ORGANIC CHEMISTRY 4 Main Organic Compounds l l








- Slides: 29

ORGANIC CHEMISTRY Ø 4 Main Organic Compounds l l Carbohydrates Fats/Lipids Proteins Nucleic Acids

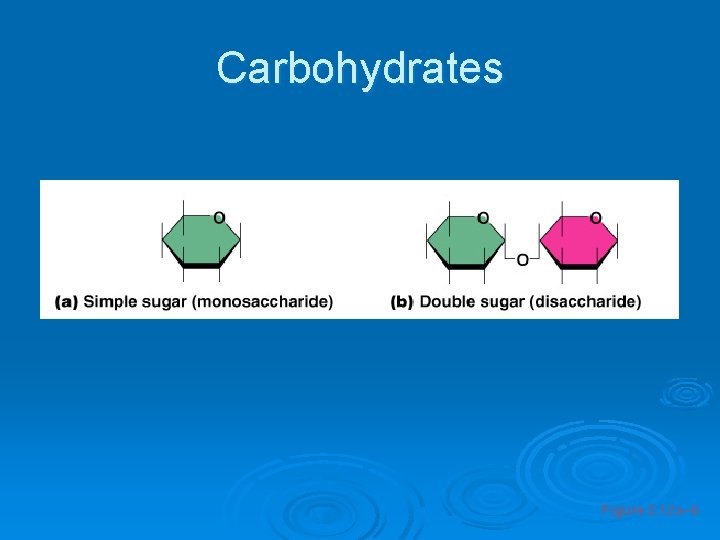
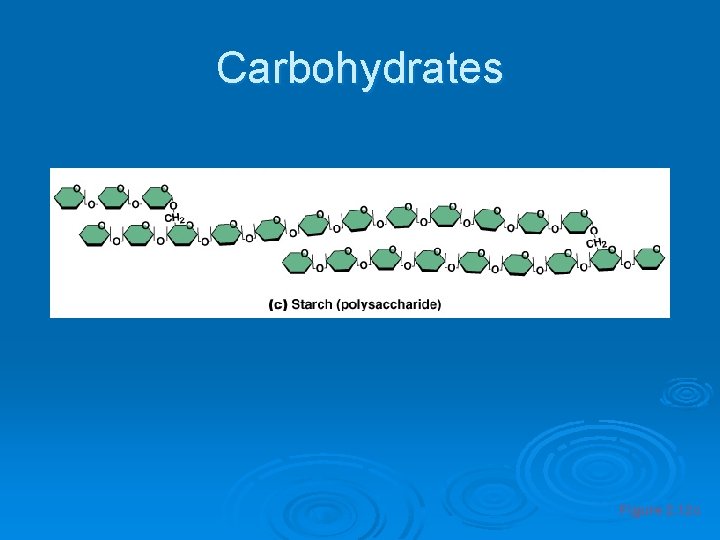
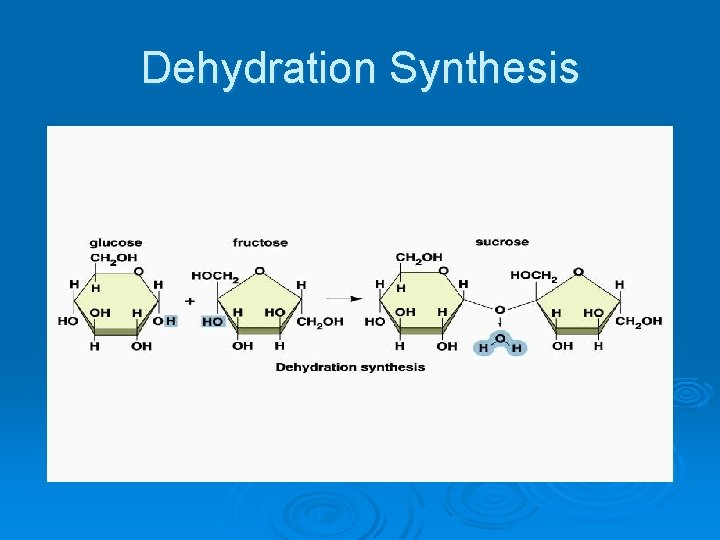
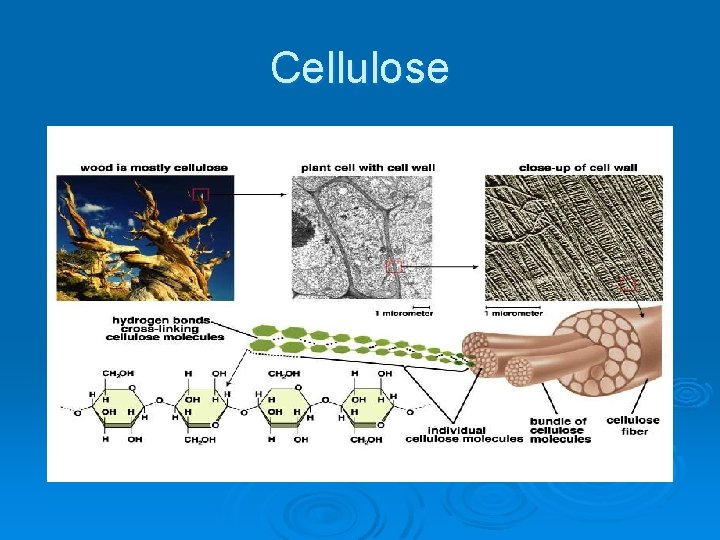
Important Organic Compounds Ø Carbohydrates l l l Contain carbon, hydrogen, and oxygen Include sugars and starches Classified according to size • Monosaccharides – simple sugars • Disaccharides – two simple sugars joined by dehydration synthesis • Polysaccharides – long branching chains of linked simple sugars

Carbohydrates Figure 2. 12 a–b

Carbohydrates Figure 2. 12 c



Dehydration Synthesis

Cellulose

Important Organic Compounds Ø Lipids l Contain carbon, hydrogen, and oxygen • Carbon and hydrogen outnumber oxygen l Insoluble in water

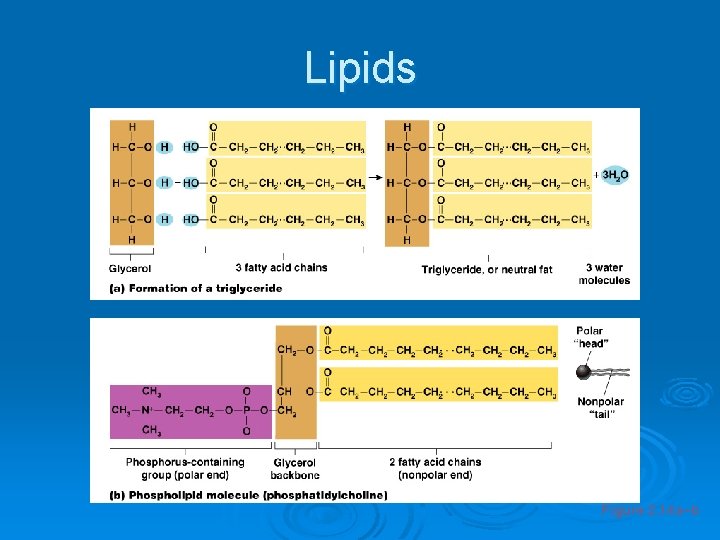
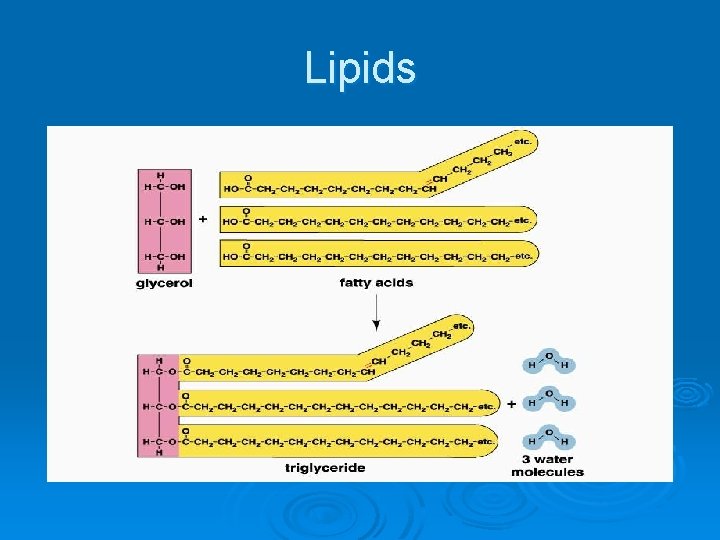
Lipids Ø Common lipids in the human body l Neutral fats (triglycerides) • • • Found in fat deposits Composed of fatty acids and glycerol Source of stored energy

Lipids Ø Common lipids in the human body (continued) l Phospholipids • Form cell membranes l Steroids • Include cholesterol, bile salts, vitamin D, and some hormones

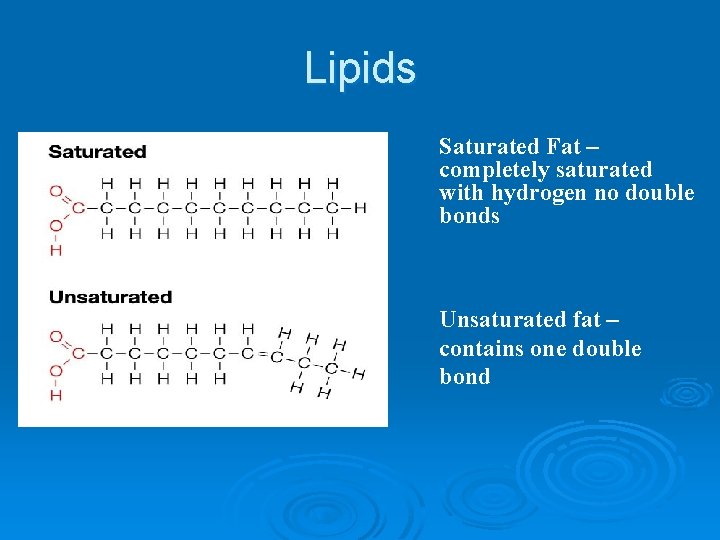
Lipids Contains chains of mostly C and H (fatty acids tails) with a backbone containing C, H, and O (glycerol head) Ø Lipids can be used to store energy. Some lipids are important parts of biological membranes and waterproof coverings. Ø Lipid, triacylglycerol (circulating fat), triglyceride(storage form of fat), phospholipid(cell membrane), steroid Ø l l l Ø Ø Saturated Fat – completely saturated with hydrogen no double bonds unsaturated fat – contains one double bond polyunsaturated fat – contains more than one double bond Fatty Acid Tails are - Hydrophobic Glycerol Heads (backbone) are - Hydrophylic

Lipids Figure 2. 14 a–b

Lipids

Lipids Saturated Fat – completely saturated with hydrogen no double bonds Unsaturated fat – contains one double bond

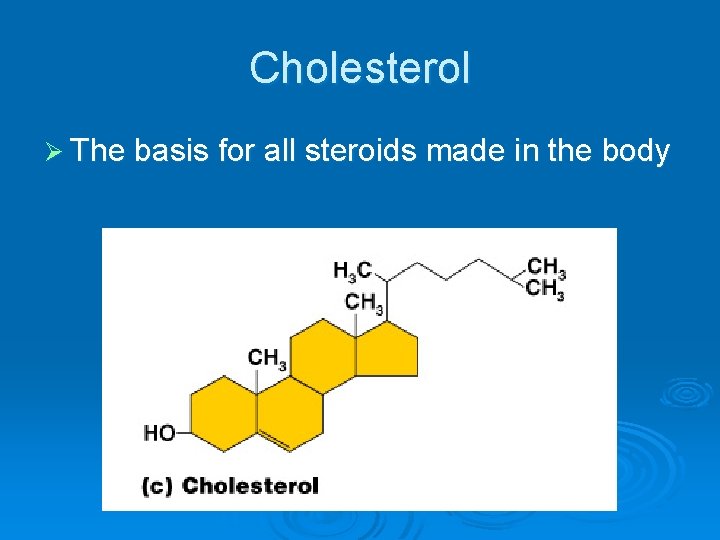
Cholesterol Ø The basis for all steroids made in the body

Important Organic Compounds Ø Proteins l Made of amino acids • Contain carbon, oxygen, hydrogen, nitrogen, and sometimes sulfur

Proteins Ø Account for over half of the body’s organic matter l l Provides for construction materials for body tissues Plays a vital role in cell function Ø Act as enzymes, hormones, and antibodies

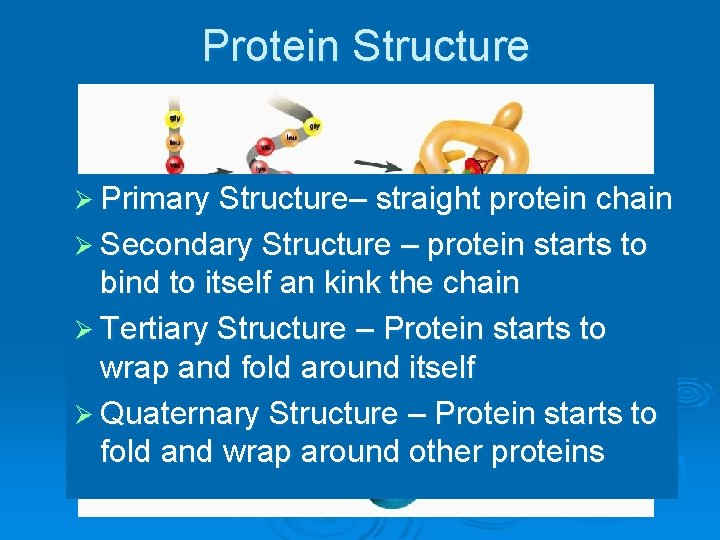
Protein Structure Ø Primary Structure– straight protein chain Ø Secondary Structure – protein starts to bind to itself an kink the chain Ø Tertiary Structure – Protein starts to wrap and fold around itself Ø Quaternary Structure – Protein starts to fold and wrap around other proteins

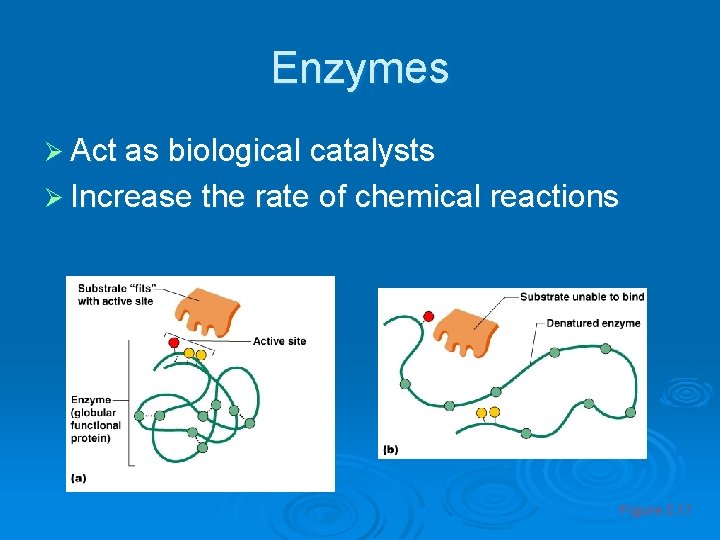
Enzymes Ø Act as biological catalysts Ø Increase the rate of chemical reactions Figure 2. 17

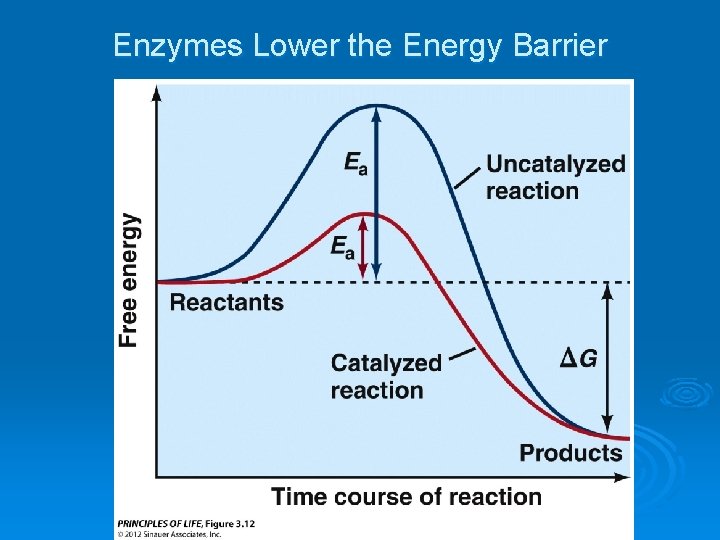
Enzymes Lower the Energy Barrier

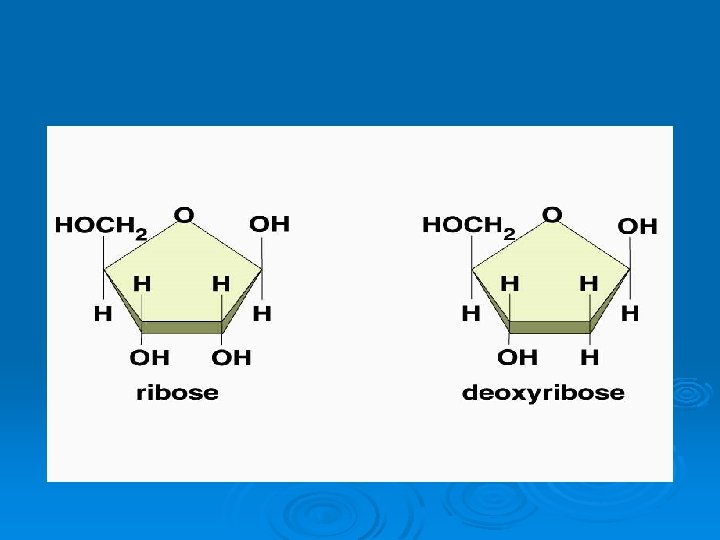
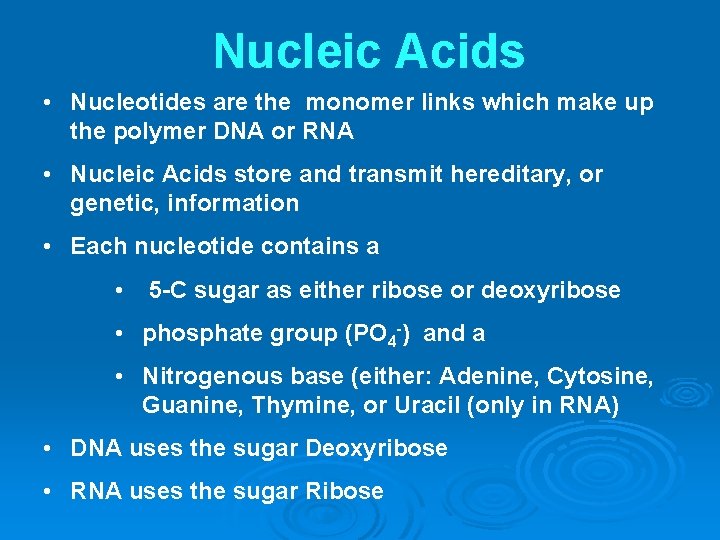
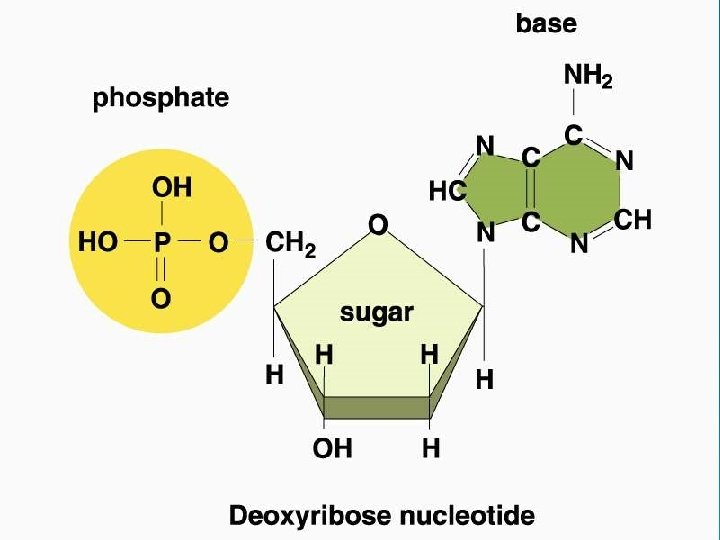
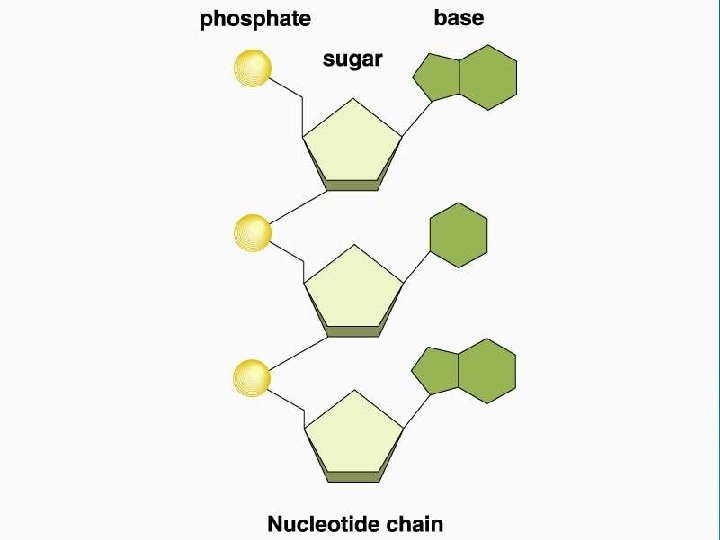
Nucleic Acids • Nucleotides are the monomer links which make up the polymer DNA or RNA • Nucleic Acids store and transmit hereditary, or genetic, information • Each nucleotide contains a • 5 -C sugar as either ribose or deoxyribose • phosphate group (PO 4 -) and a • Nitrogenous base (either: Adenine, Cytosine, Guanine, Thymine, or Uracil (only in RNA) • DNA uses the sugar Deoxyribose • RNA uses the sugar Ribose



Important Organic Compounds Ø Nucleic Acids l l Provide blueprint of life Nucleotide bases • • • l A = Adenine G = Guanine C = Cytosine T = Thymine U = Uracil Make DNA and RNA

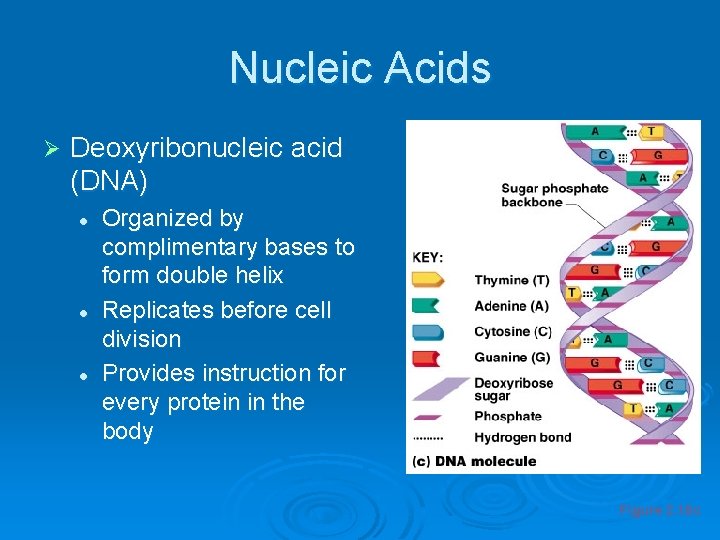
Nucleic Acids Ø Deoxyribonucleic acid (DNA) l l l Organized by complimentary bases to form double helix Replicates before cell division Provides instruction for every protein in the body Figure 2. 18 c

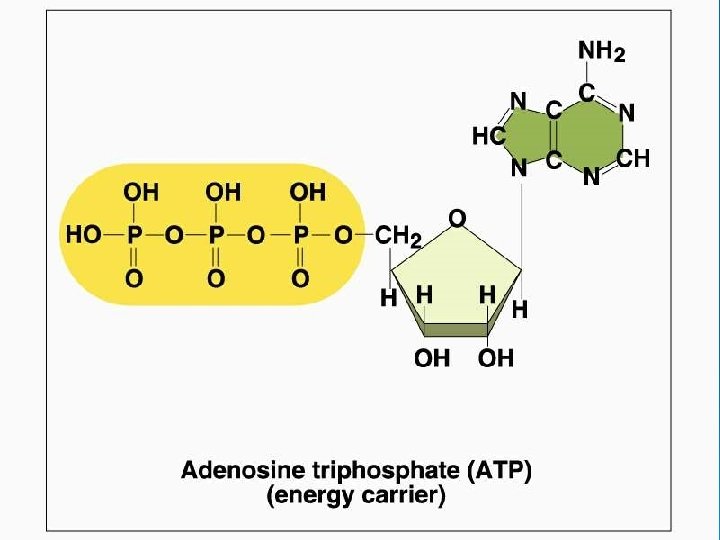
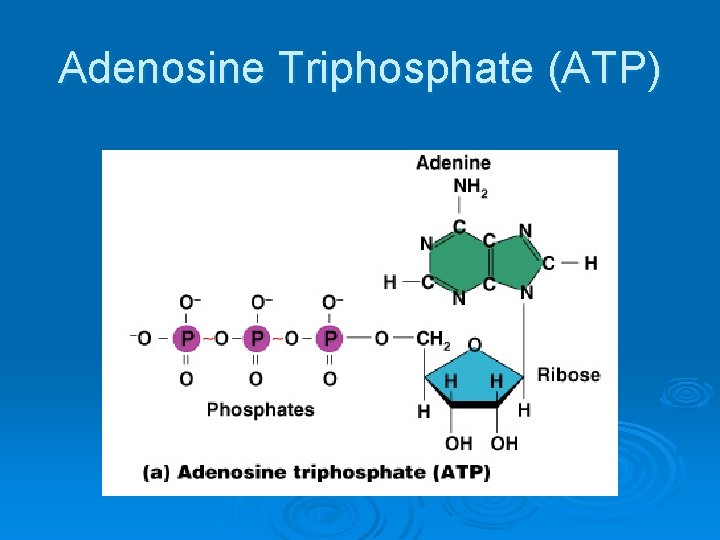
Important Organic Compounds Ø Adenosine triphosphate (ATP) l l l Chemical energy used by all cells Energy is released by breaking high energy phosphate bond ATP is replenished by oxidation of food fuels


Adenosine Triphosphate (ATP)