Organic Chemistry 5 th Edition L G Wade













- Slides: 50

Organic Chemistry, 5 th Edition L. G. Wade, Jr. Chapter 10 Structure and Synthesis of Alcohols Jo Blackburn Richland College, Dallas, TX Dallas County Community College District Chapter 10 ã 2003, Prentice Hall

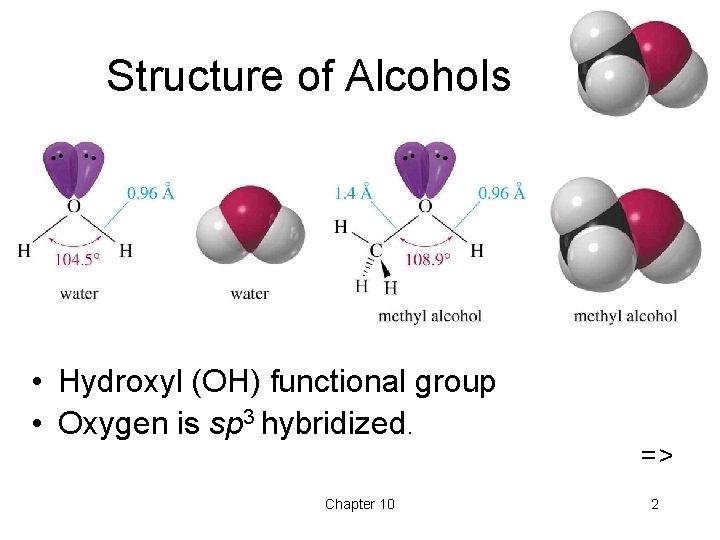
Structure of Alcohols • Hydroxyl (OH) functional group • Oxygen is sp 3 hybridized. Chapter 10 => 2

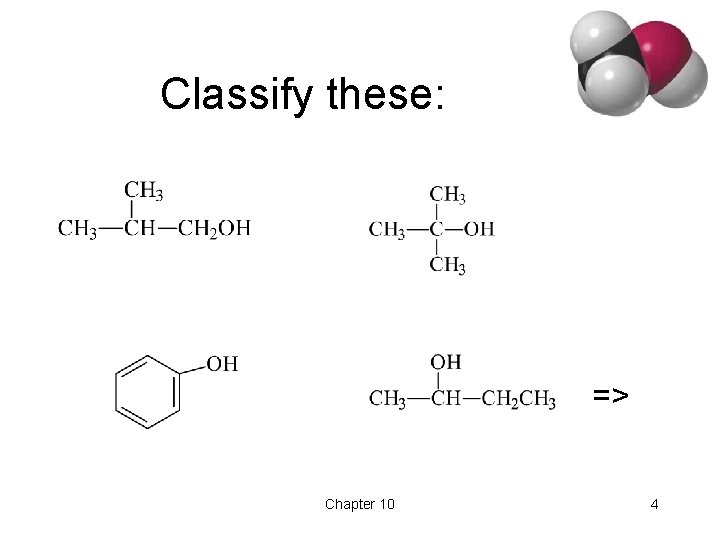
Classification • Primary: carbon with –OH is bonded to one other carbon. • Secondary: carbon with –OH is bonded to two other carbons. • Tertiary: carbon with –OH is bonded to three other carbons. • Aromatic (phenol): -OH is bonded to a benzene ring. => Chapter 10 3

Classify these: => Chapter 10 4

IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the -OH group. • Drop the -e from the alkane name, add ol. • Number the chain, starting from the end closest to the -OH group. • Number and name all substituents. => Chapter 10 5

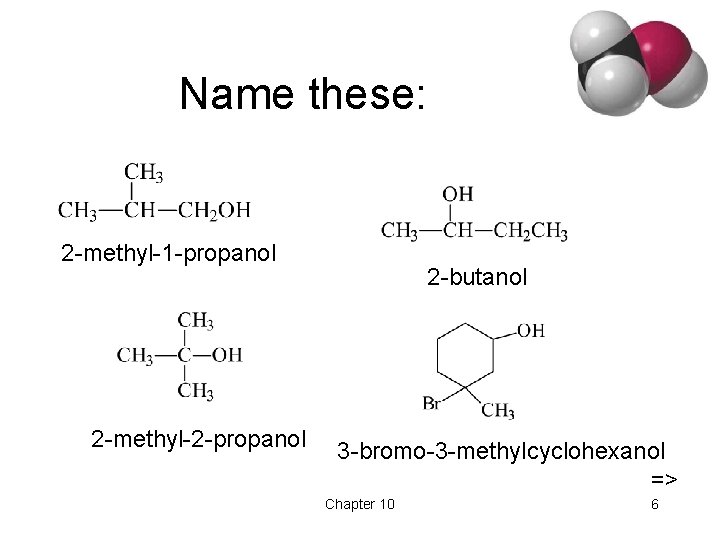
Name these: 2 -methyl-1 -propanol 2 -methyl-2 -propanol 2 -butanol 3 -bromo-3 -methylcyclohexanol => Chapter 10 6

Unsaturated Alcohols • Hydroxyl group takes precedence. Assign that carbon the lowest number. • Use alkene or alkyne name. 4 -penten-2 -ol (old) pent-4 -ene-2 -ol (1997 revision of IUPAC rules) => Chapter 10 7

Naming Priority • • • Acids Esters Aldehydes Ketones Alcohols Amines • • • Alkenes Alkynes Alkanes Ethers Halides => Chapter 10 8

Hydroxy Substituent • When -OH is part of a higher priority class of compound, it is named as hydroxy. • Example: also known as GHB 4 -hydroxybutanoic acid => Chapter 10 9

Common Names • Alcohol can be named as alkyl alcohol. • Useful only for small alkyl groups. • Examples: isobutyl alcohol sec-butyl alcohol => Chapter 10 10

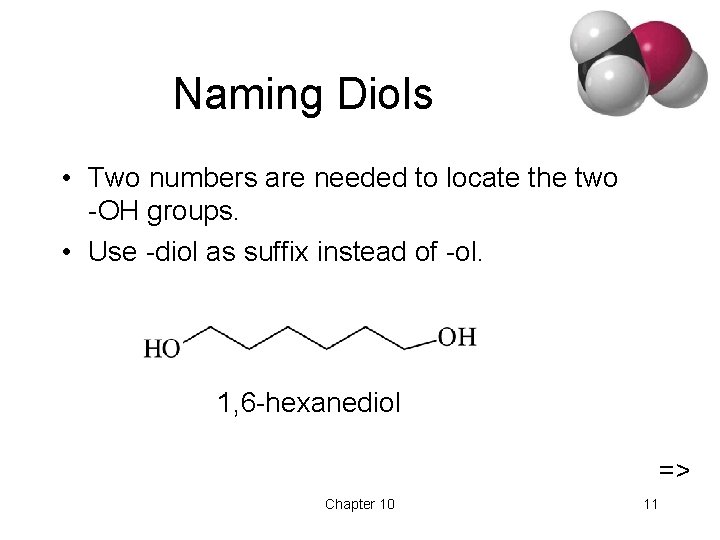
Naming Diols • Two numbers are needed to locate the two -OH groups. • Use -diol as suffix instead of -ol. 1, 6 -hexanediol => Chapter 10 11

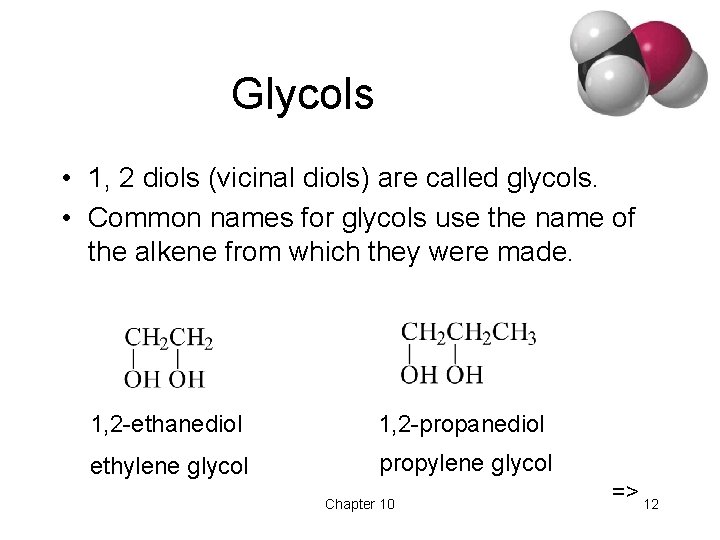
Glycols • 1, 2 diols (vicinal diols) are called glycols. • Common names for glycols use the name of the alkene from which they were made. 1, 2 -ethanediol 1, 2 -propanediol ethylene glycol propylene glycol Chapter 10 => 12

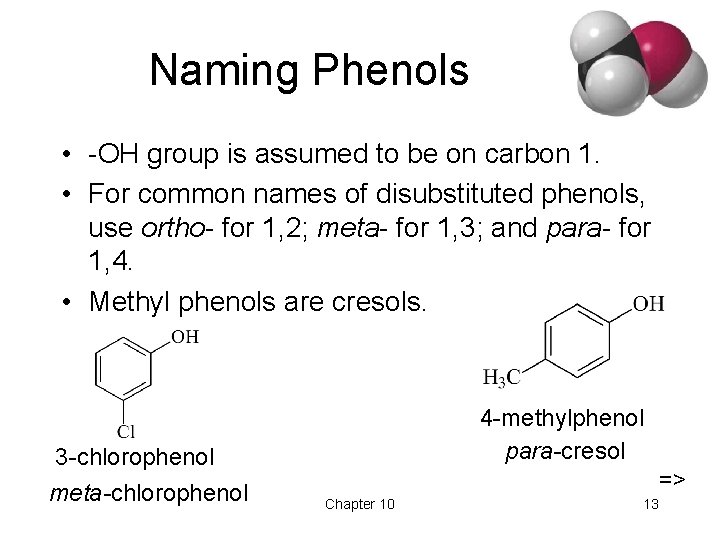
Naming Phenols • -OH group is assumed to be on carbon 1. • For common names of disubstituted phenols, use ortho- for 1, 2; meta- for 1, 3; and para- for 1, 4. • Methyl phenols are cresols. 3 -chlorophenol meta-chlorophenol 4 -methylphenol para-cresol => Chapter 10 13

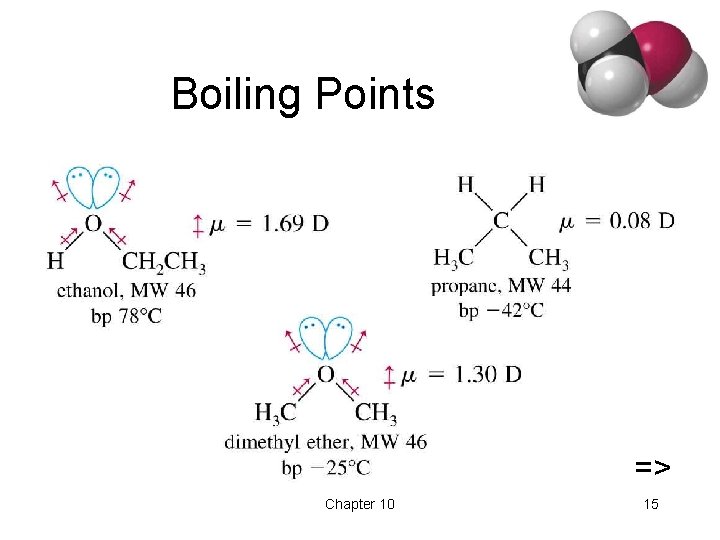
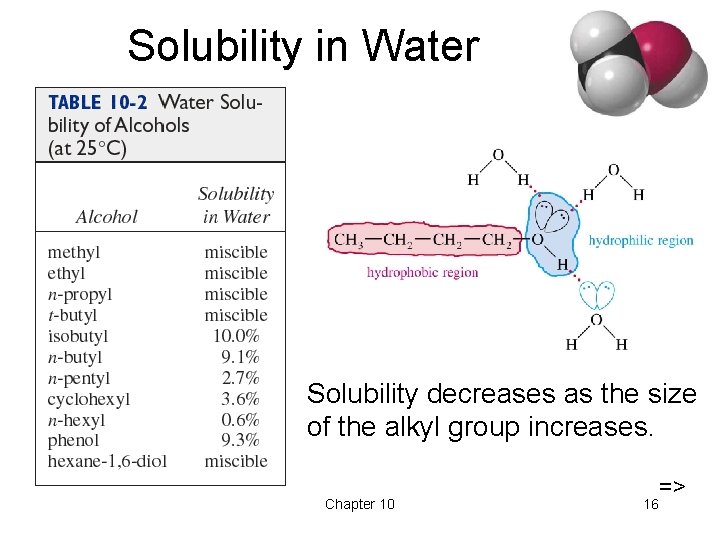
Physical Properties • Unusually high boiling points due to hydrogen bonding between molecules. • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases. => Chapter 10 14

Boiling Points => Chapter 10 15

Solubility in Water Solubility decreases as the size of the alkyl group increases. Chapter 10 16 =>

Methanol • • “Wood alcohol” Industrial production from synthesis gas Common industrial solvent Fuel at Indianapolis 500 ØFire can be extinguished with water ØHigh octane rating ØLow emissions ØBut, lower energy content ØInvisible flame Chapter 10 => 17

Ethanol • • Fermentation of sugar and starches in grains 12 -15% alcohol, then yeast cells die. Distillation produces “hard” liquors Azeotrope: 95% ethanol, constant boiling Denatured alcohol used as solvent Gasahol: 10% ethanol in gasoline Toxic dose: 200 m. L ethanol, 100 m. L methanol => Chapter 10 18

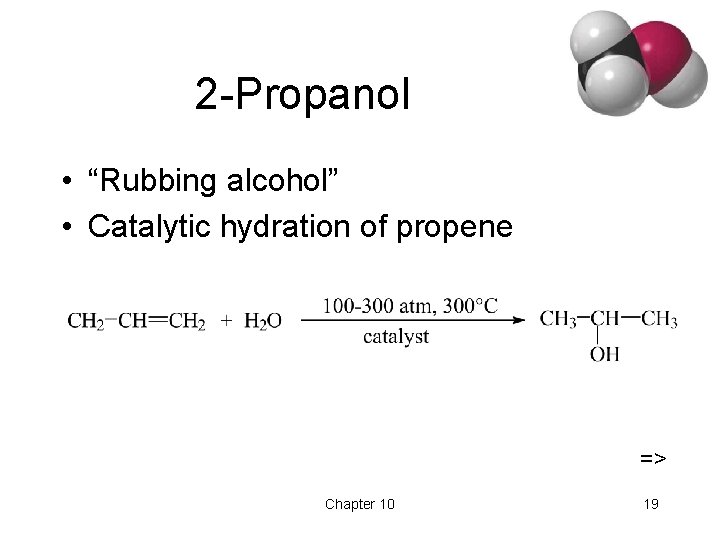
2 -Propanol • “Rubbing alcohol” • Catalytic hydration of propene => Chapter 10 19

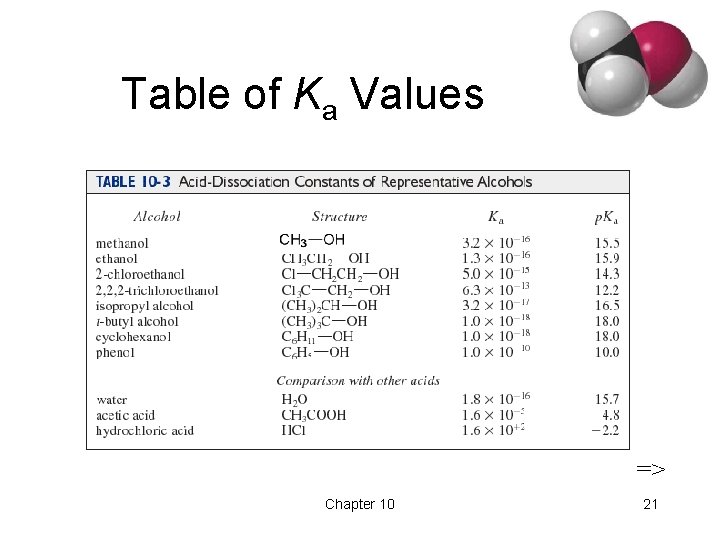
Acidity of Alcohols • p. Ka range: 15. 5 -18. 0 (water: 15. 7) • Acidity decreases as alkyl group increases. • Halogens increase the acidity. • Phenol is 100 million times more acidic than cyclohexanol! => Chapter 10 20

Table of Ka Values => Chapter 10 21

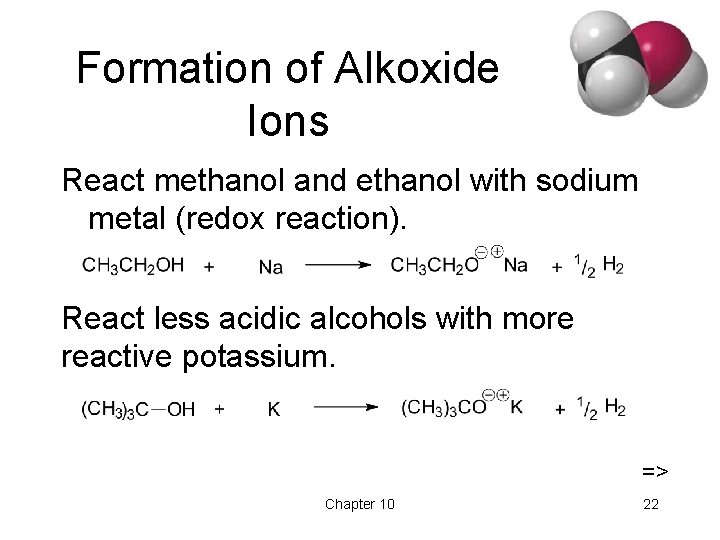
Formation of Alkoxide Ions React methanol and ethanol with sodium metal (redox reaction). React less acidic alcohols with more reactive potassium. => Chapter 10 22

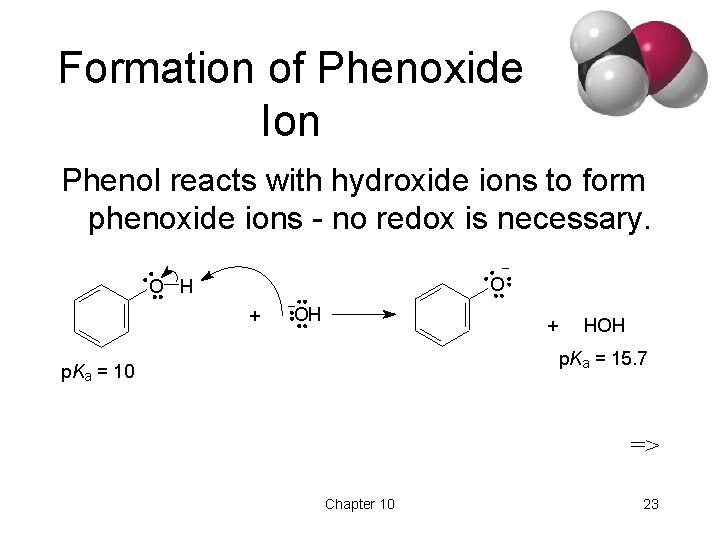
Formation of Phenoxide Ion Phenol reacts with hydroxide ions to form phenoxide ions - no redox is necessary. O O H + OH + HOH p. Ka = 15. 7 p. Ka = 10 => Chapter 10 23

Synthesis (Review) • Nucleophilic substitution of OH- on alkyl halide • Hydration of alkenes Øwater in acid solution (not very effective) Øoxymercuration - demercuration Øhydroboration - oxidation => Chapter 10 24

Glycols (Review) • Syn hydroxylation of alkenes Øosmium tetroxide, hydrogen peroxide Øcold, dilute, basic potassium permanganate • Anti hydroxylation of alkenes Øperoxyacids, hydrolysis => Chapter 10 25

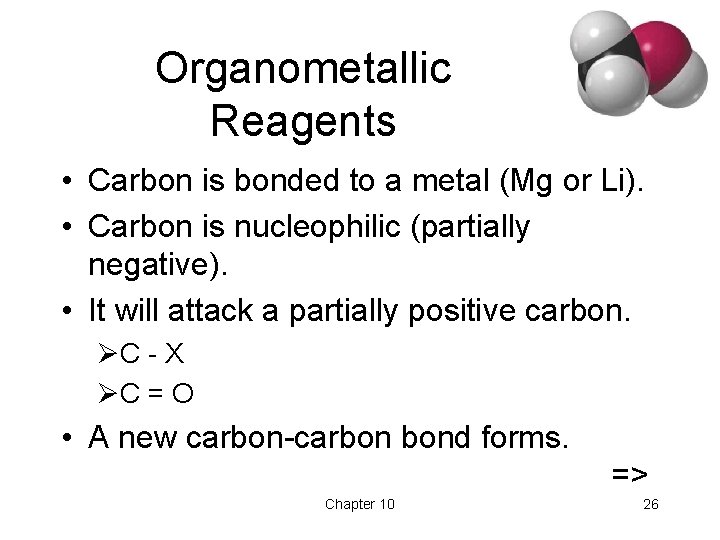
Organometallic Reagents • Carbon is bonded to a metal (Mg or Li). • Carbon is nucleophilic (partially negative). • It will attack a partially positive carbon. ØC - X ØC = O • A new carbon-carbon bond forms. => Chapter 10 26

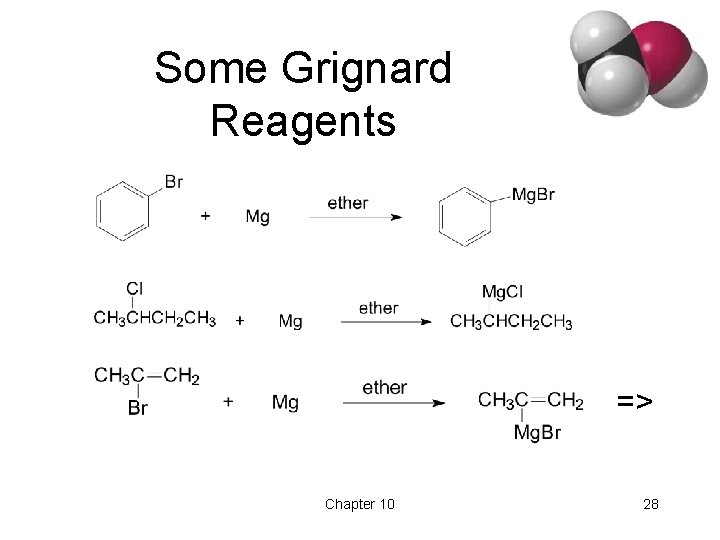
Grignard Reagents • • Formula R-Mg-X (reacts like R: - +Mg. X) Stabilized by anhydrous ether Iodides most reactive May be formed from any halide Øprimary Øsecondary Øtertiary Øvinyl Øaryl => Chapter 10 27

Some Grignard Reagents => Chapter 10 28

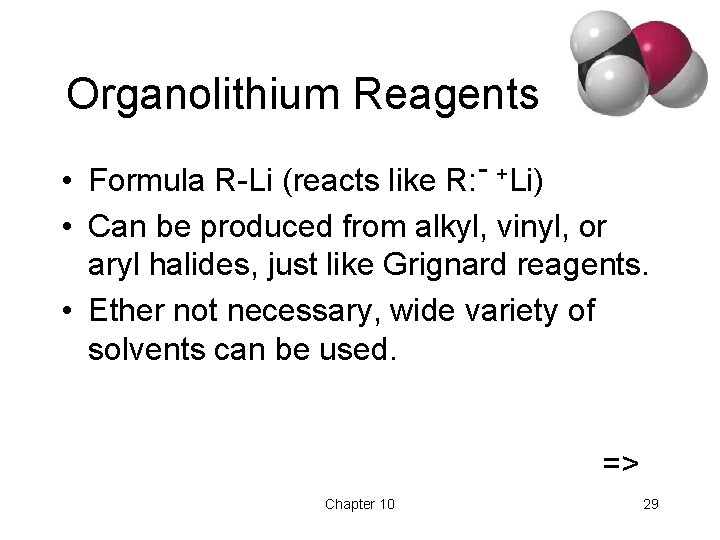
Organolithium Reagents • Formula R-Li (reacts like R: - +Li) • Can be produced from alkyl, vinyl, or aryl halides, just like Grignard reagents. • Ether not necessary, wide variety of solvents can be used. => Chapter 10 29

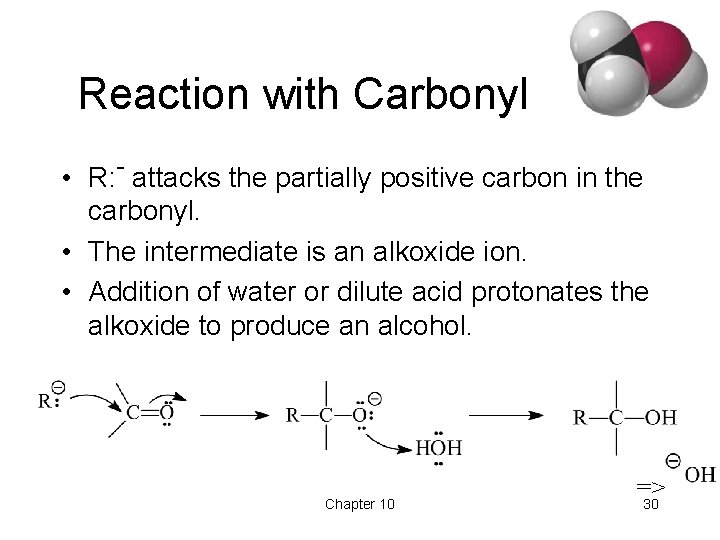
Reaction with Carbonyl • R: - attacks the partially positive carbon in the carbonyl. • The intermediate is an alkoxide ion. • Addition of water or dilute acid protonates the alkoxide to produce an alcohol. Chapter 10 => 30

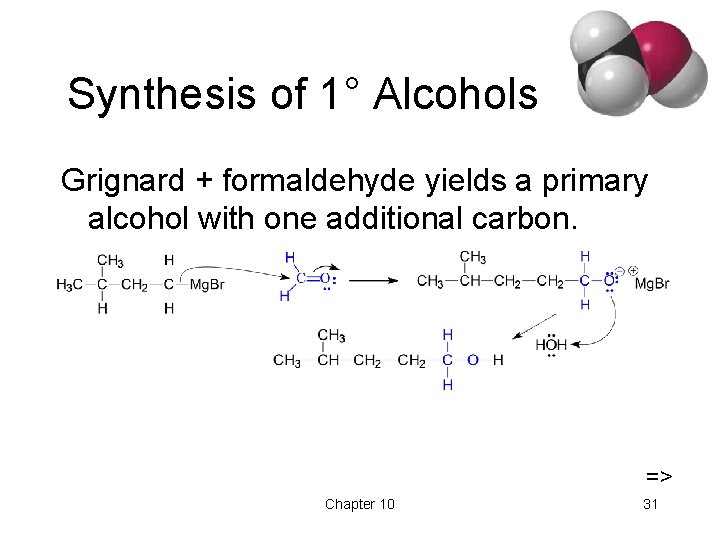
Synthesis of 1° Alcohols Grignard + formaldehyde yields a primary alcohol with one additional carbon. => Chapter 10 31

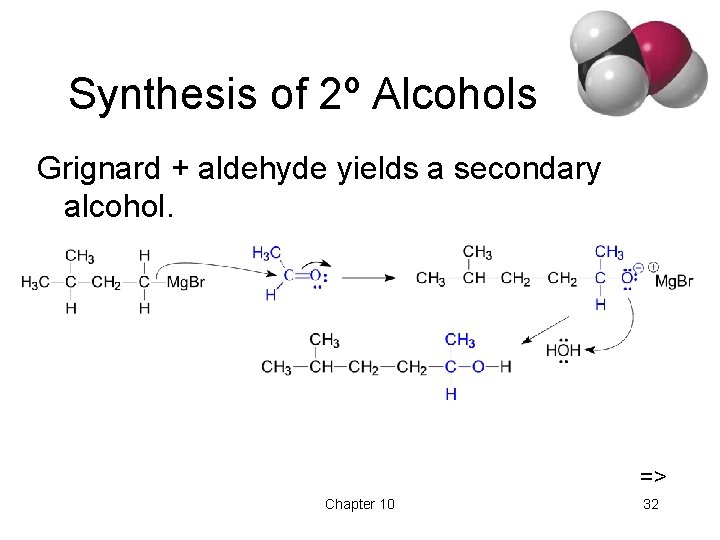
Synthesis of 2º Alcohols Grignard + aldehyde yields a secondary alcohol. => Chapter 10 32

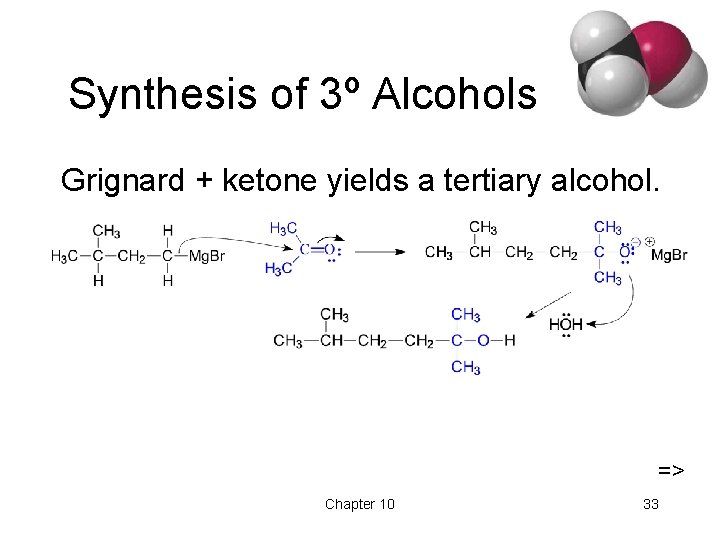
Synthesis of 3º Alcohols Grignard + ketone yields a tertiary alcohol. => Chapter 10 33

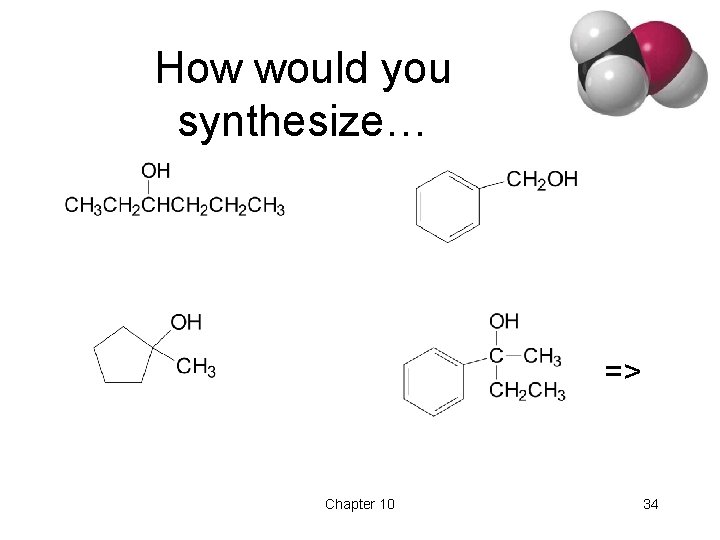
How would you synthesize… => Chapter 10 34

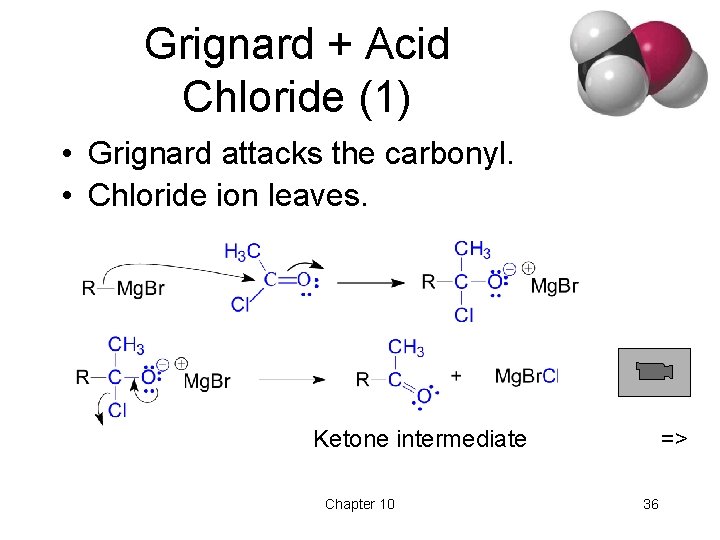
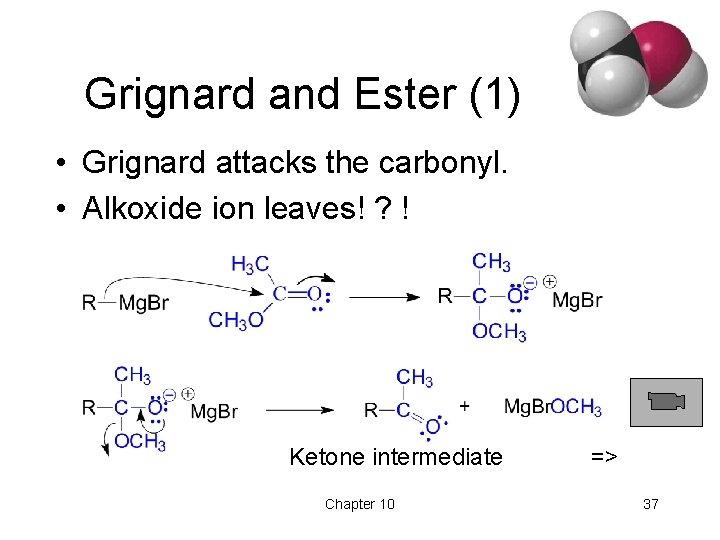
Grignard Reactions with Acid Chlorides and Esters • Use two moles of Grignard reagent. • The product is a tertiary alcohol with two identical alkyl groups. • Reaction with one mole of Grignard reagent produces a ketone intermediate, which reacts with the second mole of Grignard reagent. => Chapter 10 35

Grignard + Acid Chloride (1) • Grignard attacks the carbonyl. • Chloride ion leaves. Ketone intermediate Chapter 10 => 36

Grignard and Ester (1) • Grignard attacks the carbonyl. • Alkoxide ion leaves! ? ! Ketone intermediate Chapter 10 => 37

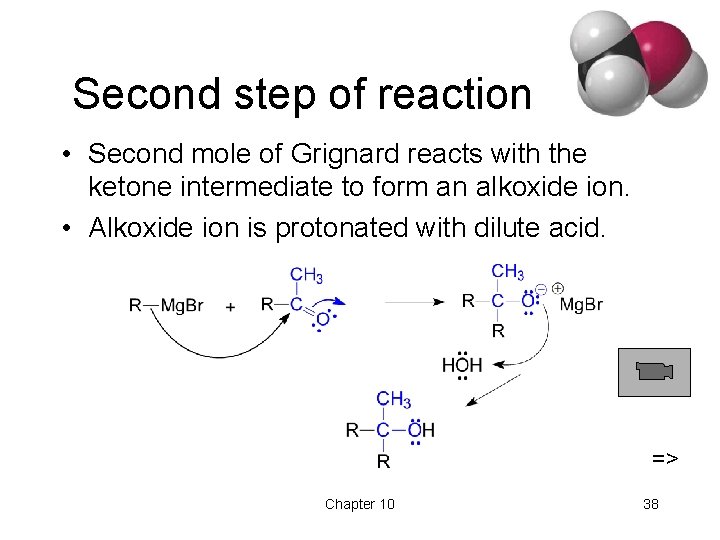
Second step of reaction • Second mole of Grignard reacts with the ketone intermediate to form an alkoxide ion. • Alkoxide ion is protonated with dilute acid. => Chapter 10 38

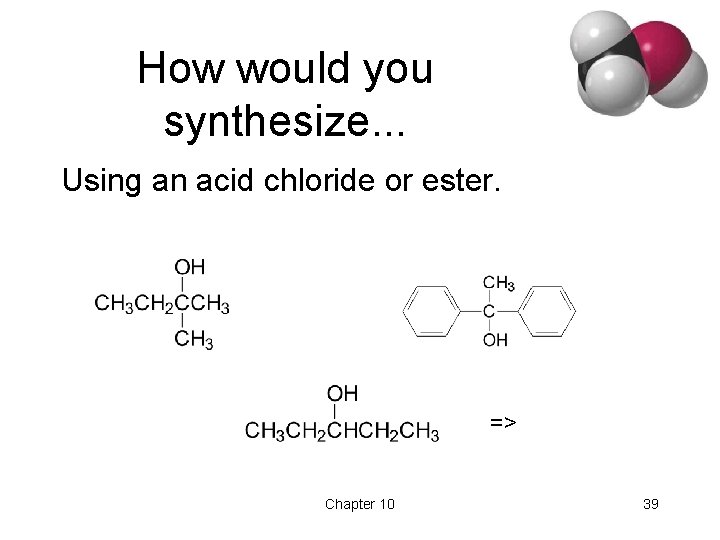
How would you synthesize. . . Using an acid chloride or ester. => Chapter 10 39

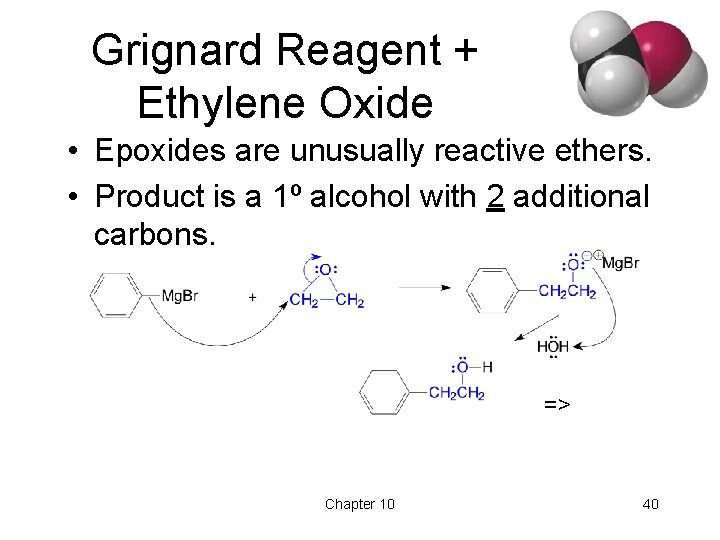
Grignard Reagent + Ethylene Oxide • Epoxides are unusually reactive ethers. • Product is a 1º alcohol with 2 additional carbons. => Chapter 10 40

Limitations of Grignard • No water or other acidic protons like O-H, N-H, S-H, or -C—C-H. Grignard reagent is destroyed, becomes an alkane. • No other electrophilic multiple bonds, like C=N, C—N, S=O, or N=O. => Chapter 10 41

Reduction of Carbonyl • Reduction of aldehyde yields 1º alcohol. • Reduction of ketone yields 2º alcohol. • Reagents: ØSodium borohydride, Na. BH 4 ØLithium aluminum hydride, Li. Al. H 4 ØRaney nickel => Chapter 10 42

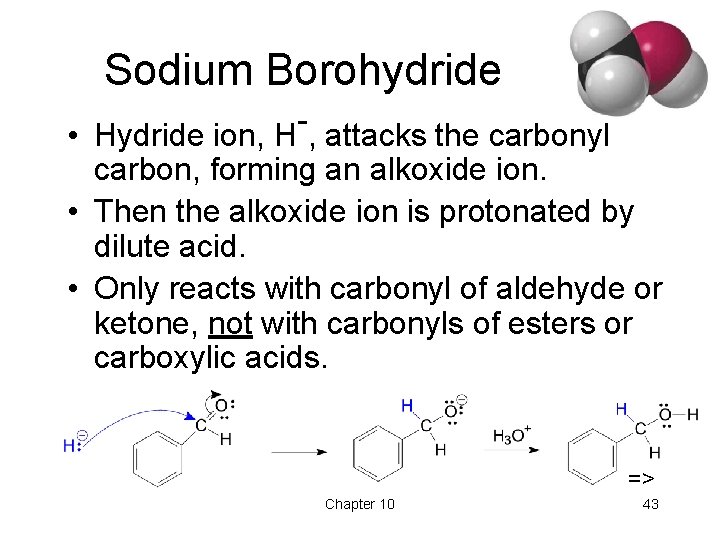
Sodium Borohydride • Hydride ion, H , attacks the carbonyl carbon, forming an alkoxide ion. • Then the alkoxide ion is protonated by dilute acid. • Only reacts with carbonyl of aldehyde or ketone, not with carbonyls of esters or carboxylic acids. => Chapter 10 43

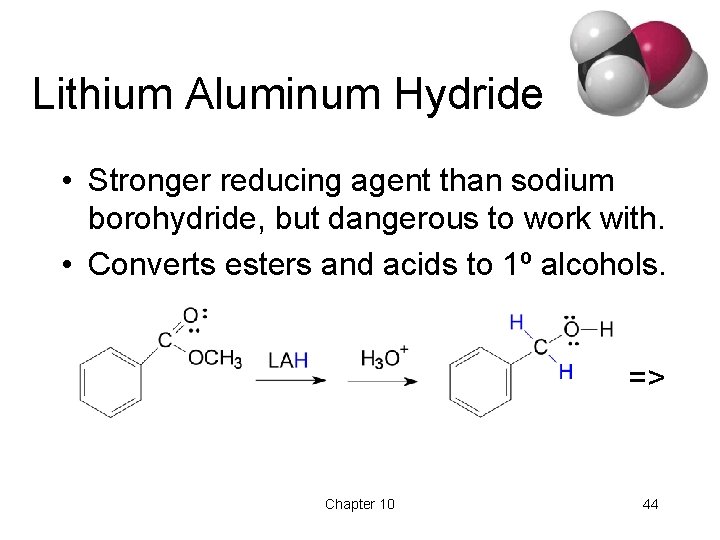
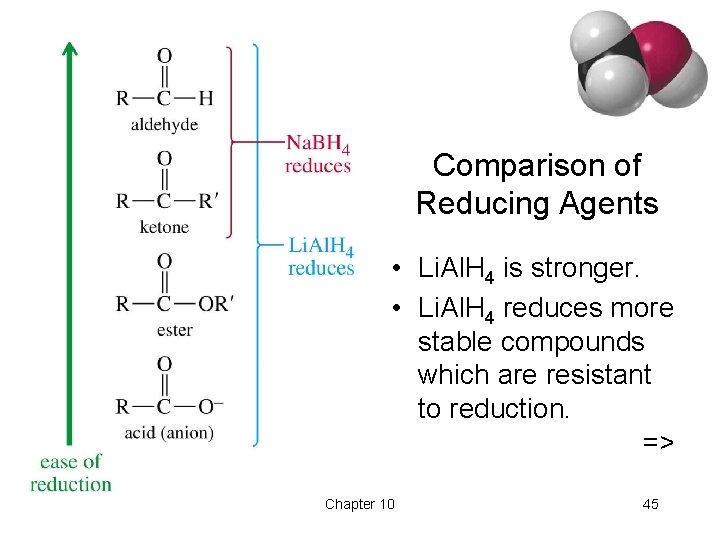
Lithium Aluminum Hydride • Stronger reducing agent than sodium borohydride, but dangerous to work with. • Converts esters and acids to 1º alcohols. => Chapter 10 44

Comparison of Reducing Agents • Li. Al. H 4 is stronger. • Li. Al. H 4 reduces more stable compounds which are resistant to reduction. => Chapter 10 45

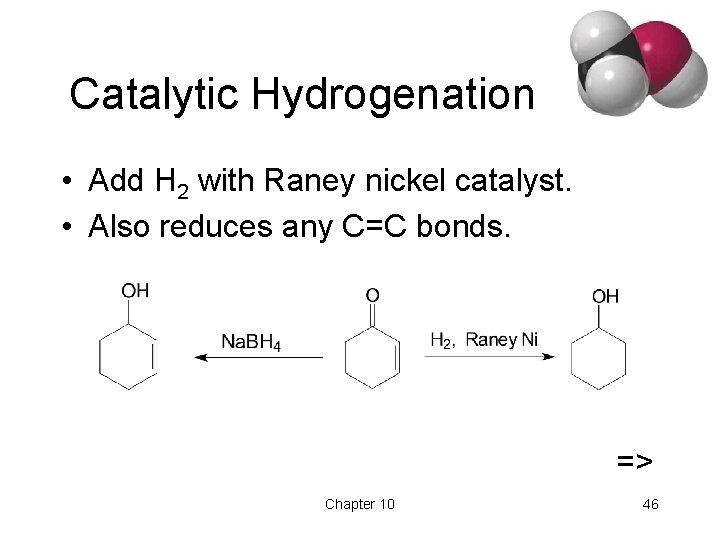
Catalytic Hydrogenation • Add H 2 with Raney nickel catalyst. • Also reduces any C=C bonds. => Chapter 10 46

Thiols (Mercaptans) • • • Sulfur analogues of alcohols, -SH. Named by adding -thiol to alkane name. The -SH group is called mercapto. Complex with heavy metals: Hg, As, Au. More acidic than alcohols, react with Na. OH to form thiolate ion. • Stinks! => Chapter 10 47

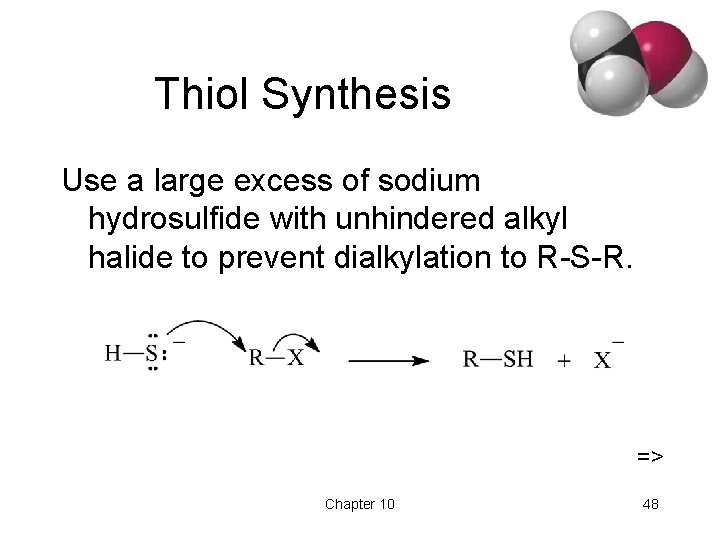
Thiol Synthesis Use a large excess of sodium hydrosulfide with unhindered alkyl halide to prevent dialkylation to R-S-R. => Chapter 10 48

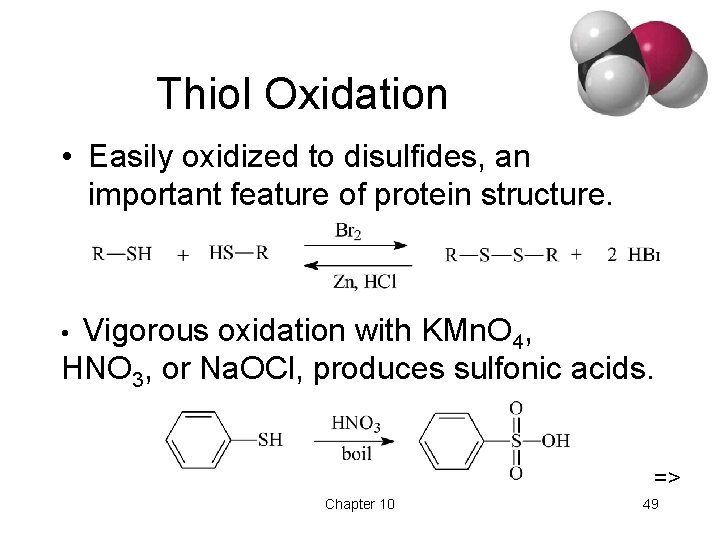
Thiol Oxidation • Easily oxidized to disulfides, an important feature of protein structure. Vigorous oxidation with KMn. O 4, HNO 3, or Na. OCl, produces sulfonic acids. • => Chapter 10 49

End of Chapter 10 50