Organic Chemistry 4 th Edition Paula Yurkanis Bruice
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 23 Amino Acids, Peptides, and Proteins Irene Lee Case Western Reserve University Cleveland, OH © 2004, Prentice Hall
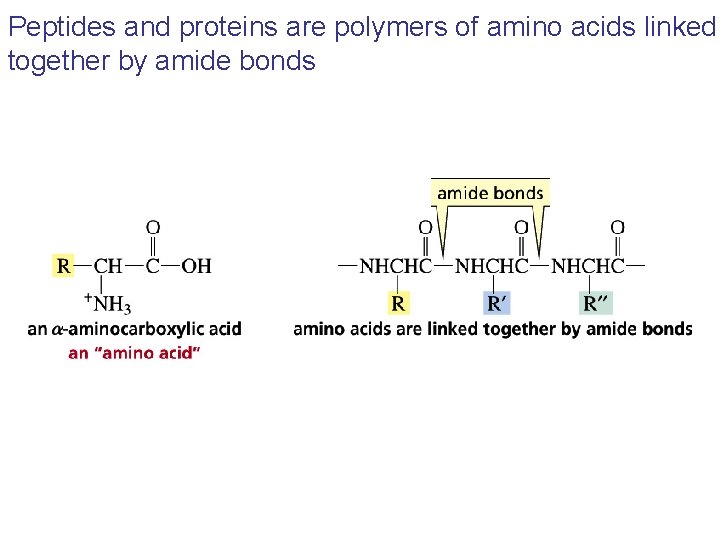
Peptides and proteins are polymers of amino acids linked together by amide bonds
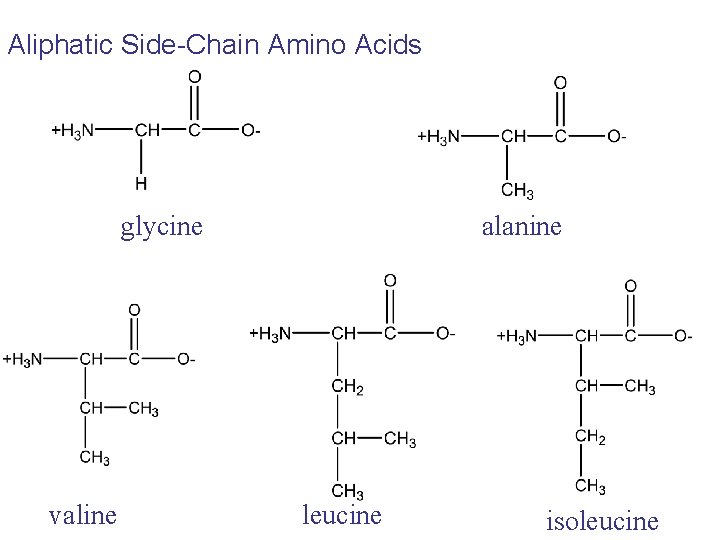
Aliphatic Side-Chain Amino Acids glycine valine alanine leucine isoleucine
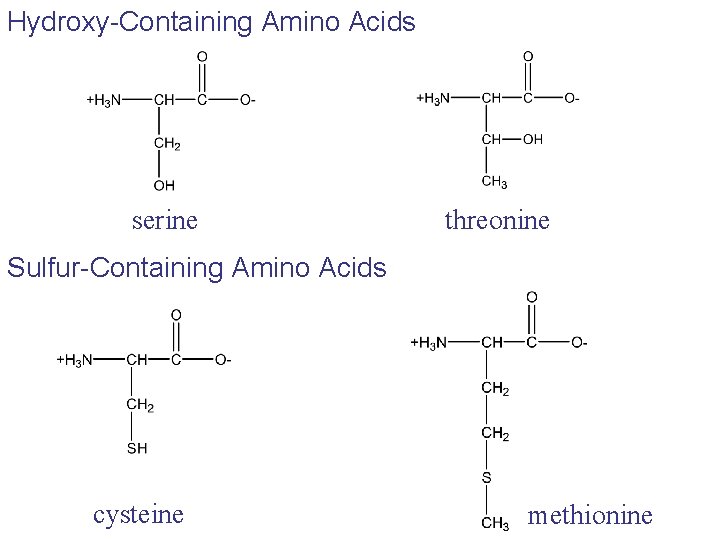
Hydroxy-Containing Amino Acids serine threonine Sulfur-Containing Amino Acids cysteine methionine
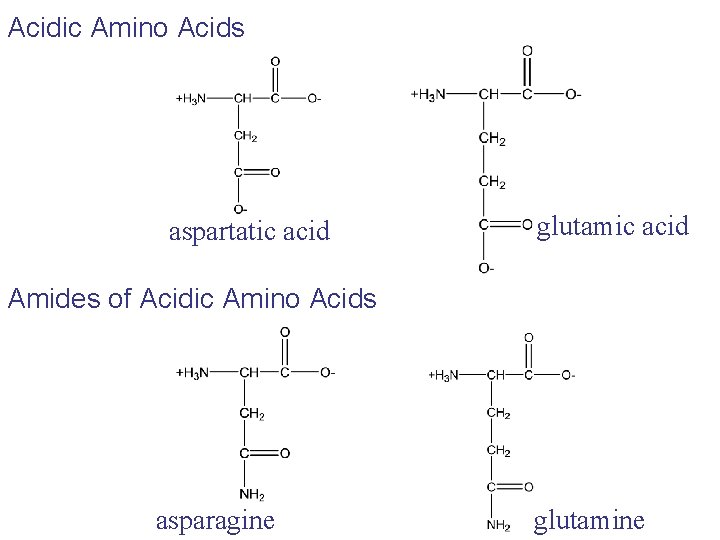
Acidic Amino Acids aspartatic acid glutamic acid Amides of Acidic Amino Acids asparagine glutamine
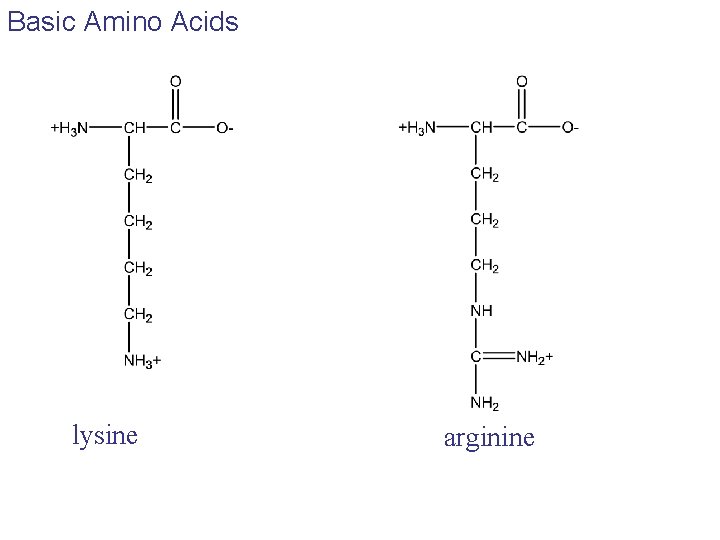
Basic Amino Acids lysine arginine
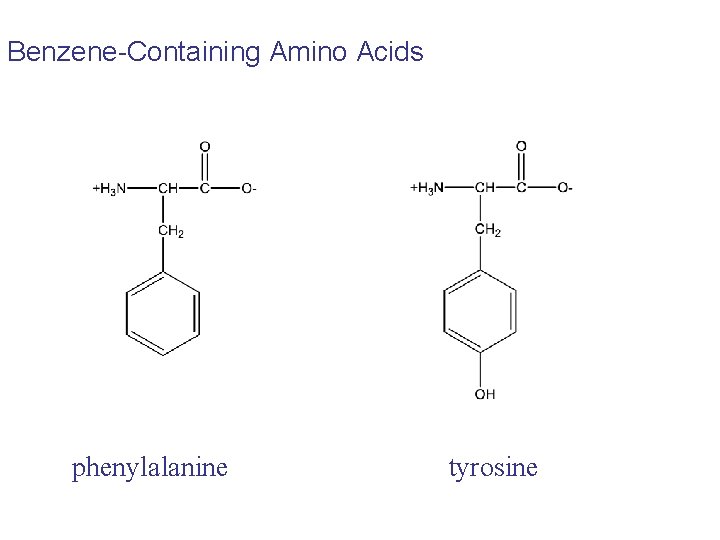
Benzene-Containing Amino Acids phenylalanine tyrosine
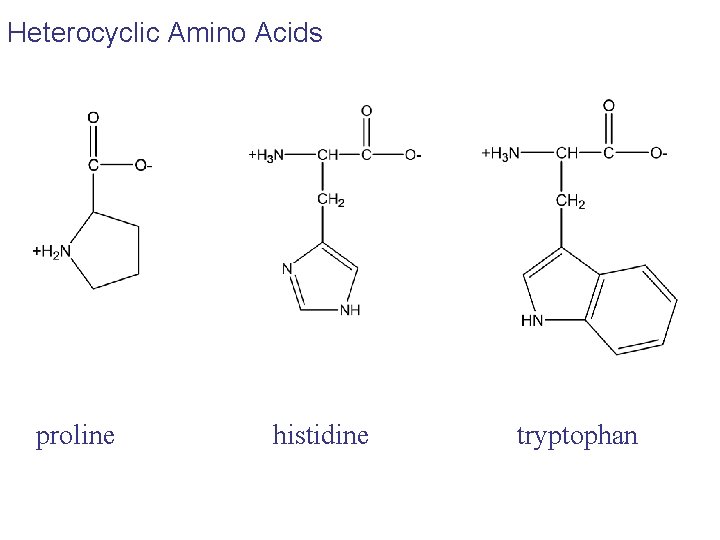
Heterocyclic Amino Acids proline histidine tryptophan
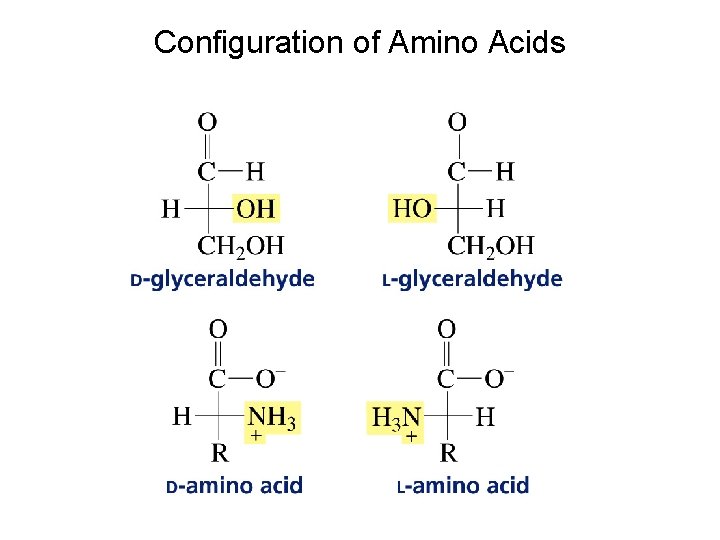
Configuration of Amino Acids
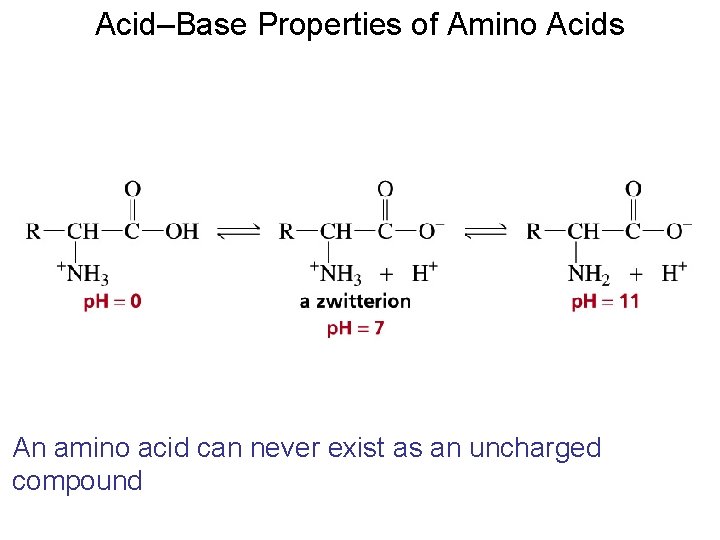
Acid–Base Properties of Amino Acids An amino acid can never exist as an uncharged compound
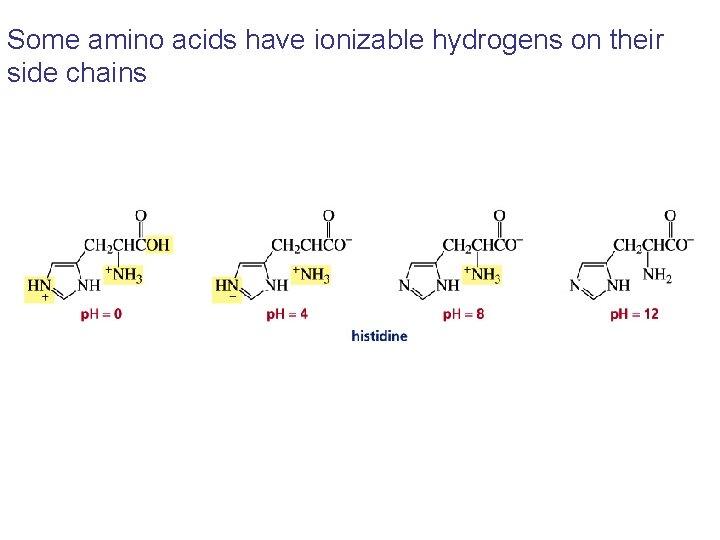
Some amino acids have ionizable hydrogens on their side chains
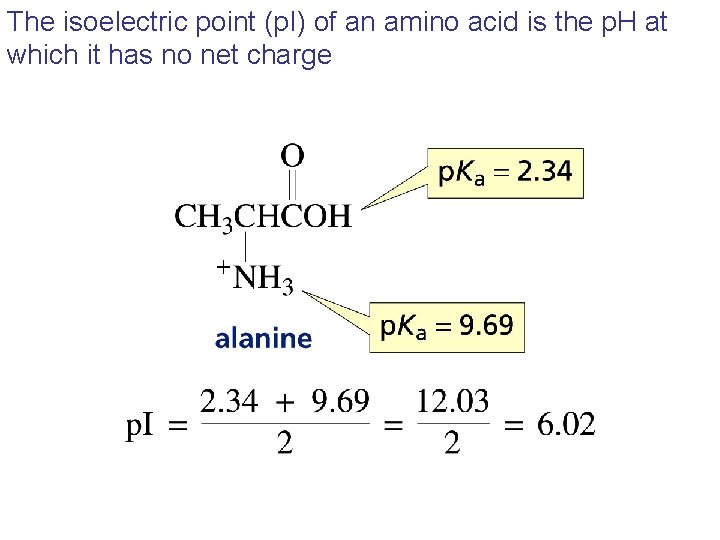
The isoelectric point (p. I) of an amino acid is the p. H at which it has no net charge
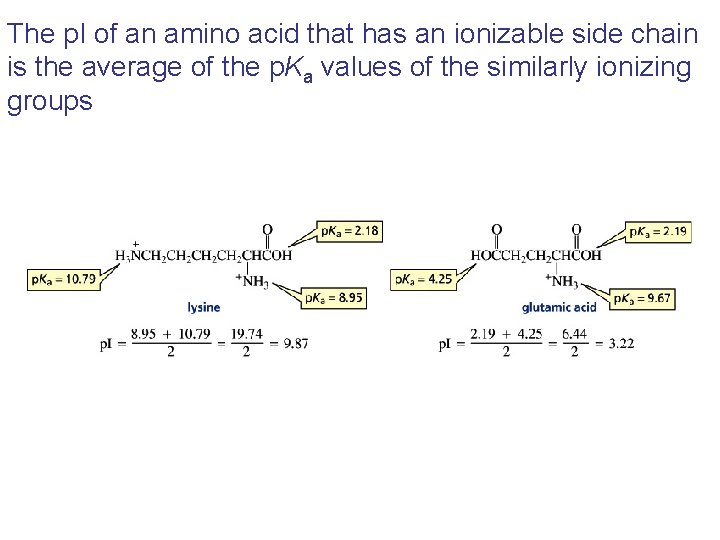
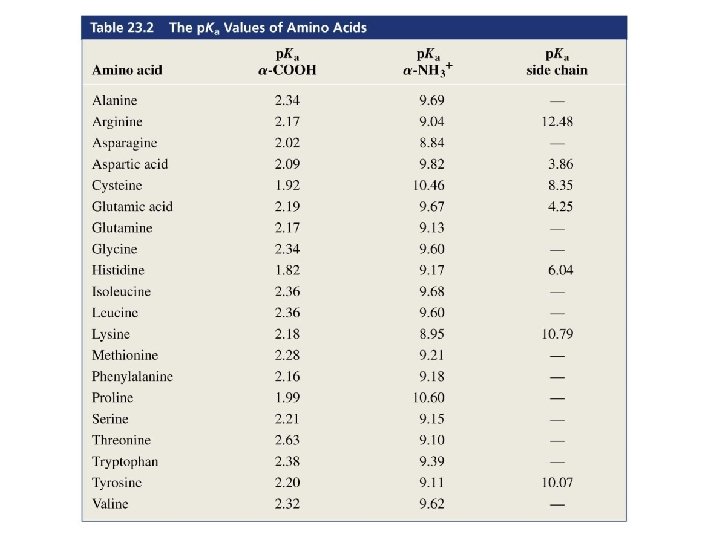
The p. I of an amino acid that has an ionizable side chain is the average of the p. Ka values of the similarly ionizing groups
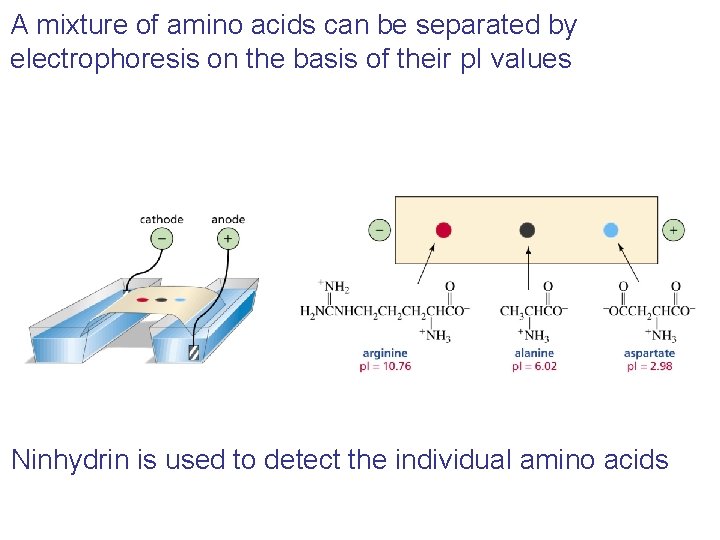
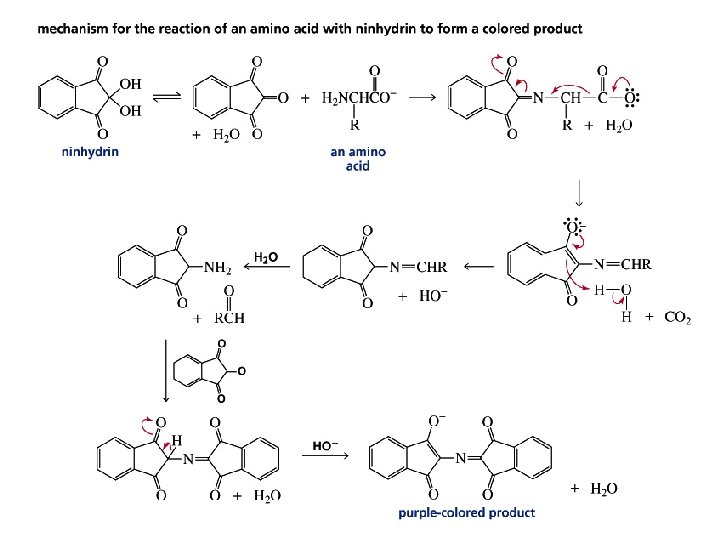
A mixture of amino acids can be separated by electrophoresis on the basis of their p. I values Ninhydrin is used to detect the individual amino acids
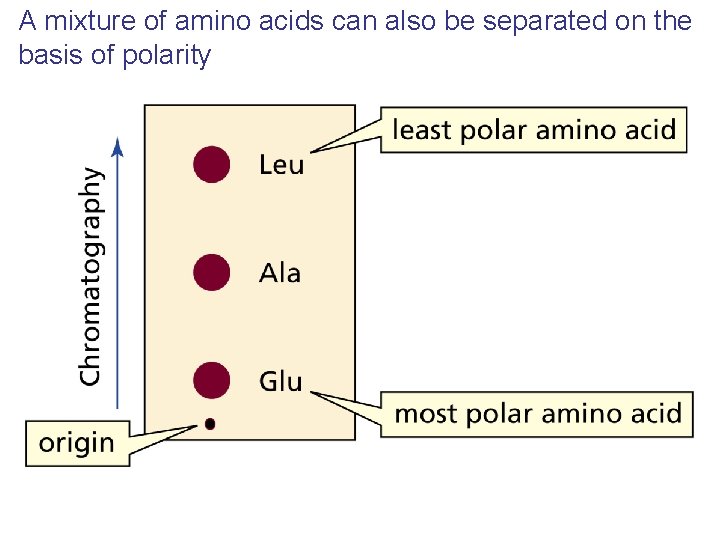
A mixture of amino acids can also be separated on the basis of polarity
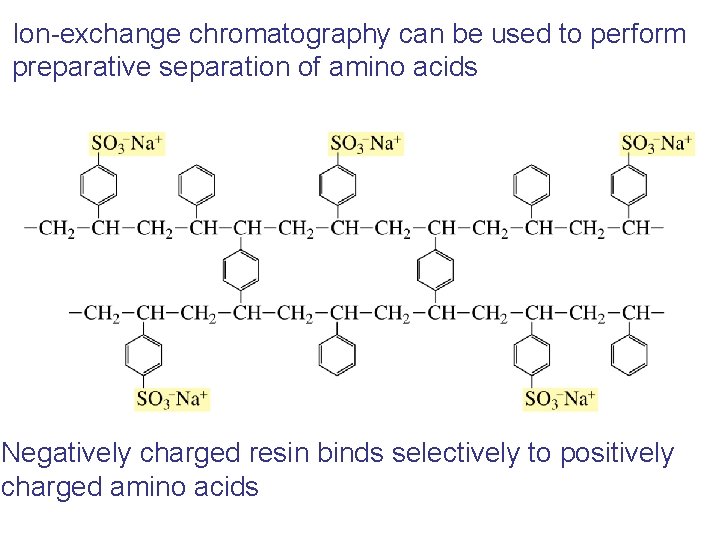
Ion-exchange chromatography can be used to perform preparative separation of amino acids Negatively charged resin binds selectively to positively charged amino acids
Ion-Exchange Chromatography • Cations bind most strongly to cation-exchange resins • Anions bind most strongly to anion-exchange resins • An amino acid analyzer is an instrument that automates ion-exchange chromatography
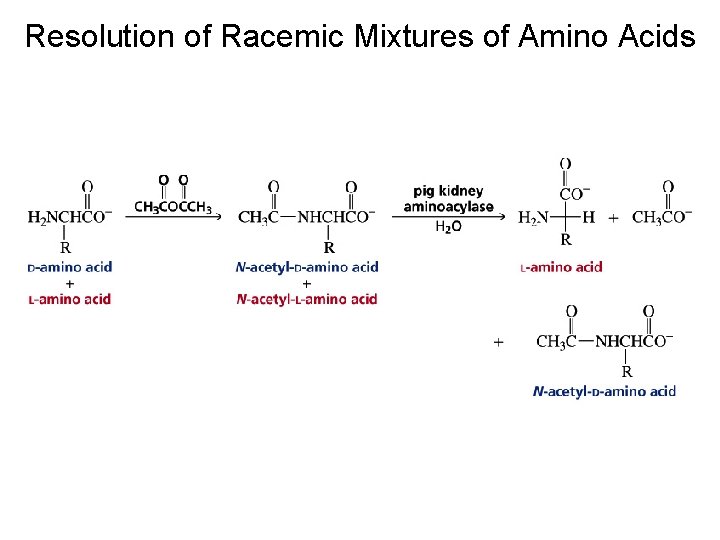
Resolution of Racemic Mixtures of Amino Acids
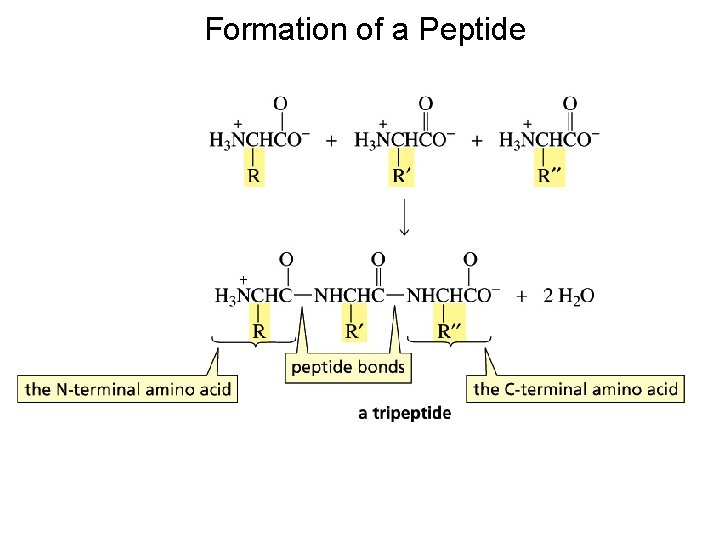
Formation of a Peptide
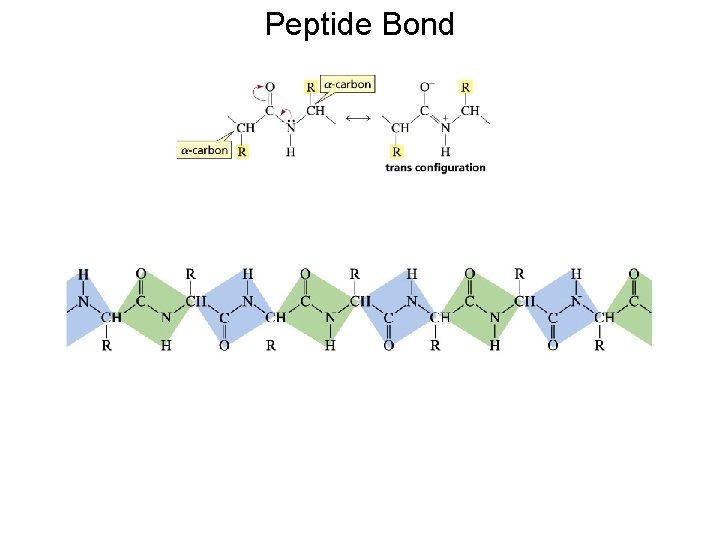
Peptide Bond
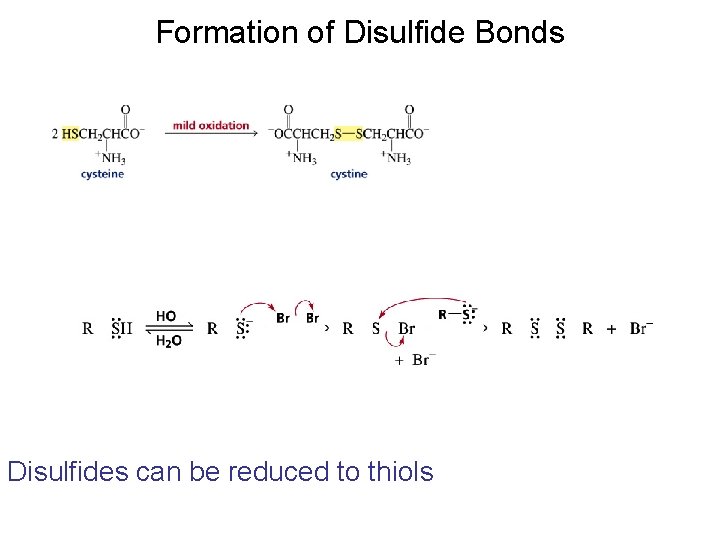
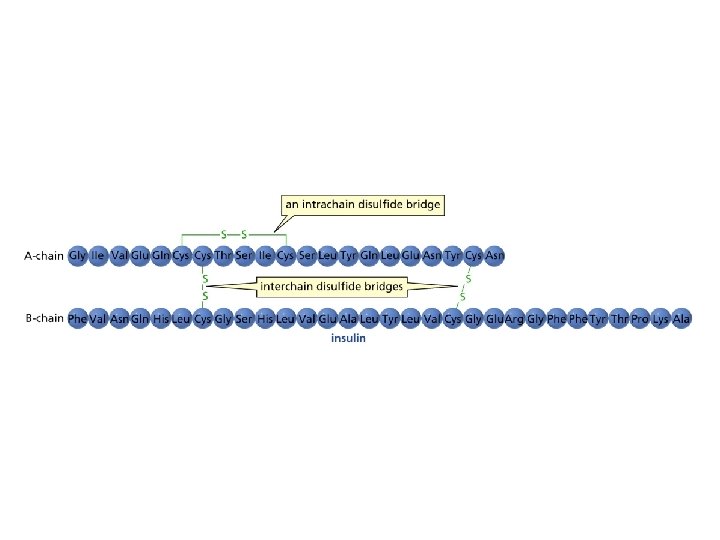
Formation of Disulfide Bonds Disulfides can be reduced to thiols
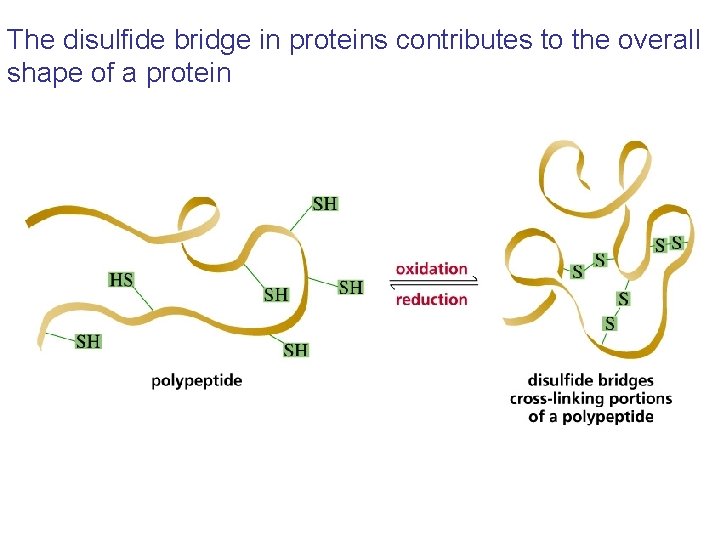
The disulfide bridge in proteins contributes to the overall shape of a protein
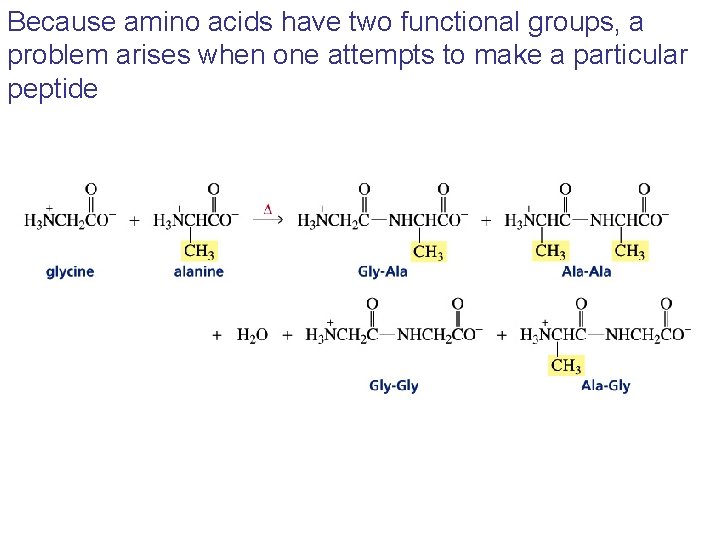
Because amino acids have two functional groups, a problem arises when one attempts to make a particular peptide
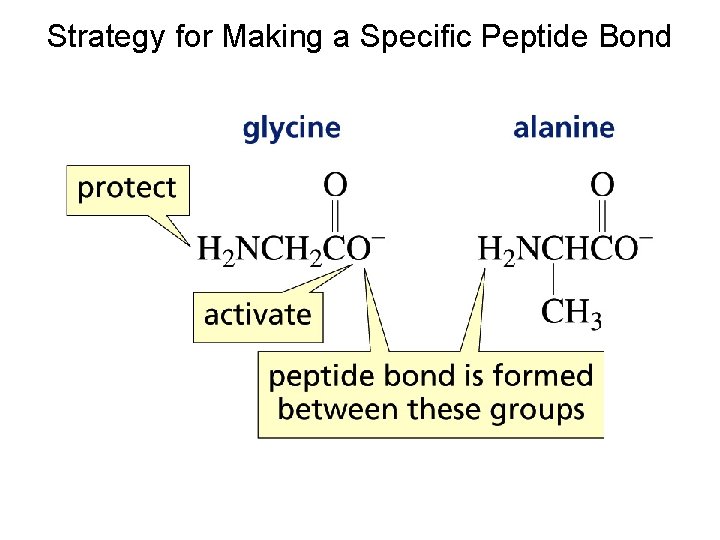
Strategy for Making a Specific Peptide Bond
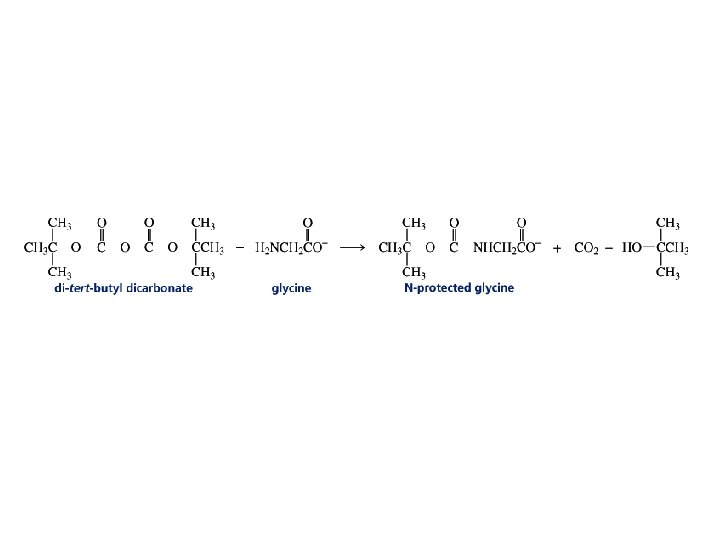
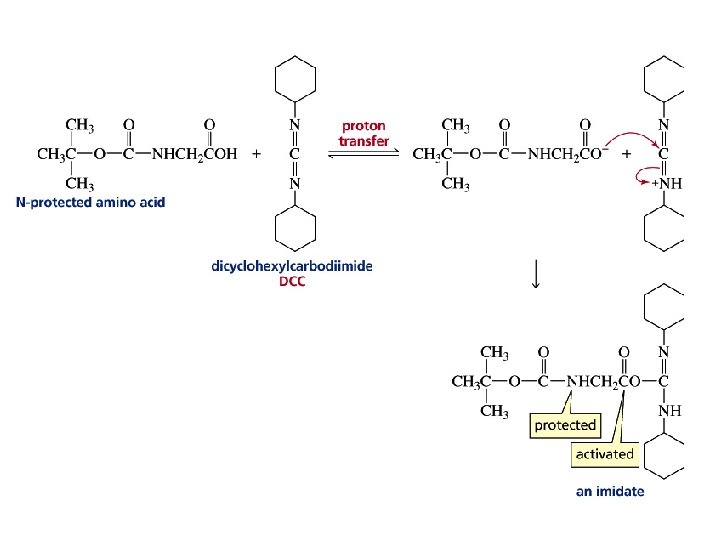
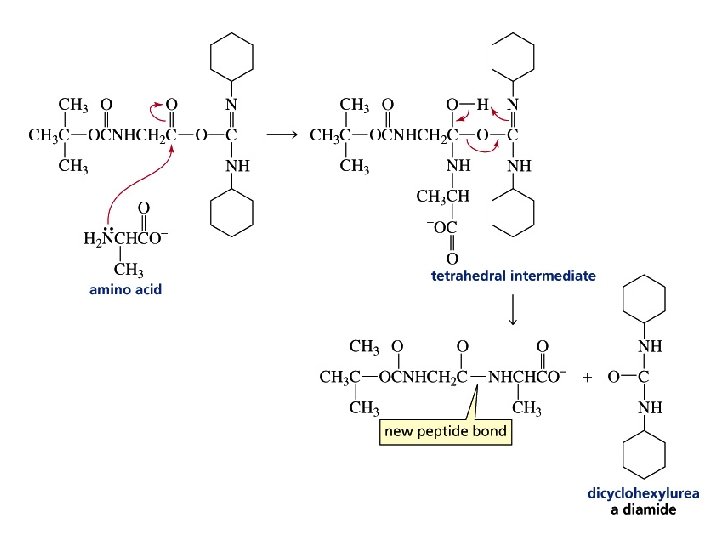
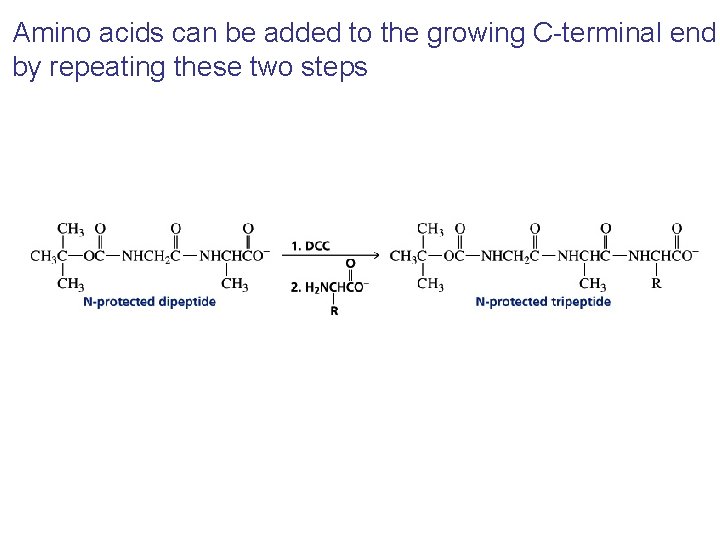
Amino acids can be added to the growing C-terminal end by repeating these two steps
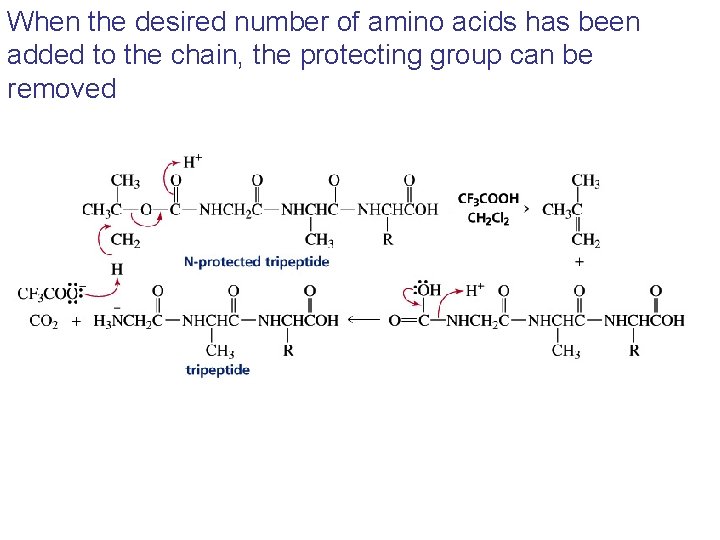
When the desired number of amino acids has been added to the chain, the protecting group can be removed
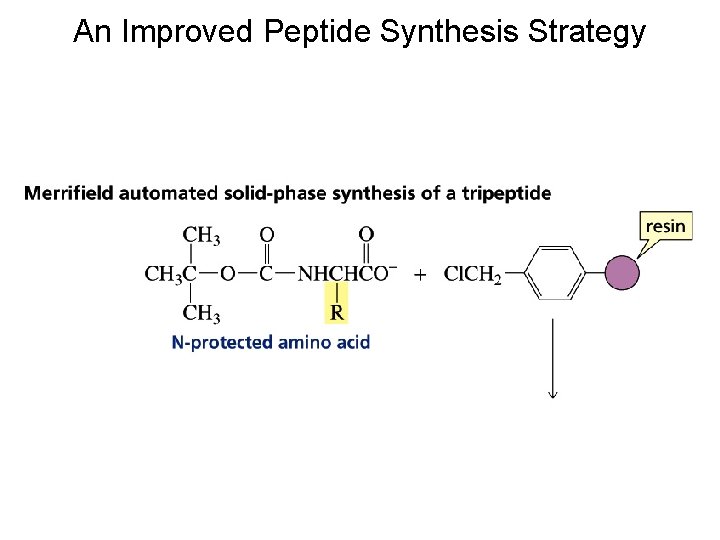
An Improved Peptide Synthesis Strategy
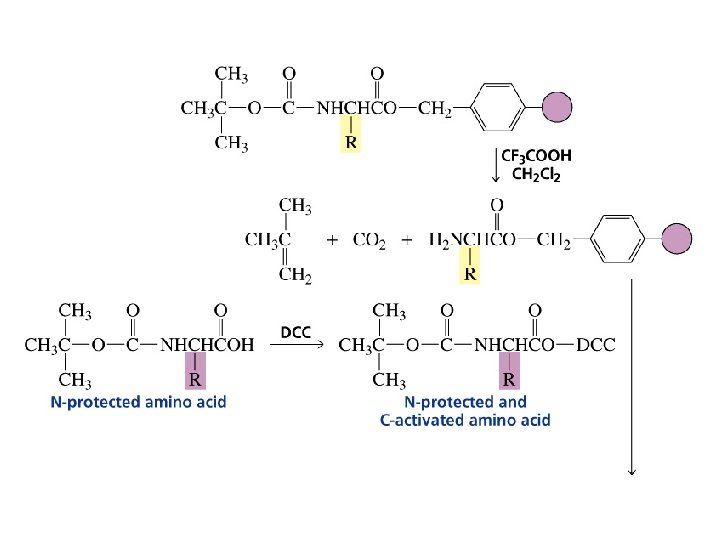
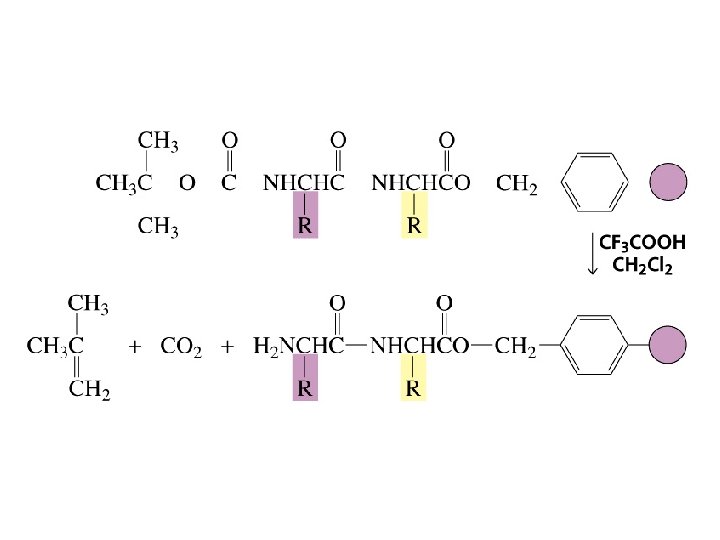
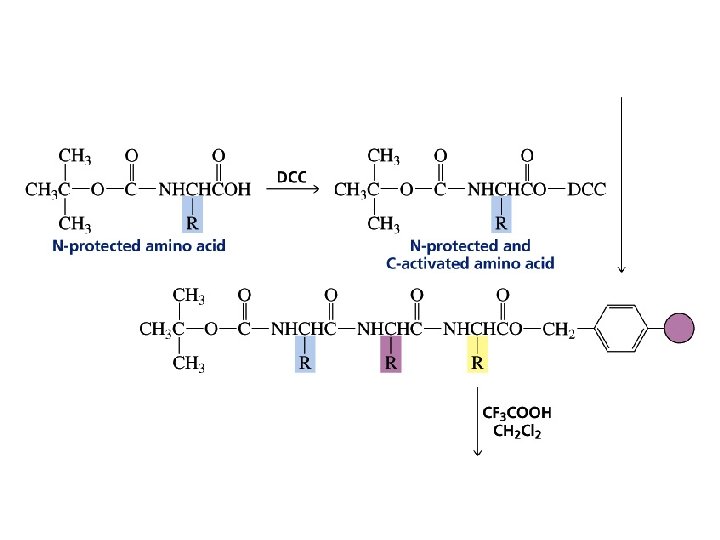
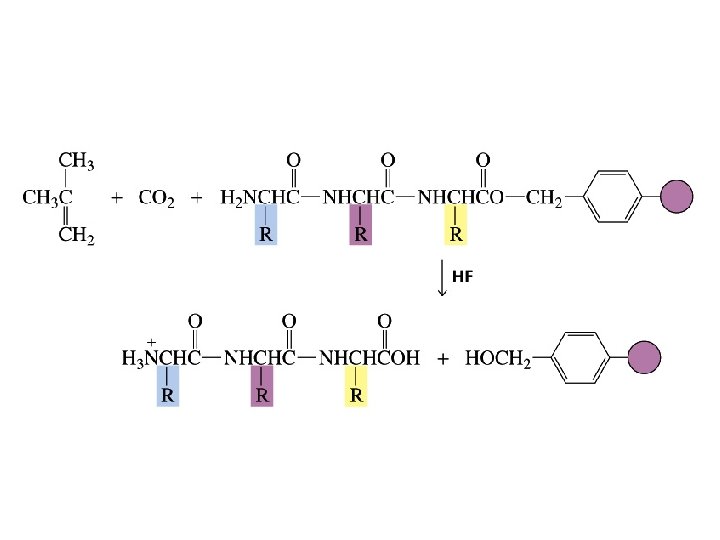
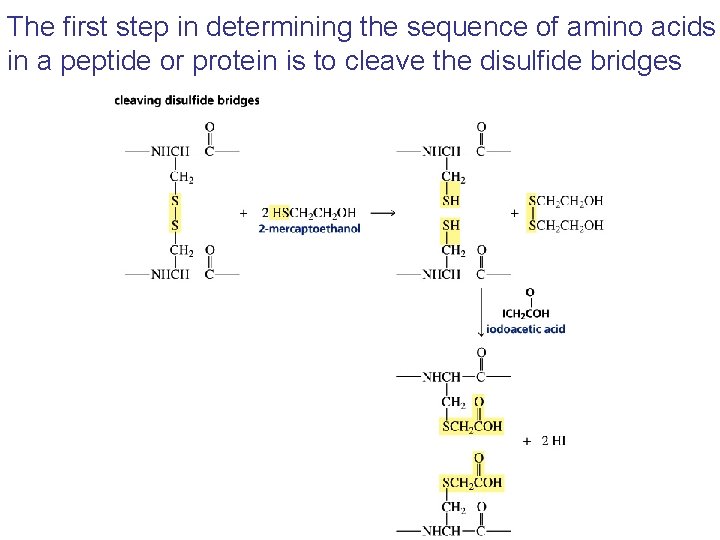
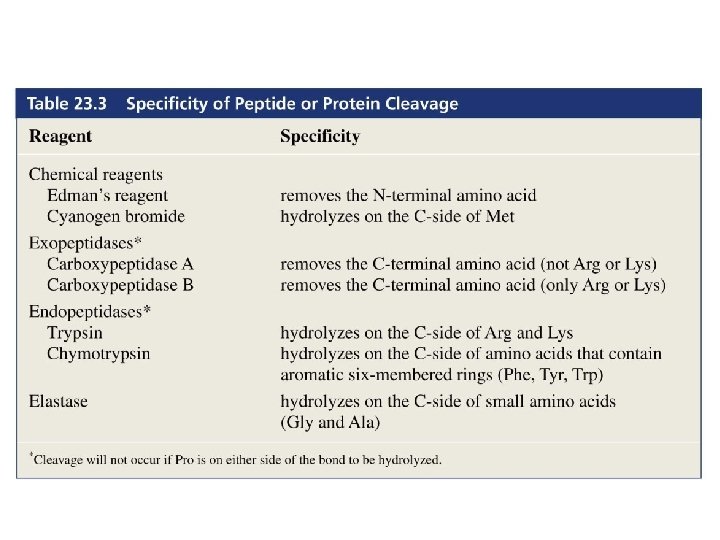
The first step in determining the sequence of amino acids in a peptide or protein is to cleave the disulfide bridges
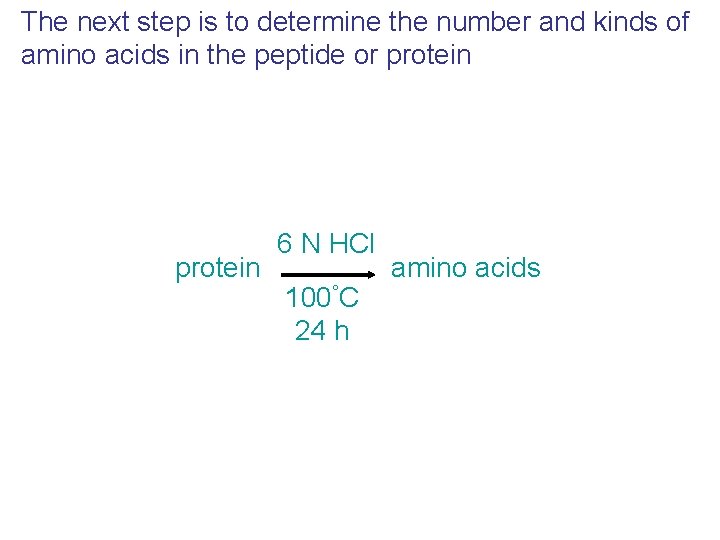
The next step is to determine the number and kinds of amino acids in the peptide or protein 6 N HCl 100°C 24 h amino acids
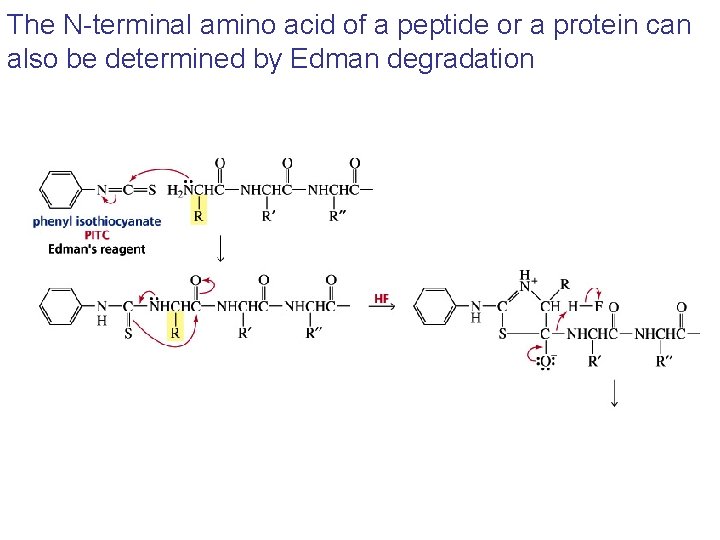
The N-terminal amino acid of a peptide or a protein can also be determined by Edman degradation
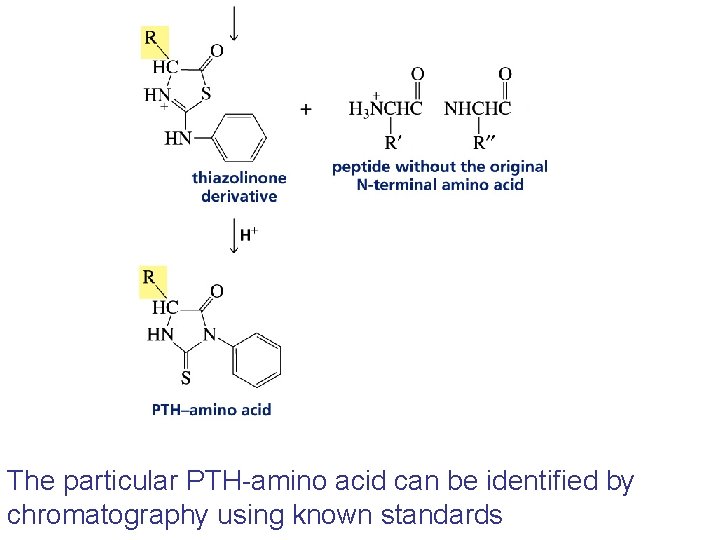
The particular PTH-amino acid can be identified by chromatography using known standards
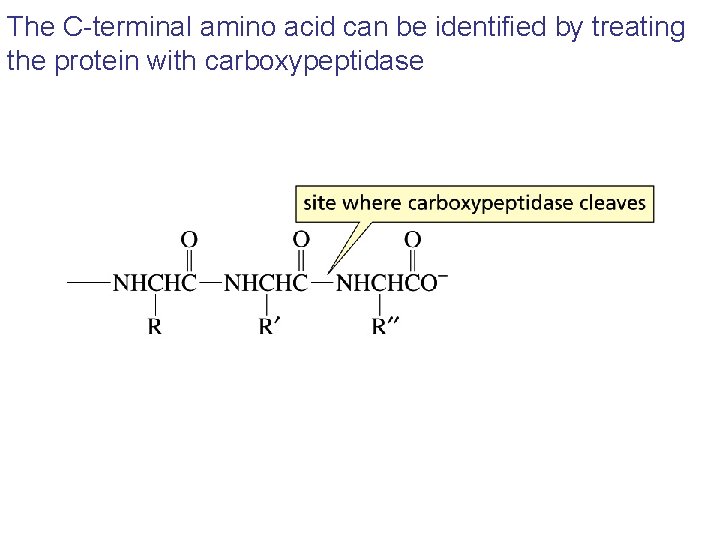
The C-terminal amino acid can be identified by treating the protein with carboxypeptidase
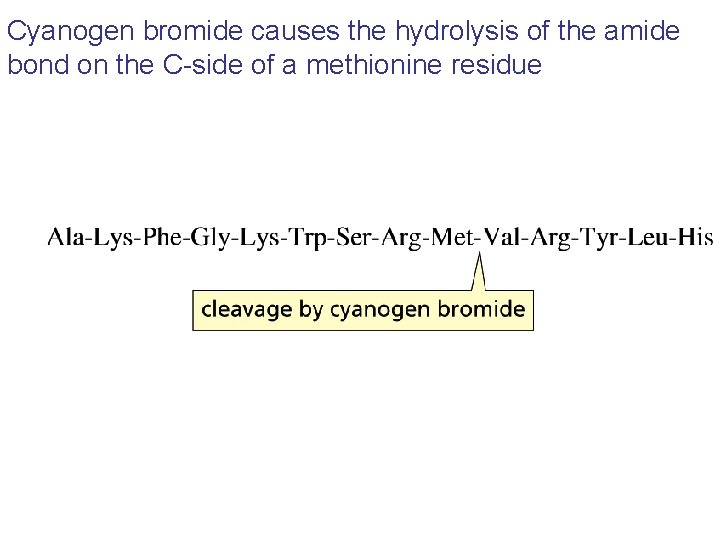
Cyanogen bromide causes the hydrolysis of the amide bond on the C-side of a methionine residue
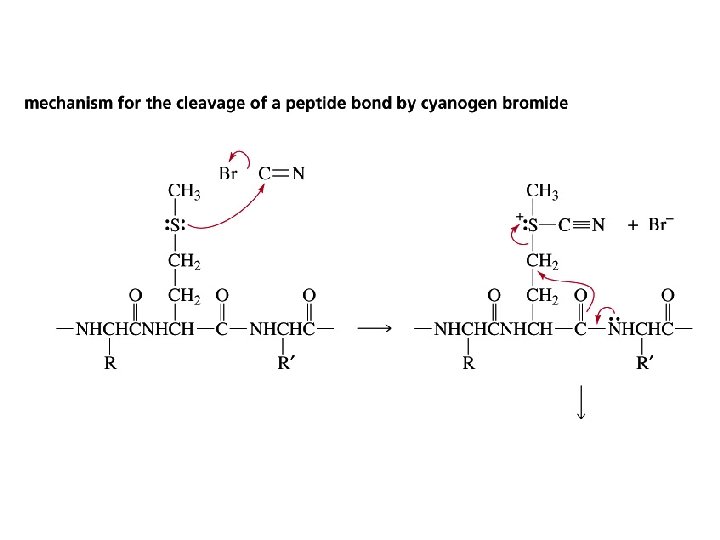
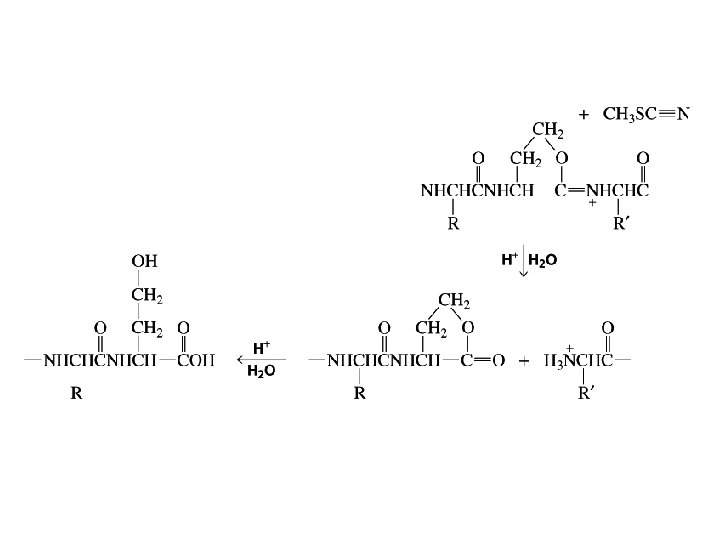
Secondary Structure of Proteins Describe the conformation of segments of the backbone chain of a peptide or protein Three factors determine the choice of secondary structure: • the regional planarity about each peptide bond • maximization of the number of peptide groups that engage in hydrogen bonding • adequate separation between nearby R groups
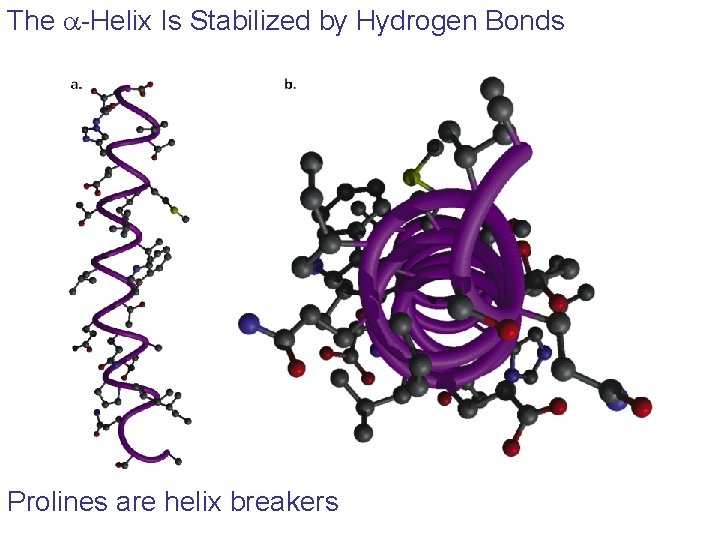
The a-Helix Is Stabilized by Hydrogen Bonds Prolines are helix breakers
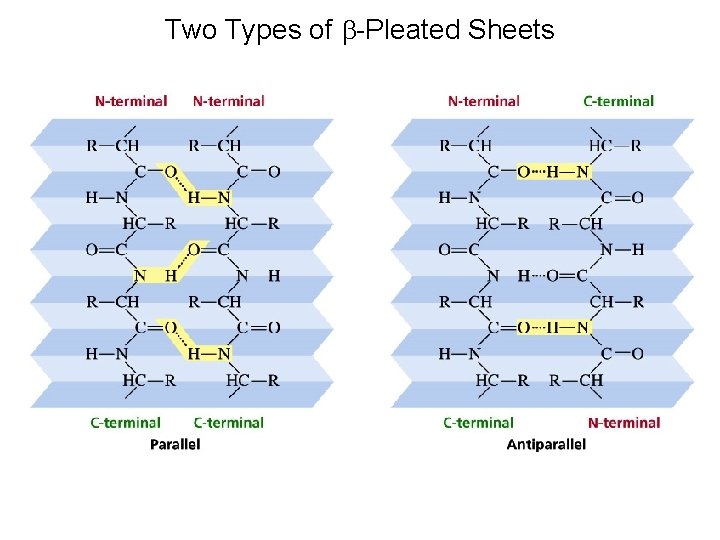
Two Types of b-Pleated Sheets
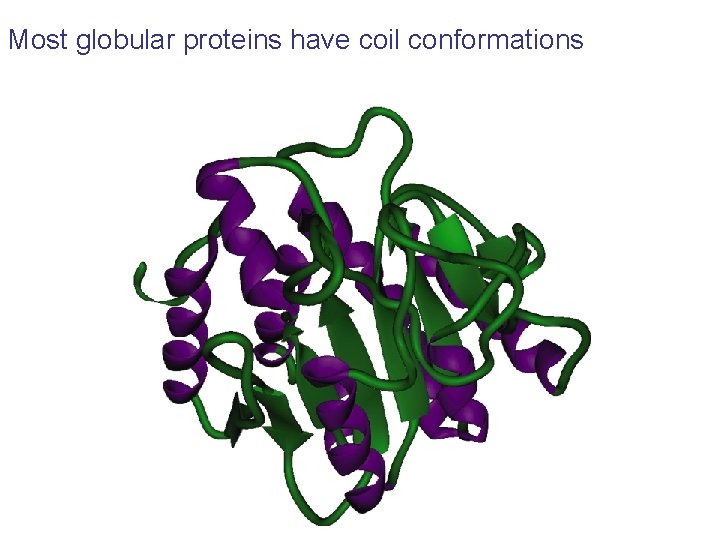
Most globular proteins have coil conformations
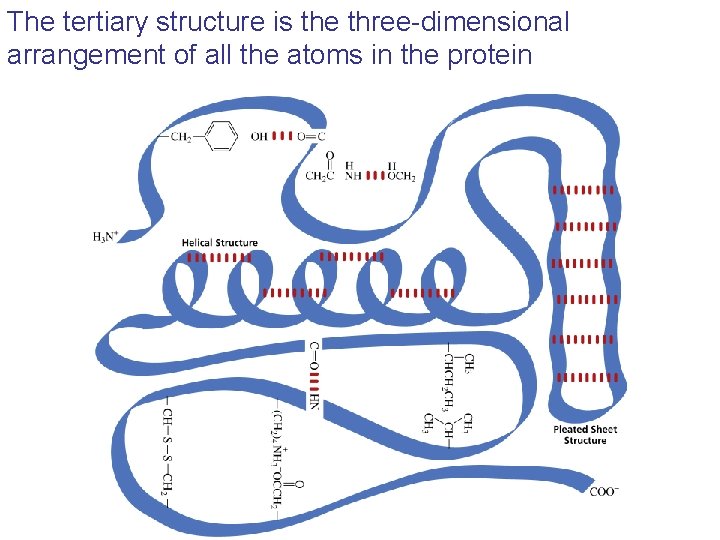
The tertiary structure is the three-dimensional arrangement of all the atoms in the protein

The tertiary structure is defined by the primary structure The stabilizing interactions include covalent bonds, hydrogen bonds, electrostatic attractions, and hydrophobic interactions Disulfide bonds are the only covalent bonds that can form when a protein folds Proteins that have more than one peptide chain are called oligomers
- Slides: 52