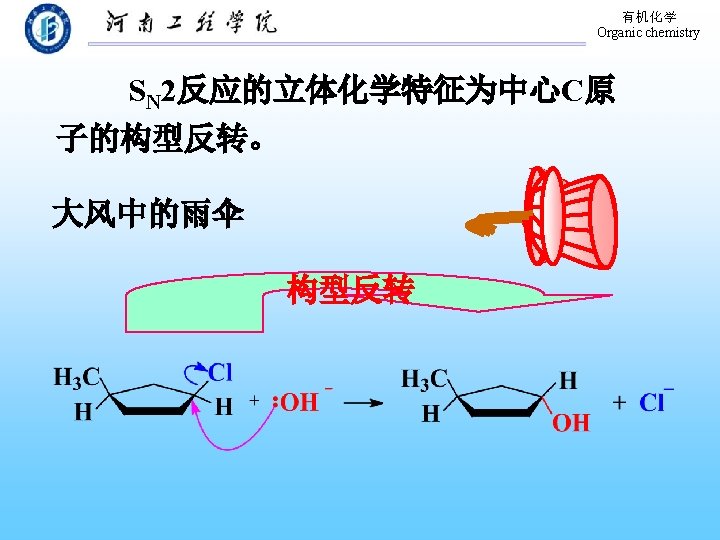
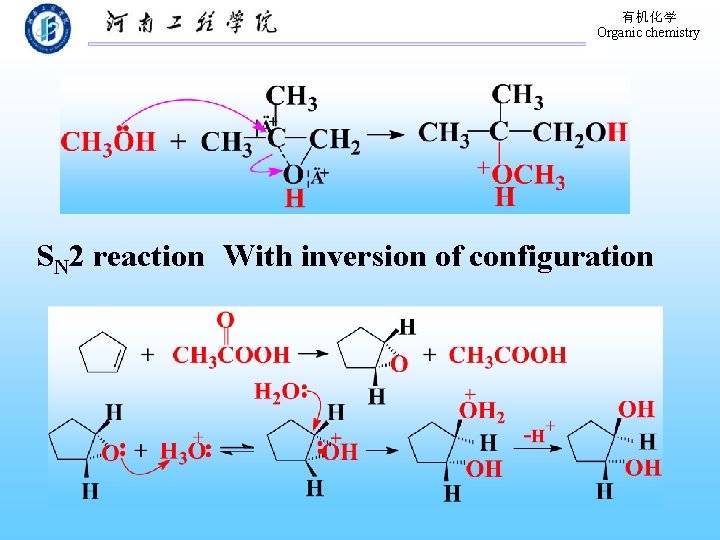
有机化学 Organic chemistry SN 2 reaction With inversion of configuration

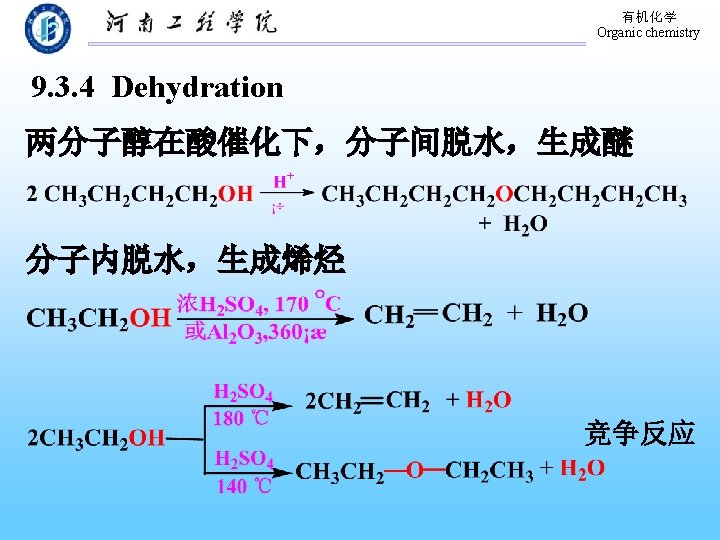
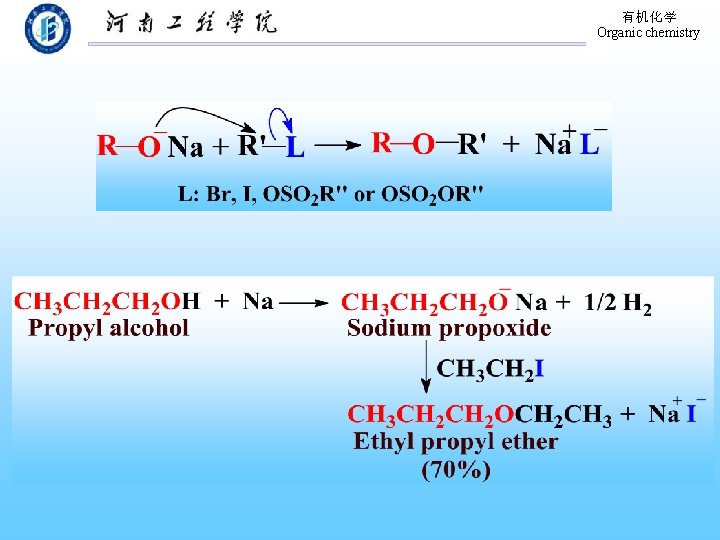
有机化学 Organic chemistry 9. 16 Preparation of Ethers 1. By intermolecular dehydration of alcohols Substrate: Primary alcohols Acid-catalyzed Products: symmetric ethers 2. The Williamson Synthesis of Ethers Sodium alkoxide, Alkyl halide and derivatives (Asymmetric) Mixed ethers

有机化学 Organic chemistry

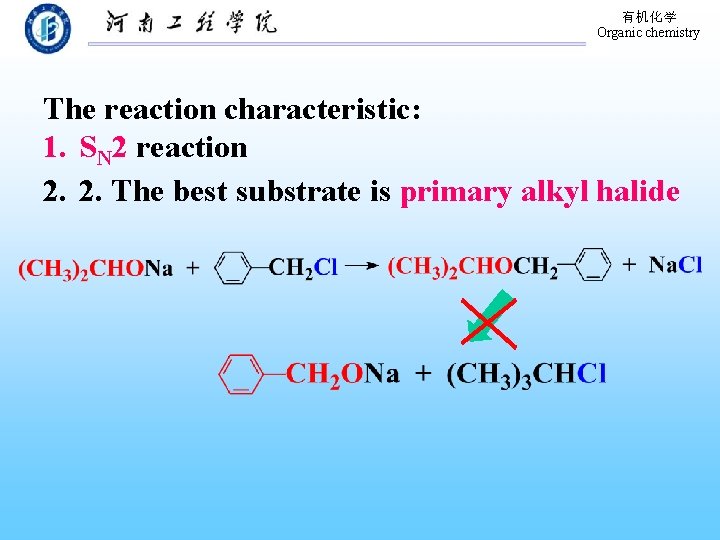
有机化学 Organic chemistry The reaction characteristic: 1. SN 2 reaction 2. 2. The best substrate is primary alkyl halide

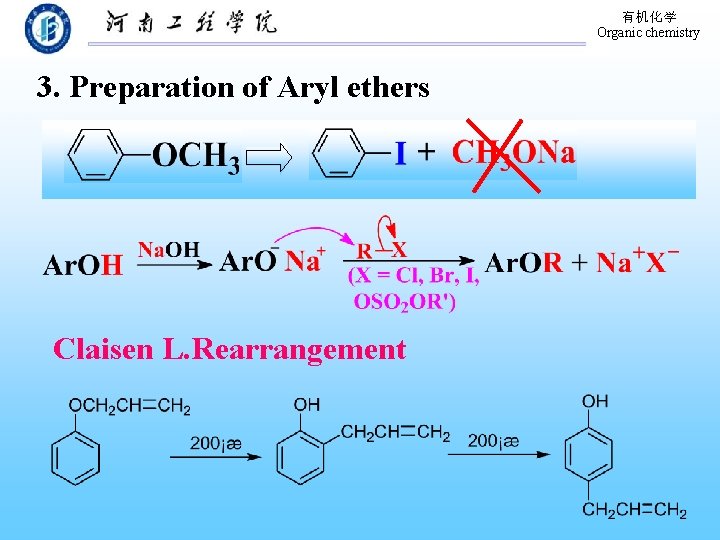
有机化学 Organic chemistry 3. Preparation of Aryl ethers Claisen L. Rearrangement

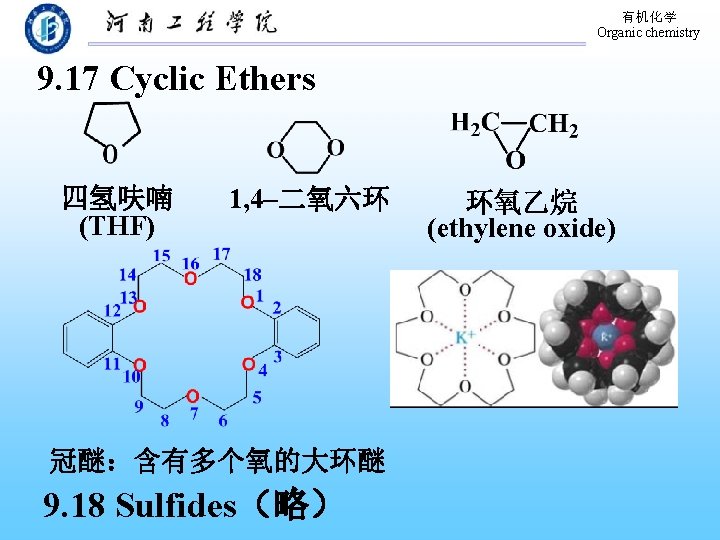
有机化学 Organic chemistry 9. 17 Cyclic Ethers 四氢呋喃 (THF) 1, 4–二氧六环 冠醚:含有多个氧的大环醚 9. 18 Sulfides(略) 环氧乙烷 (ethylene oxide)

 Alcohols phenols thiols and ethers
Alcohols phenols thiols and ethers Naming esters
Naming esters Simple phenols
Simple phenols Ib organic chemistry
Ib organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Butaanzuur
Butaanzuur Ether cleavage
Ether cleavage Ethers naming
Ethers naming Ether naming
Ether naming Ethers boiling point
Ethers boiling point David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane Why are ethers relatively inert compounds
Why are ethers relatively inert compounds Acidic cleavage of ethers
Acidic cleavage of ethers Primary alcohol secondary alcohol
Primary alcohol secondary alcohol Grignard reagent formula
Grignard reagent formula Cppp nucleus
Cppp nucleus Primary alcohol vs secondary alcohol
Primary alcohol vs secondary alcohol Lucas test reagent
Lucas test reagent Lucas reagent equation
Lucas reagent equation Preparing haloalkanes from alcohols
Preparing haloalkanes from alcohols Butanone and acidified kcn
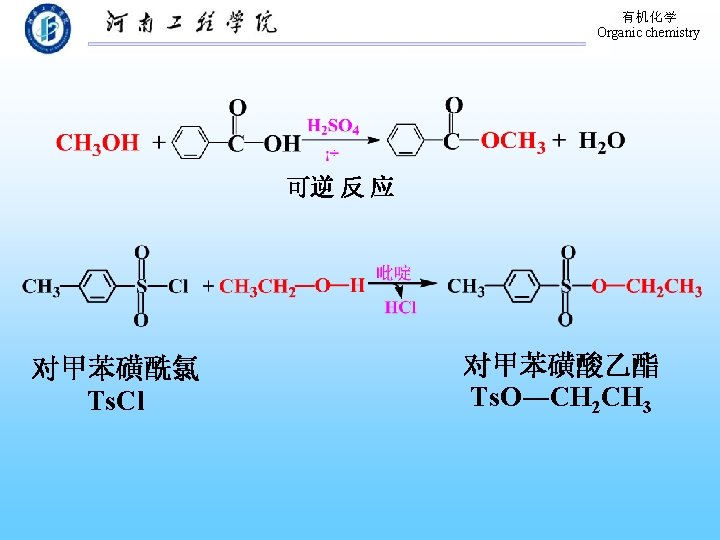
Butanone and acidified kcn Tscl py reaction
Tscl py reaction Sp
Sp Sugar alcohol names
Sugar alcohol names Alcohols nomenclature
Alcohols nomenclature Naming alkyl halides
Naming alkyl halides Ethanol to ethanal to ethanoic acid
Ethanol to ethanal to ethanoic acid Alcohols nomenclature
Alcohols nomenclature Chemistry
Chemistry Compound lipids definition
Compound lipids definition Structure and bonding in organic chemistry
Structure and bonding in organic chemistry Enols and enolates organic chemistry
Enols and enolates organic chemistry Numbering carbon chains
Numbering carbon chains Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Displayed formula
Displayed formula Transition state energy diagram
Transition state energy diagram Ee organic chemistry
Ee organic chemistry Leveling effect organic chemistry
Leveling effect organic chemistry Pericyclic
Pericyclic Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein What is the leveling effect organic chemistry
What is the leveling effect organic chemistry Seniority of functional groups
Seniority of functional groups Organic chemistry lab report sample
Organic chemistry lab report sample Www.masterorganicchemistry.com
Www.masterorganicchemistry.com Organic chemistry grade 10
Organic chemistry grade 10 Organic chemistry
Organic chemistry Organic chemistry introduction
Organic chemistry introduction Kiliani fischer synthesis
Kiliani fischer synthesis Meth eth prop but
Meth eth prop but Chemistry cracking
Chemistry cracking Meth eth prop but
Meth eth prop but Organic chemistry myanmar
Organic chemistry myanmar Br2aq
Br2aq M+1 peak
M+1 peak Hono organic chemistry
Hono organic chemistry Leaving group ability
Leaving group ability Organic chemistry topic 11
Organic chemistry topic 11 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry reaction pathways
Organic chemistry reaction pathways Alkene alcohol naming
Alkene alcohol naming What is organic chemistry like
What is organic chemistry like Is ch4o organic or inorganic
Is ch4o organic or inorganic Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry (3rd) edition chapter 1 problem 20s
Organic chemistry (3rd) edition chapter 1 problem 20s Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Ario acidity
Ario acidity How to calculate percent yield
How to calculate percent yield Polarimetry organic chemistry
Polarimetry organic chemistry Organic chemistry third edition david klein
Organic chemistry third edition david klein Radicals
Radicals Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Organic chemistry
Organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Organic chemistry case studies
Organic chemistry case studies Chapter 7 chemistry review
Chapter 7 chemistry review This name
This name Analytical chemistry chapters
Analytical chemistry chapters Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Carbohydrates organic chemistry
Carbohydrates organic chemistry Resonance in benzyl carbocation
Resonance in benzyl carbocation Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry John wiley & sons, inc.
John wiley & sons, inc. Organic chemistry cheat sheet
Organic chemistry cheat sheet Mindup mind map
Mindup mind map