Organic chemistry A Chapter 6 Alcohols and Ethers















- Slides: 60

Organic chemistry A Chapter 6 Alcohols and Ethers By Prof. Dr. Adel M. Awadallah Islamic University of Gaza

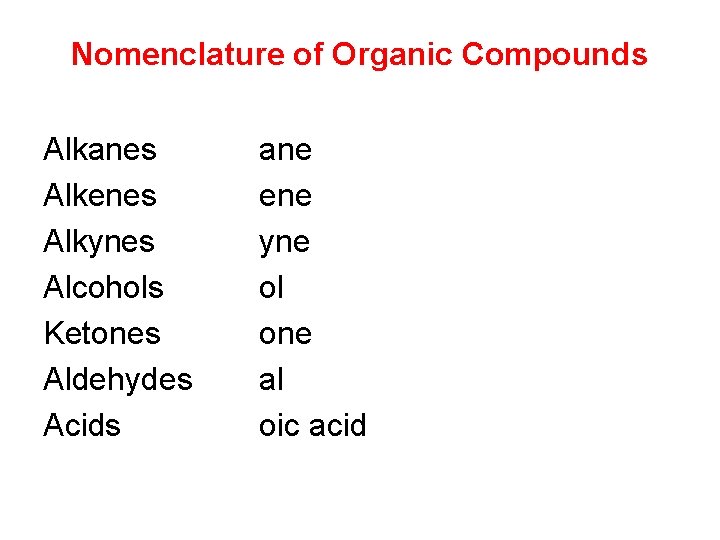
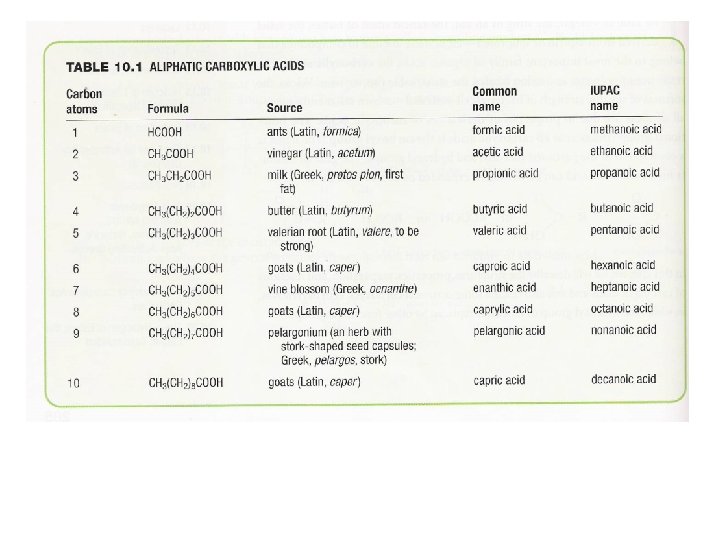
Nomenclature of Organic Compounds Alkanes Alkenes Alkynes Alcohols Ketones Aldehydes Acids ane ene yne ol one al oic acid

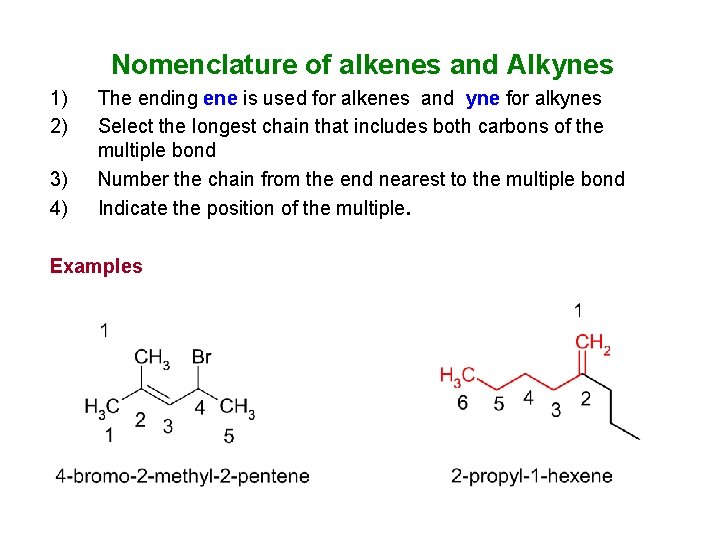
Nomenclature of alkenes and Alkynes 1) 2) 3) 4) The ending ene is used for alkenes and yne for alkynes Select the longest chain that includes both carbons of the multiple bond Number the chain from the end nearest to the multiple bond Indicate the position of the multiple. Examples

Isomers and common names of simple alkenes

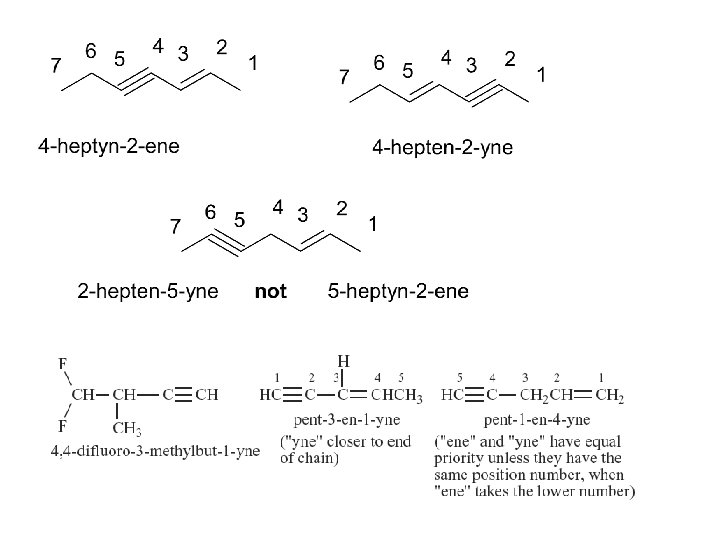
Assigning Priority • Alkenes and alkynes are considered to have equal priority • In a molecule with both a double and a triple bond, whichever is closer to the end of the chain determines the direction of numbering. • In the case where each would have the same position number, the double bond takes the lower number. • In the name, “ene” comes before “yne” because of alphabetization.


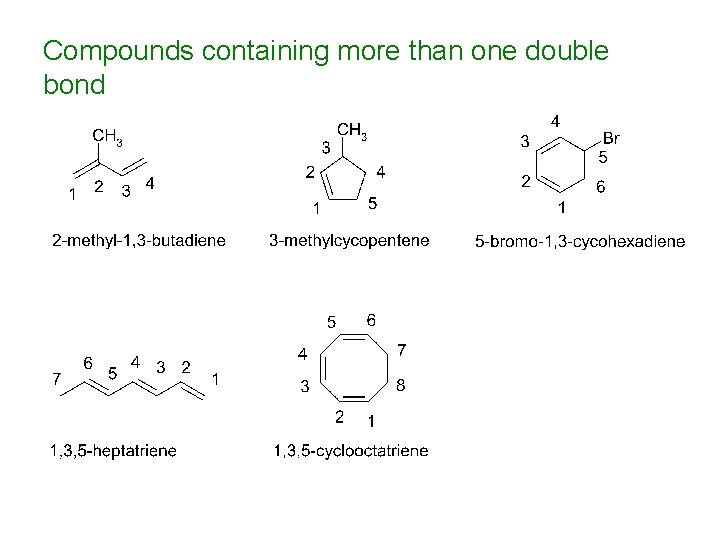
Compounds containing more than one double bond

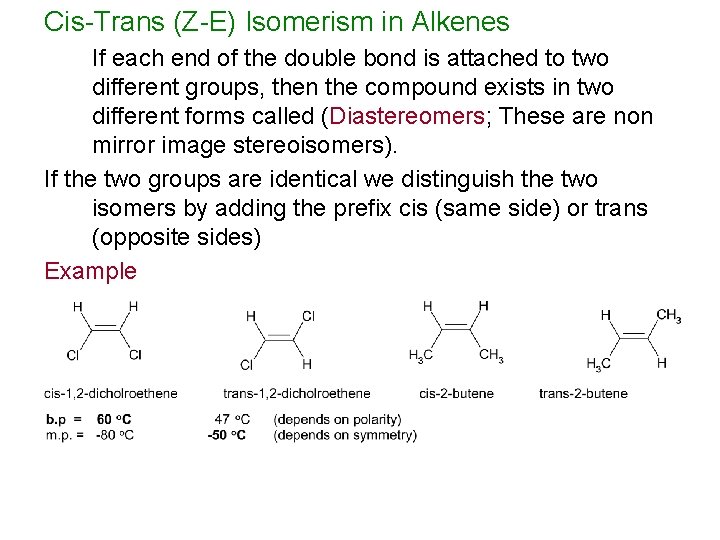
Cis-Trans (Z-E) Isomerism in Alkenes If each end of the double bond is attached to two different groups, then the compound exists in two different forms called (Diastereomers; These are non mirror image stereoisomers). If the two groups are identical we distinguish the two isomers by adding the prefix cis (same side) or trans (opposite sides) Example

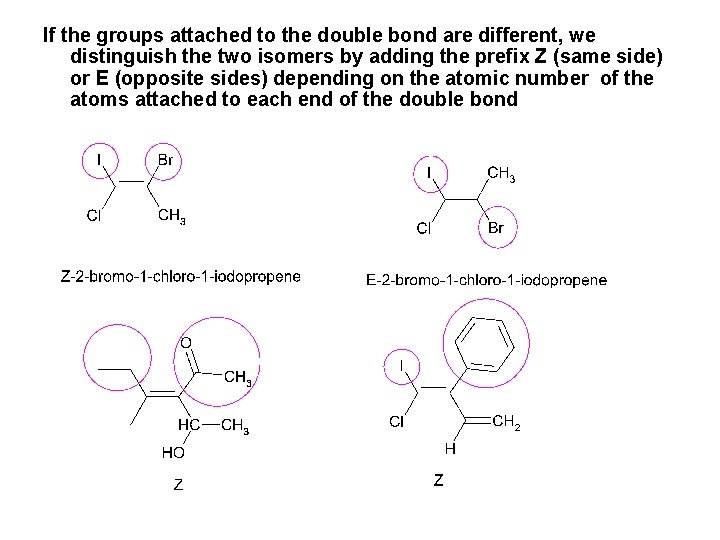
If the groups attached to the double bond are different, we distinguish the two isomers by adding the prefix Z (same side) or E (opposite sides) depending on the atomic number of the atoms attached to each end of the double bond

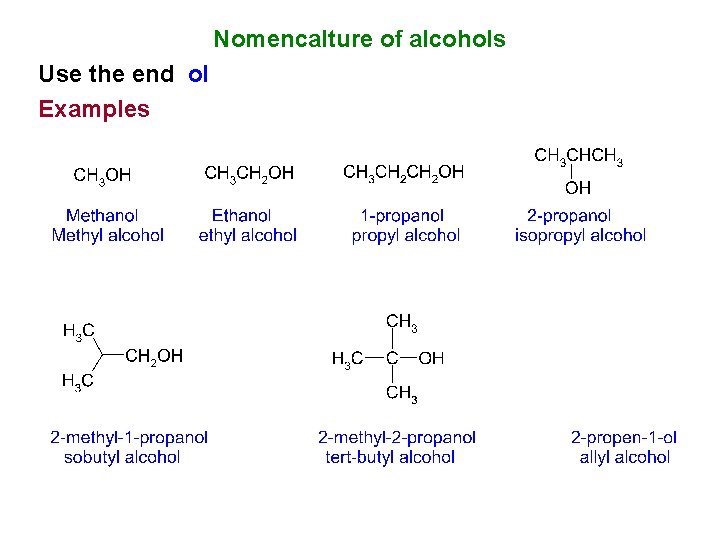
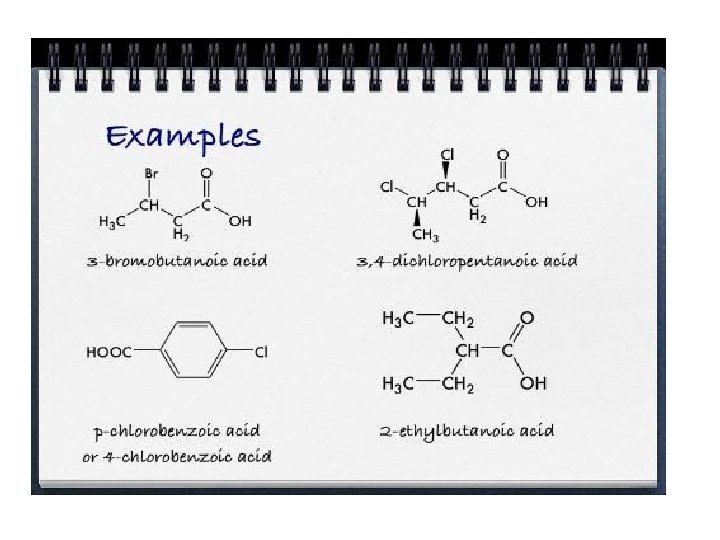
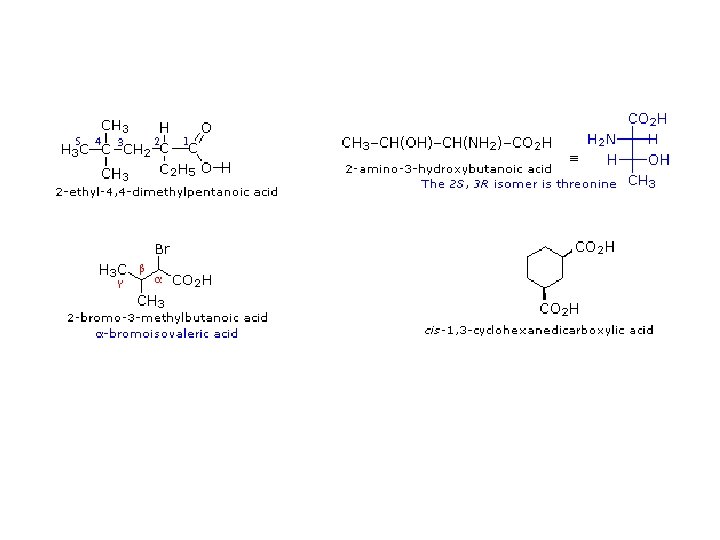
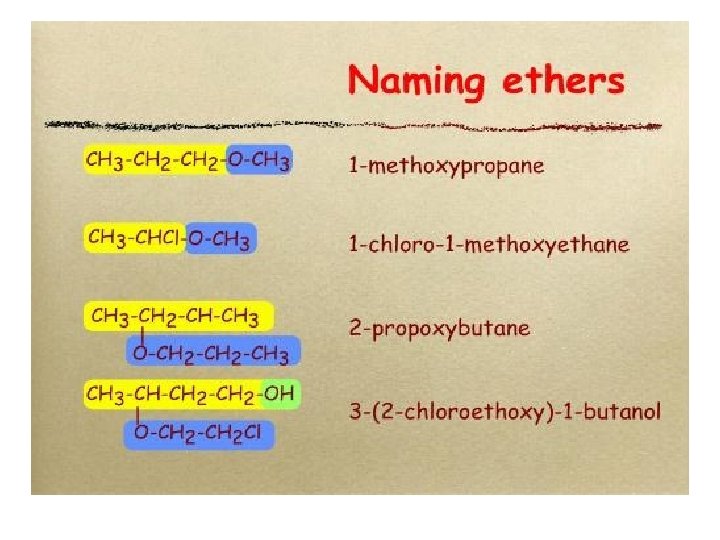
Nomencalture of alcohols Use the end ol Examples

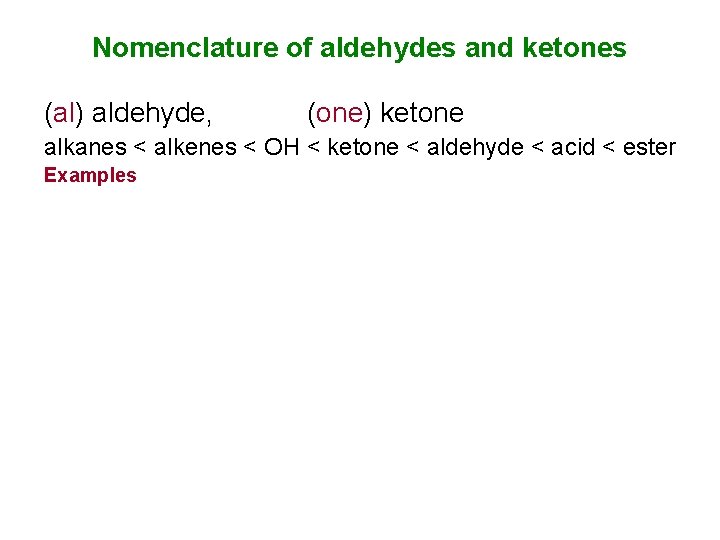
Assigning Priority Halogens < alkanes < alkenes (alkynes) < amines < OH < ketone < aldehyde < acid < ester

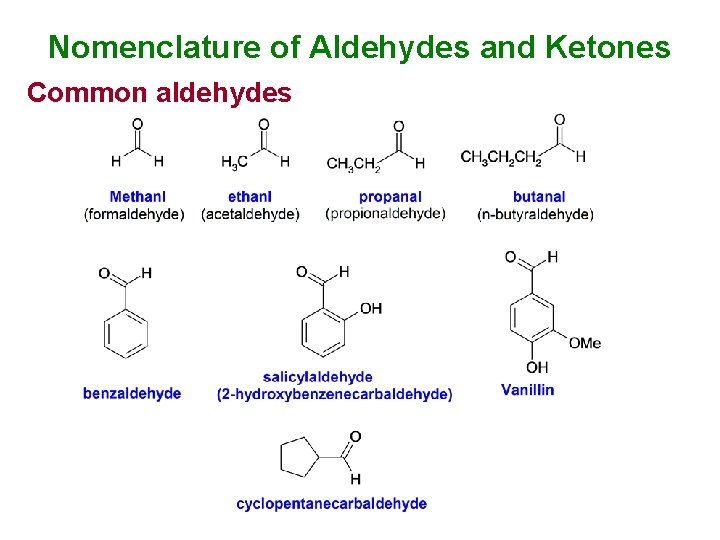
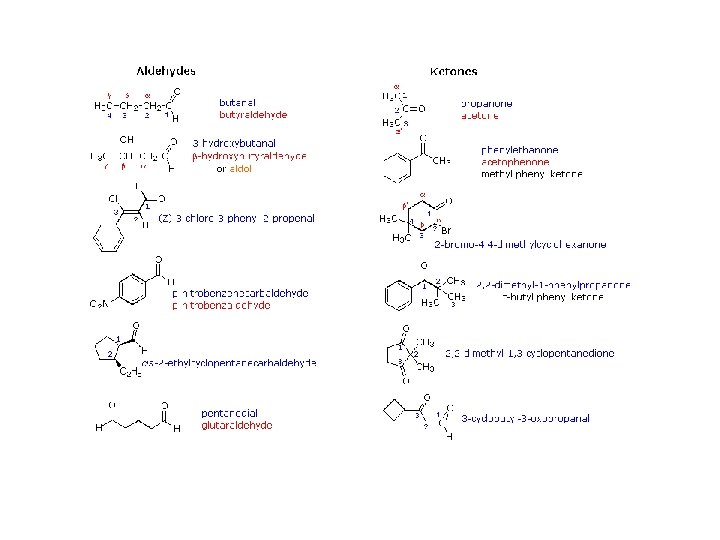
Nomenclature of Aldehydes and Ketones Common aldehydes

Common Ketones

Nomenclature of aldehydes and ketones (al) aldehyde, (one) ketone alkanes < alkenes < OH < ketone < aldehyde < acid < ester Examples







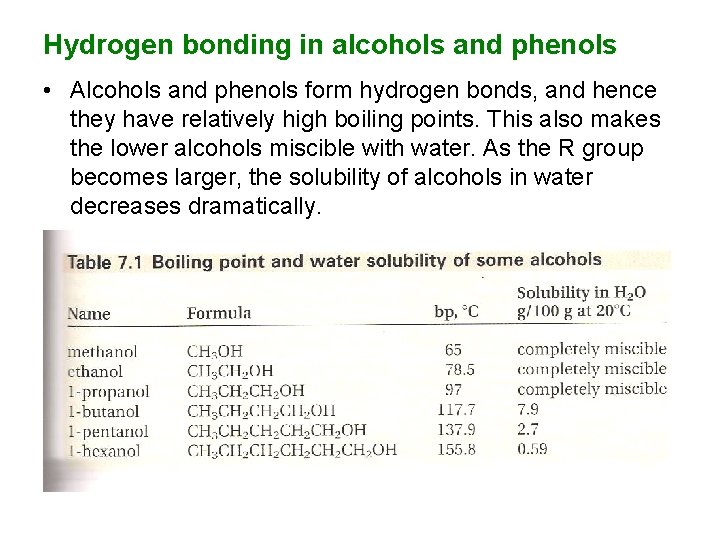
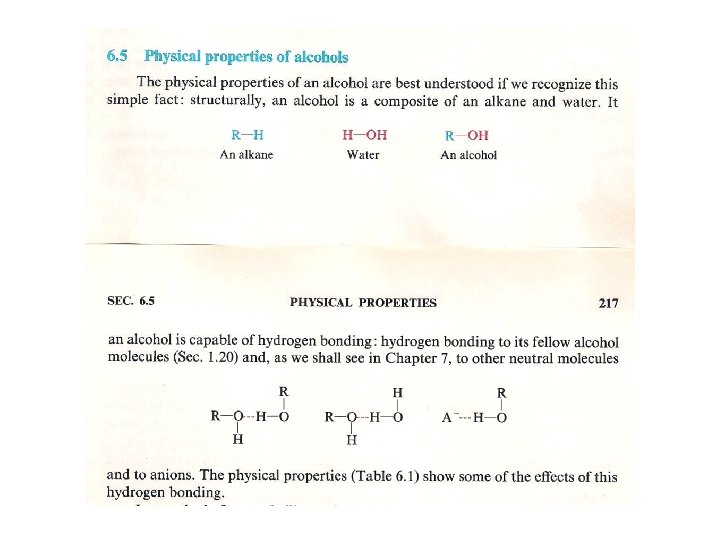
Hydrogen bonding in alcohols and phenols • Alcohols and phenols form hydrogen bonds, and hence they have relatively high boiling points. This also makes the lower alcohols miscible with water. As the R group becomes larger, the solubility of alcohols in water decreases dramatically.


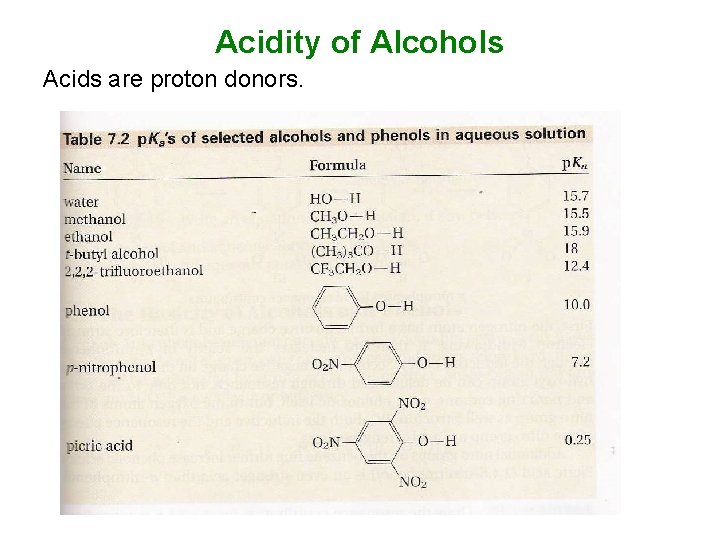
Acidity of Alcohols Acids are proton donors.

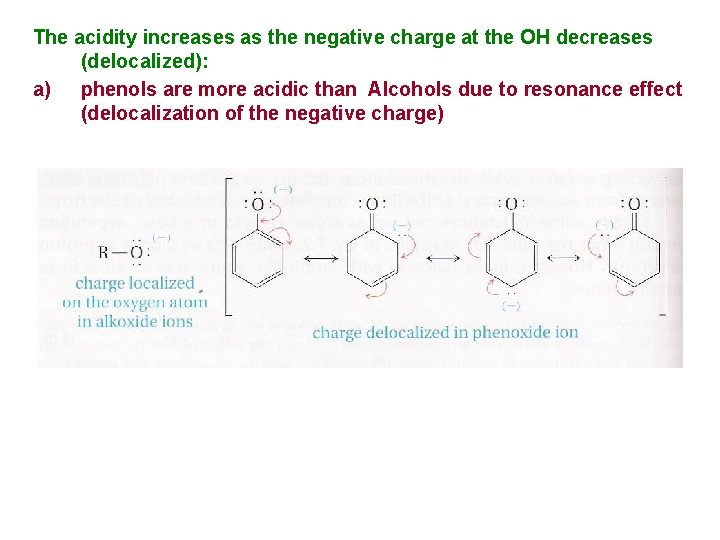
The acidity increases as the negative charge at the OH decreases (delocalized): a) phenols are more acidic than Alcohols due to resonance effect (delocalization of the negative charge)

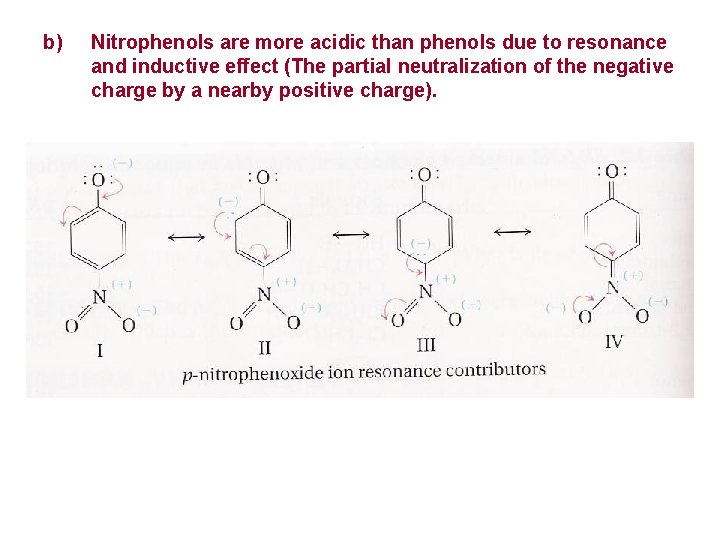
b) Nitrophenols are more acidic than phenols due to resonance and inductive effect (The partial neutralization of the negative charge by a nearby positive charge).

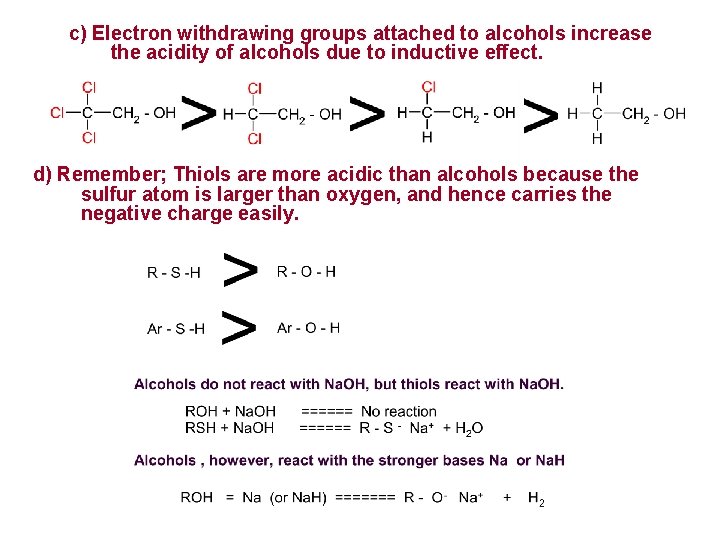
c) Electron withdrawing groups attached to alcohols increase the acidity of alcohols due to inductive effect. d) Remember; Thiols are more acidic than alcohols because the sulfur atom is larger than oxygen, and hence carries the negative charge easily.

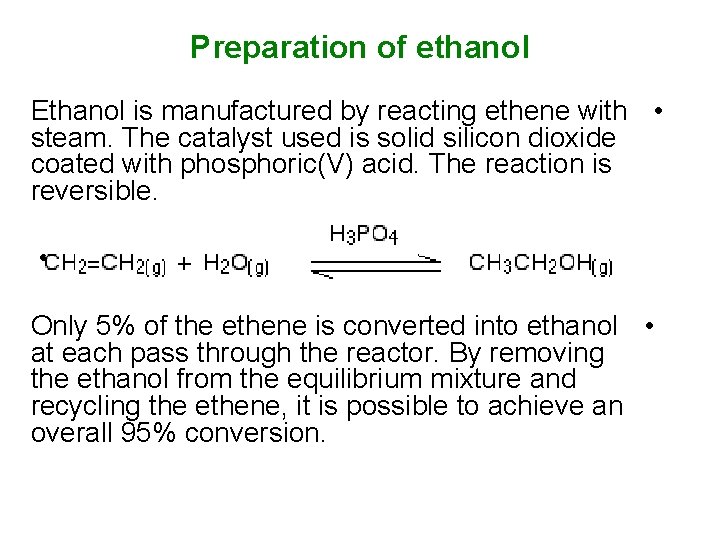
Preparation of ethanol Ethanol is manufactured by reacting ethene with • steam. The catalyst used is solid silicon dioxide coated with phosphoric(V) acid. The reaction is reversible. • Only 5% of the ethene is converted into ethanol • at each pass through the reactor. By removing the ethanol from the equilibrium mixture and recycling the ethene, it is possible to achieve an overall 95% conversion.

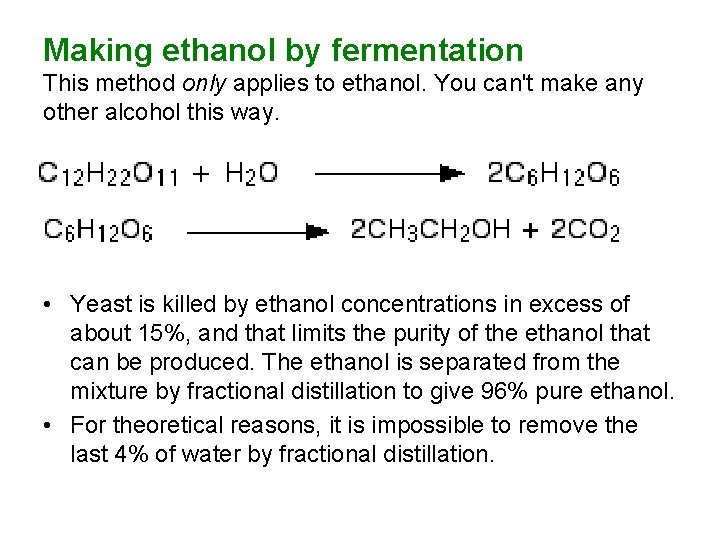
Making ethanol by fermentation This method only applies to ethanol. You can't make any other alcohol this way. • Yeast is killed by ethanol concentrations in excess of about 15%, and that limits the purity of the ethanol that can be produced. The ethanol is separated from the mixture by fractional distillation to give 96% pure ethanol. • For theoretical reasons, it is impossible to remove the last 4% of water by fractional distillation.


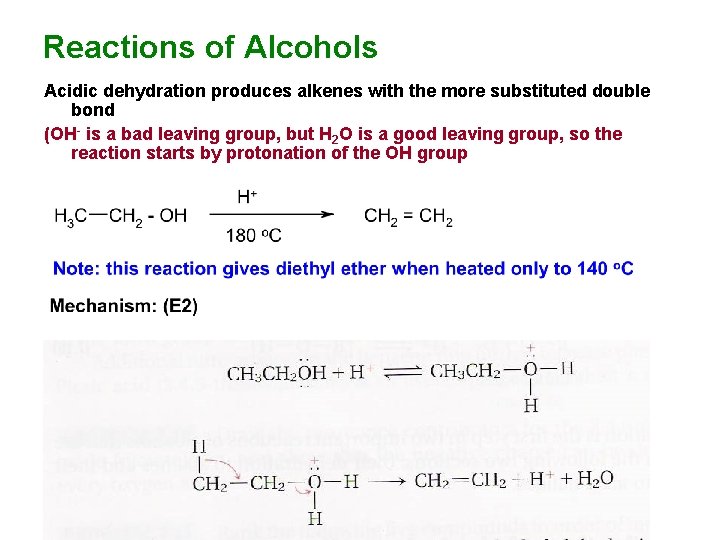
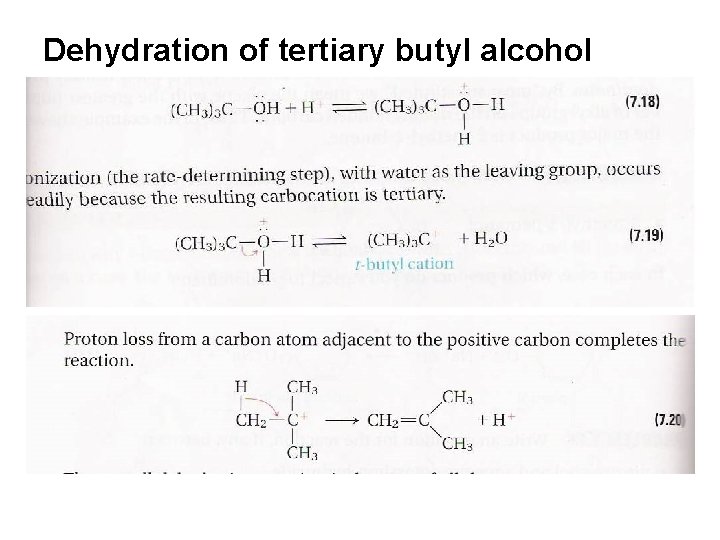
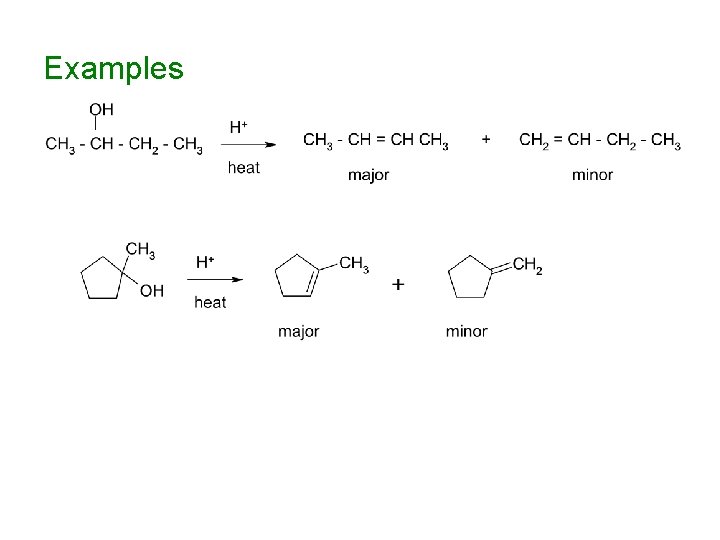
Reactions of Alcohols Acidic dehydration produces alkenes with the more substituted double bond (OH- is a bad leaving group, but H 2 O is a good leaving group, so the reaction starts by protonation of the OH group

Dehydration of tertiary butyl alcohol

Examples

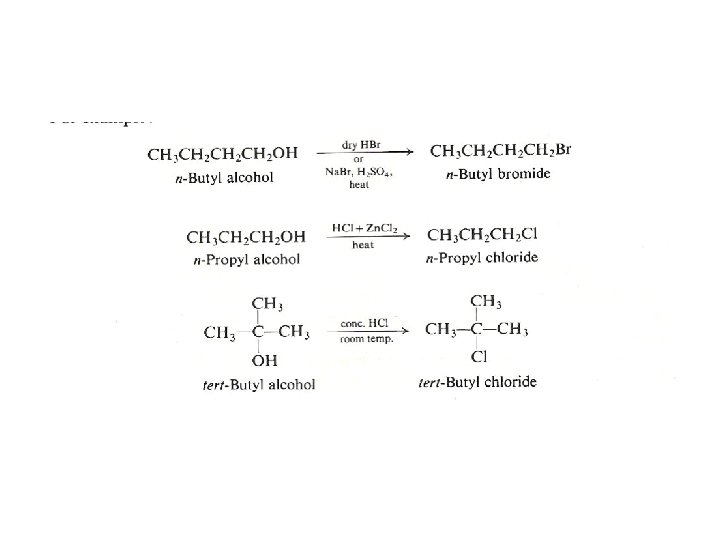
Reaction of Alcohols with Hydrogen Halides The general reaction looks like this: A tertiary alcohol reacts if it is shaken with concentrated hydrochloric acid at room temperature. This reaction occurs by SN 1 mechanism, so the reaction rate is almost the same with HCl, HBr or HI, since the addition of the halide nucleophile occurs in the second fast step.


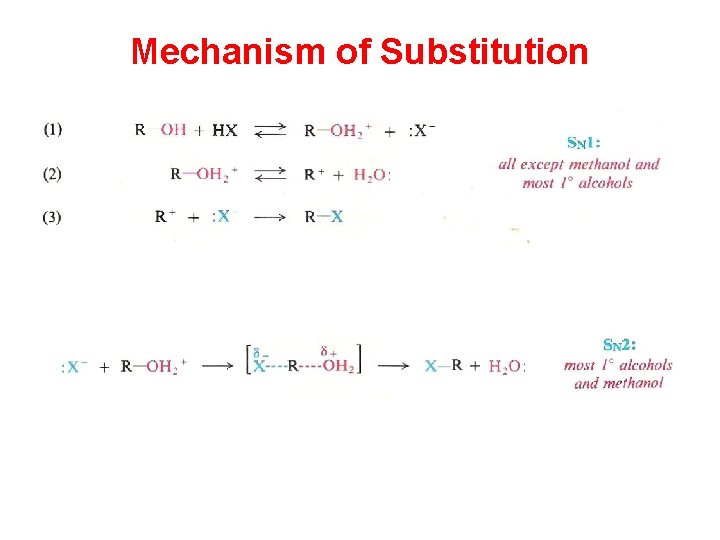
Mechanism of Substitution

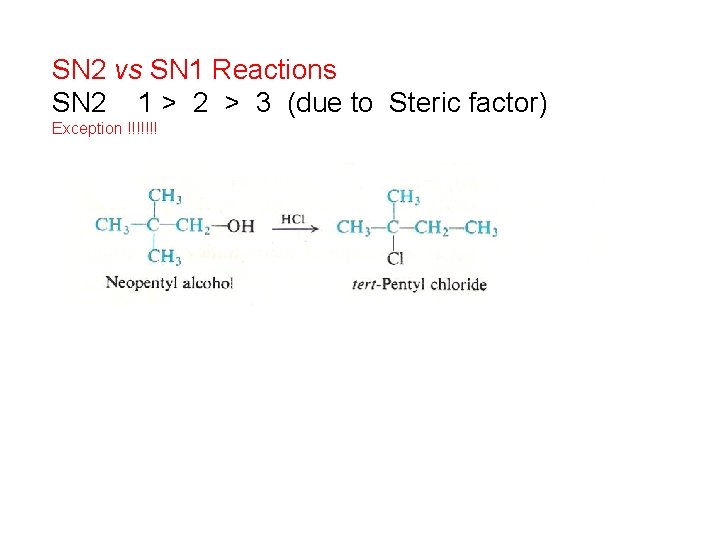
SN 2 vs SN 1 Reactions SN 2 1 > 2 > 3 (due to Steric factor) Exception !!!!!!!

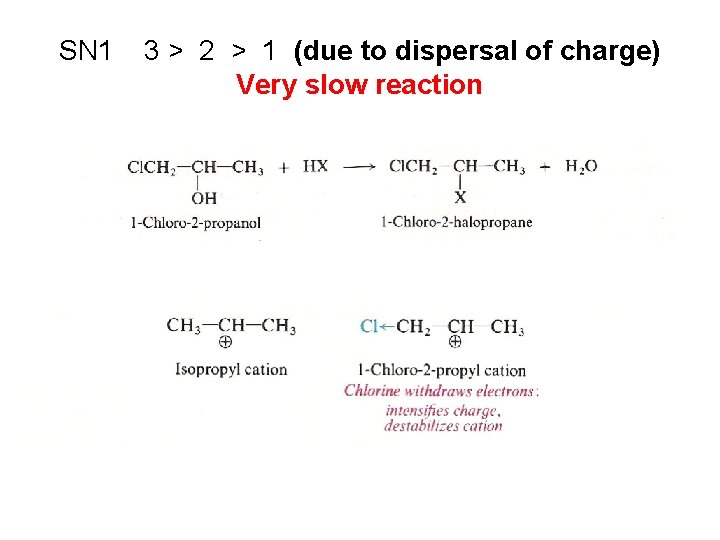
SN 1 3 > 2 > 1 (due to dispersal of charge) Very slow reaction

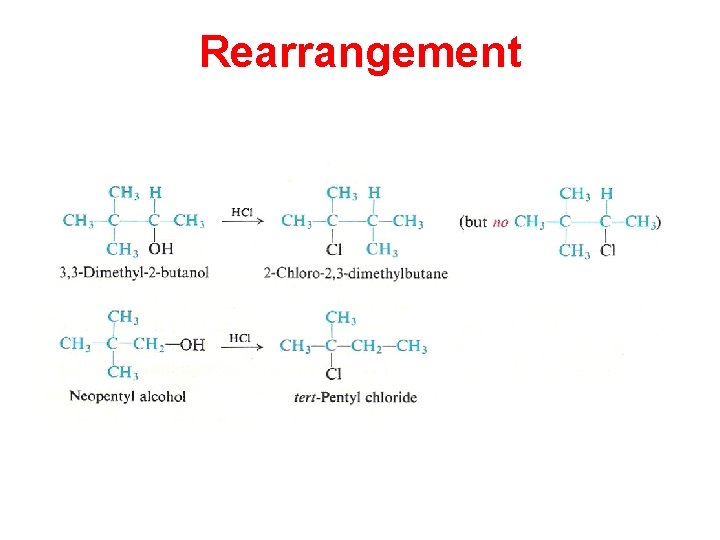
Rearrangement

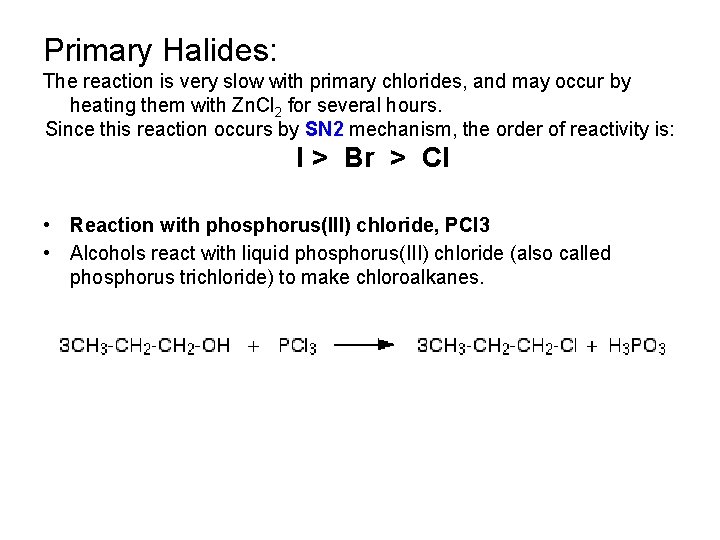
Primary Halides: The reaction is very slow with primary chlorides, and may occur by heating them with Zn. Cl 2 for several hours. Since this reaction occurs by SN 2 mechanism, the order of reactivity is: I > Br > Cl • Reaction with phosphorus(III) chloride, PCl 3 • Alcohols react with liquid phosphorus(III) chloride (also called phosphorus trichloride) to make chloroalkanes.

Reacting alcohols with sulphur dichloride oxide (thionyl chloride) • The reaction • Sulphur dichloride oxide (thionyl chloride) has the formula SOCl 2. • The two other products of the reaction (sulphur dioxide and HCl) are both gases. That means that they separate themselves from the reaction mixture. • Hydrogen halides, phosphorous halides or thionyl halides cannot replace the hydroxyl group of phenols by halogens

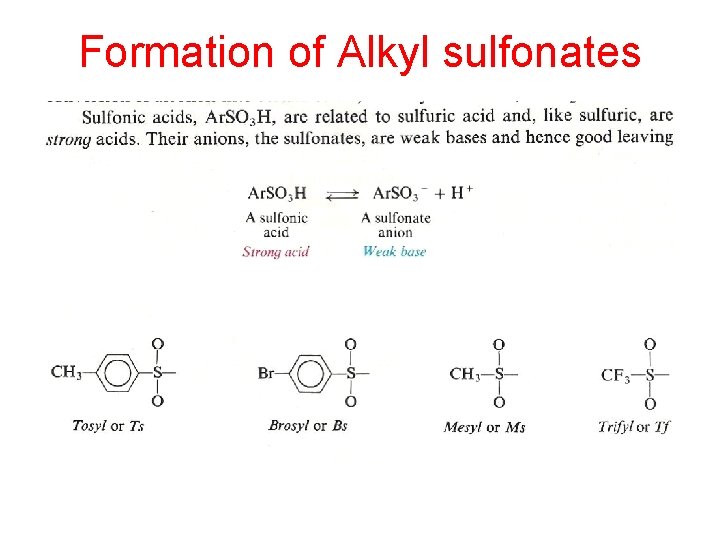
Formation of Alkyl sulfonates

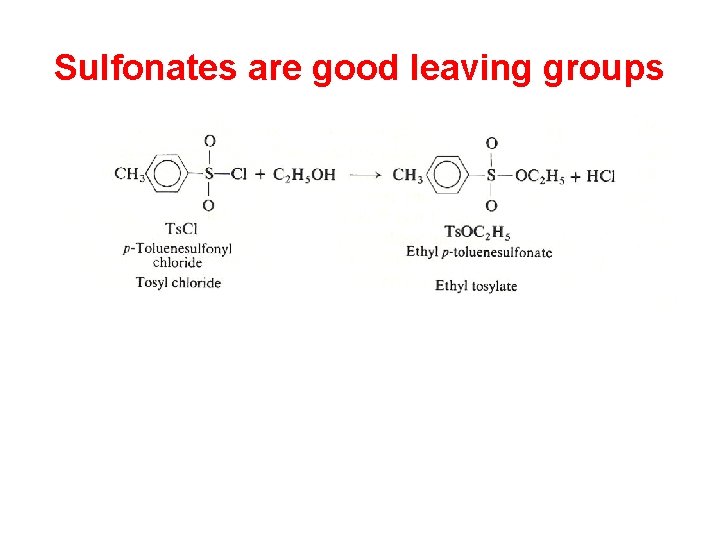
Sulfonates are good leaving groups

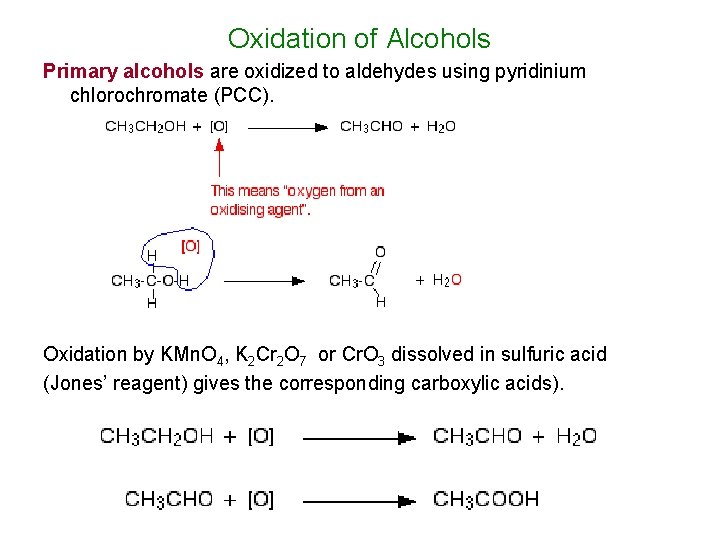
Oxidation of Alcohols Primary alcohols are oxidized to aldehydes using pyridinium chlorochromate (PCC). Oxidation by KMn. O 4, K 2 Cr 2 O 7 or Cr. O 3 dissolved in sulfuric acid (Jones’ reagent) gives the corresponding carboxylic acids).

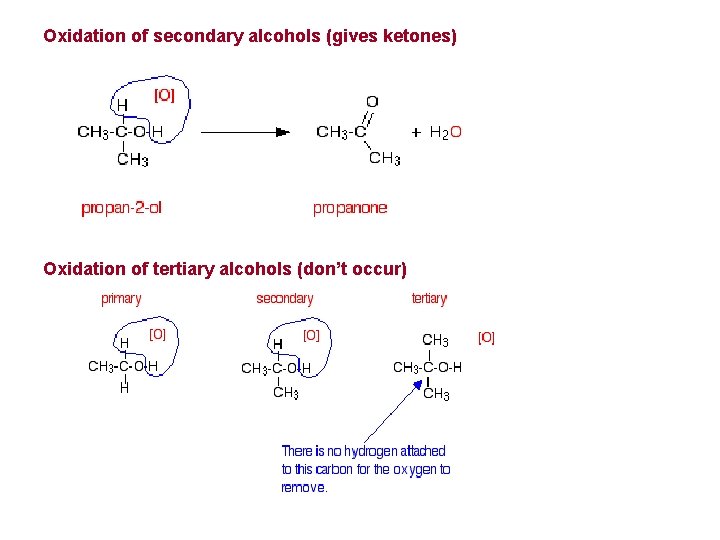
Oxidation of secondary alcohols (gives ketones) Oxidation of tertiary alcohols (don’t occur)

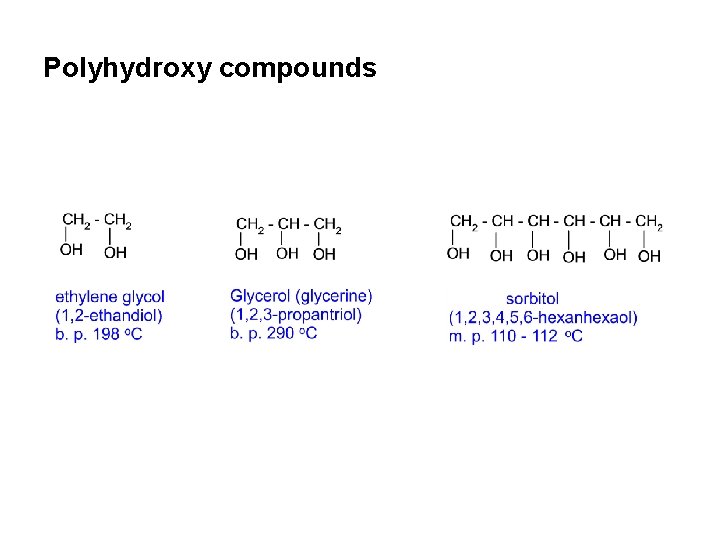
Polyhydroxy compounds

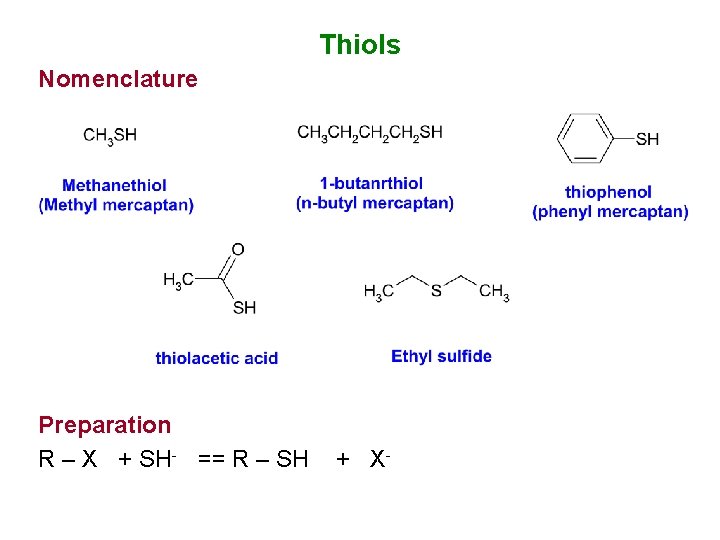
Thiols Nomenclature Preparation R – X + SH- == R – SH + X-

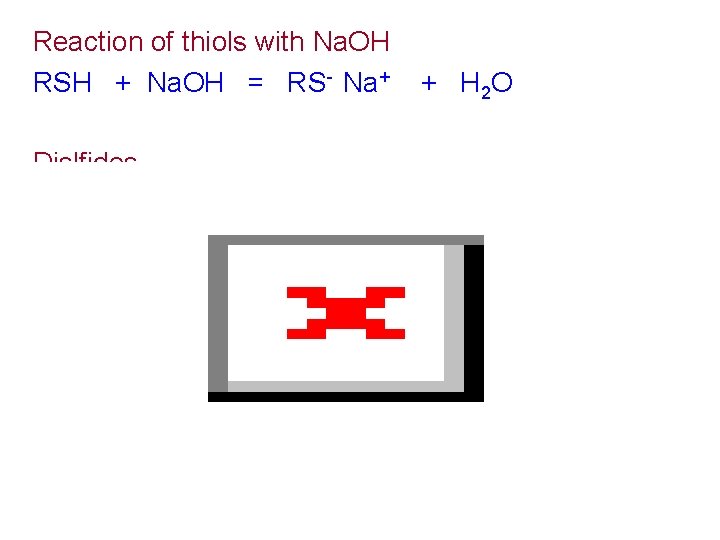
Reaction of thiols with Na. OH RSH + Na. OH = RS- Na+ Dislfides + H 2 O







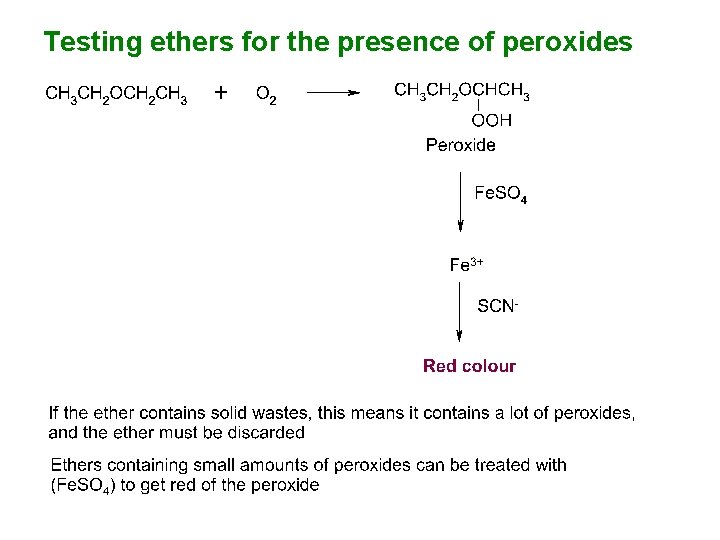
Testing ethers for the presence of peroxides

The Grignard Reagent

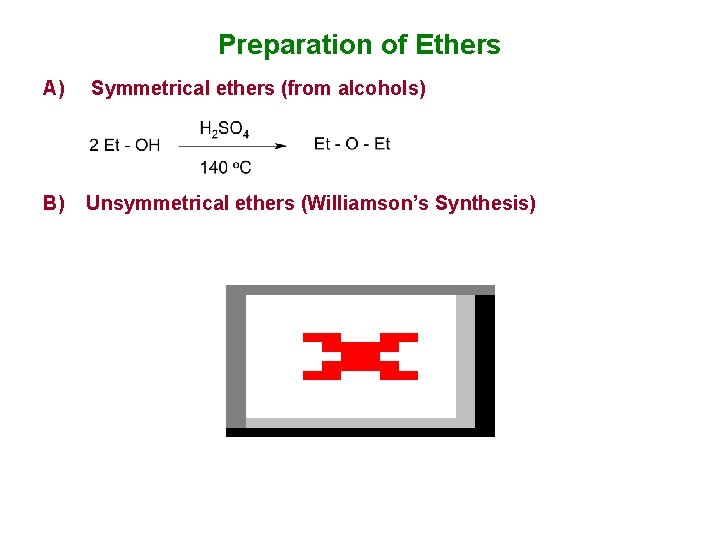
Preparation of Ethers A) Symmetrical ethers (from alcohols) B) Unsymmetrical ethers (Williamson’s Synthesis)

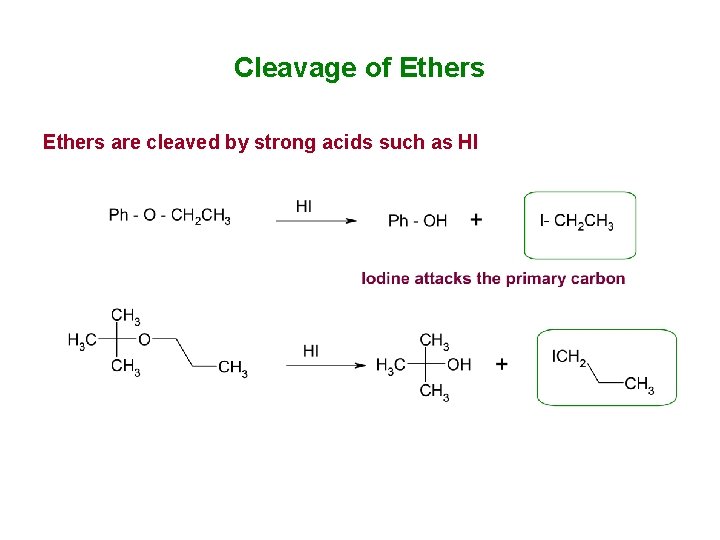
Cleavage of Ethers are cleaved by strong acids such as HI

Epoxides (Oxiranes) Cyclic ethers with a three-membered rings

Reactions of Epoxides When attacked by nucleophiles, epoxides undergo acid catalized ring opening.

Cyclic Ethers