ORGANIC CHEMISTRY 1 Prof Janina E Kamiska d






























- Slides: 82

ORGANIC CHEMISTRY 1 Prof. Janina E. Kamińska Łódź University of Technology Faculty of Biotechnology and Food Sciences Institute of General Food Chemistry ul. Stefanowskiego 4/10 Room no 209 (Consultation hours: Wed. 11: 15 -12: 00, Fri. 14: 15 -15: 00) Phone: 42 6313412 E-mail: janina. kaminska@p. lodz. pl

ORGANIC CHEMISTRY 1 sem. II. 2018/19 Lecture 30 h Tutorials 15 h Workload outside classroom 75 h ECTS credits: 4

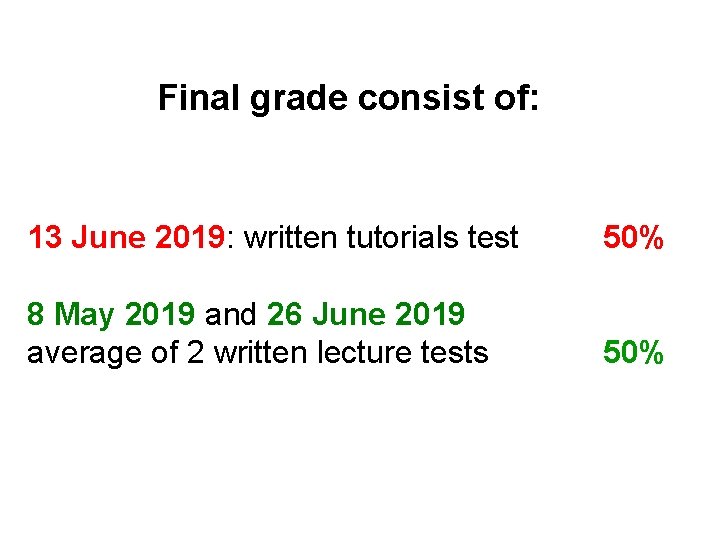
Final grade consist of: 13 June 2019: written tutorials test 50% 8 May 2019 and 26 June 2019 average of 2 written lecture tests 50%

PERIODIC TABLE OF ELEMENTS

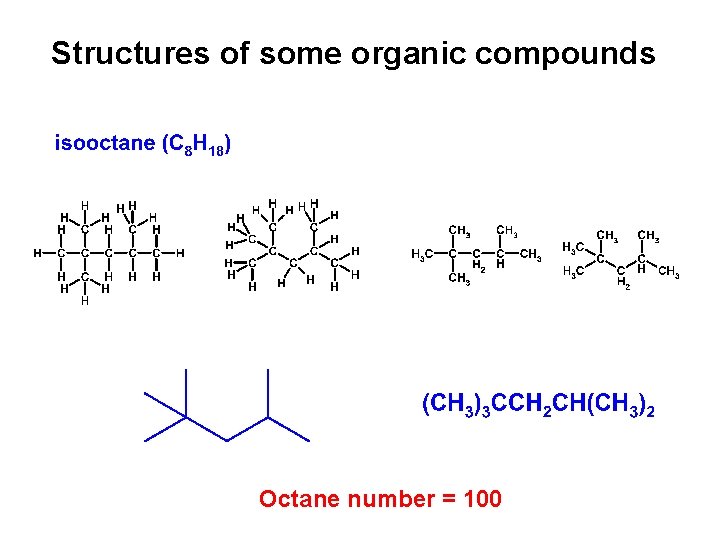
Structures of some organic compounds Octane number = 100

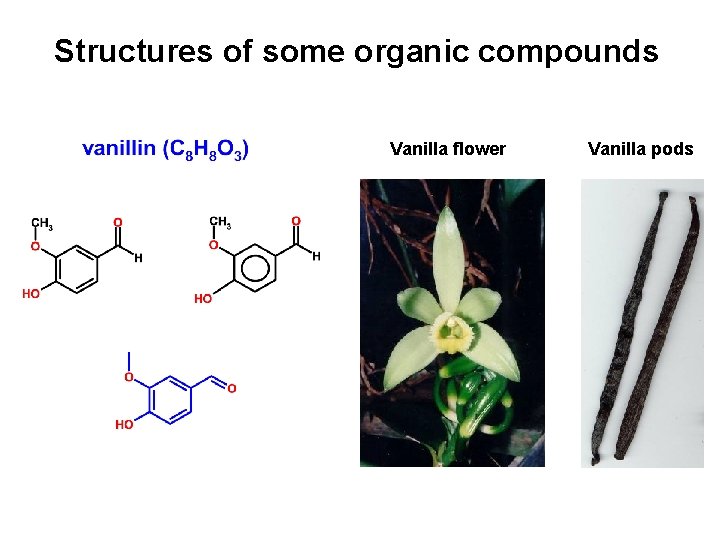
Structures of some organic compounds Vanilla flower Vanilla pods

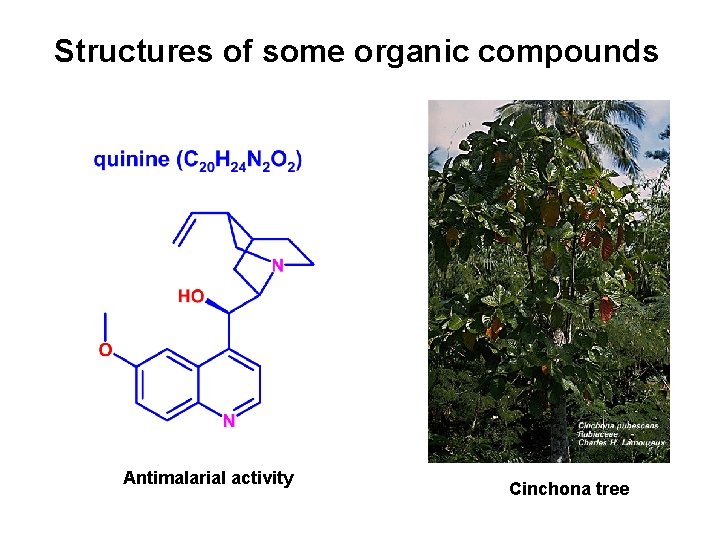
Structures of some organic compounds Antimalarial activity Cinchona tree

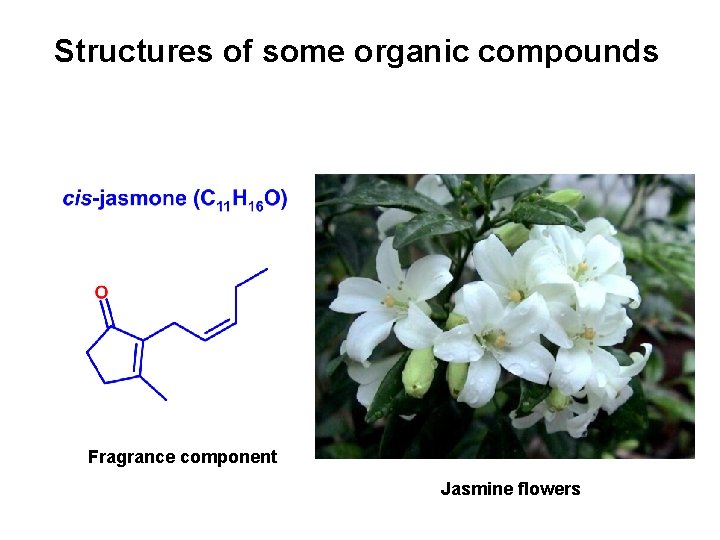
Structures of some organic compounds Fragrance component Jasmine flowers

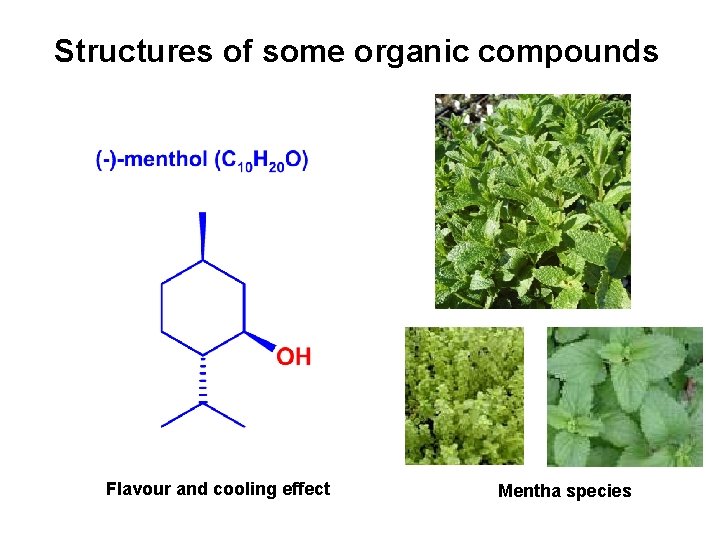
Structures of some organic compounds Flavour and cooling effect Mentha species

Structures of some organic compounds Sugar beet Sugar cane

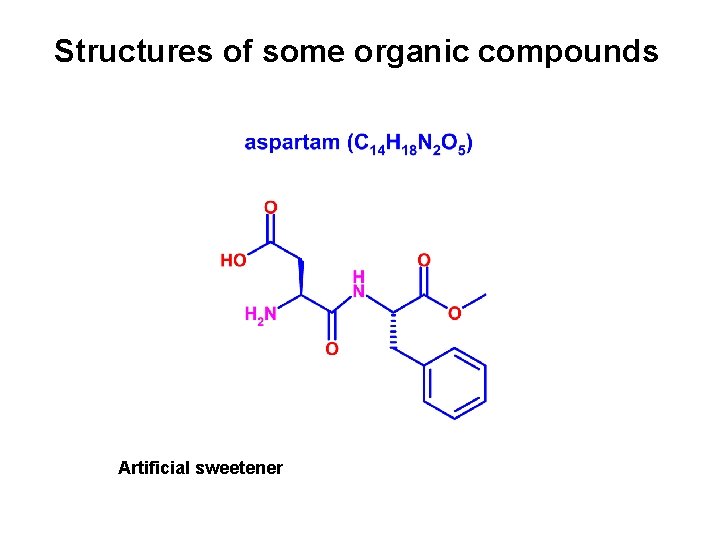
Structures of some organic compounds Artificial sweetener

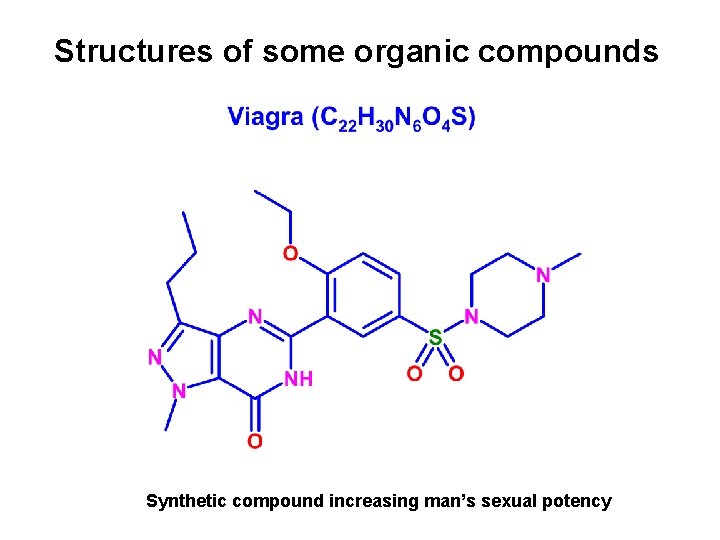
Structures of some organic compounds Synthetic compound increasing man’s sexual potency

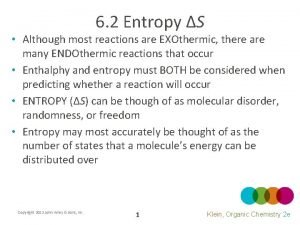
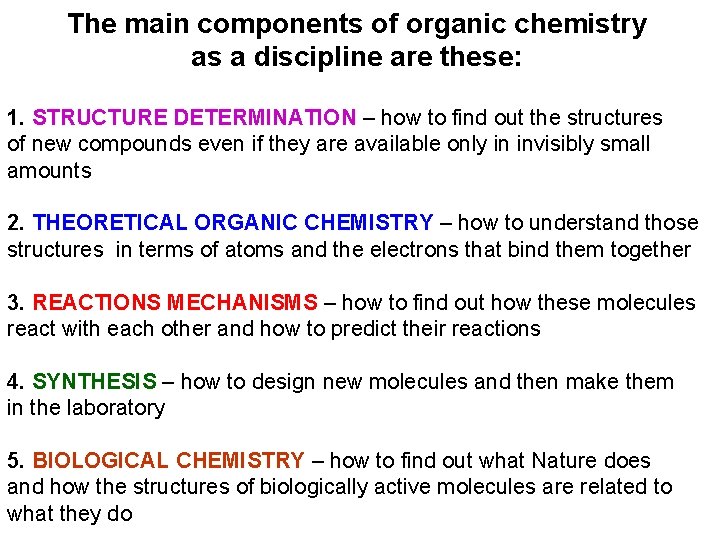
The main components of organic chemistry as a discipline are these: 1. STRUCTURE DETERMINATION – how to find out the structures of new compounds even if they are available only in invisibly small amounts 2. THEORETICAL ORGANIC CHEMISTRY – how to understand those structures in terms of atoms and the electrons that bind them together 3. REACTIONS MECHANISMS – how to find out how these molecules react with each other and how to predict their reactions 4. SYNTHESIS – how to design new molecules and then make them in the laboratory 5. BIOLOGICAL CHEMISTRY – how to find out what Nature does and how the structures of biologically active molecules are related to what they do

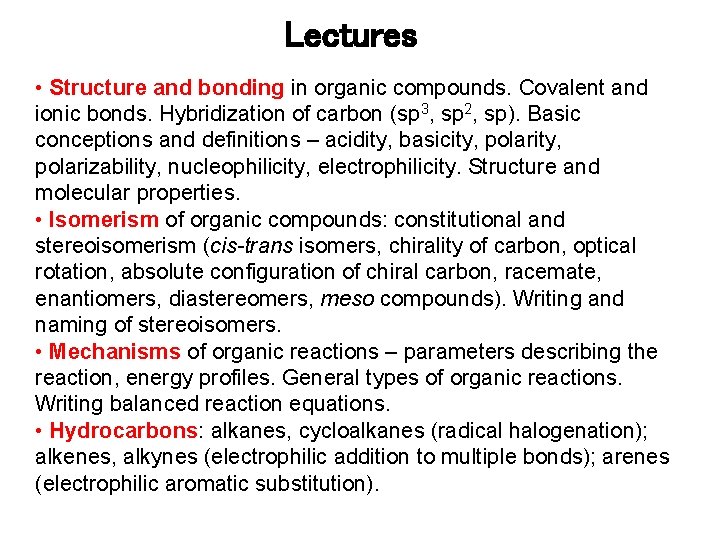
Lectures • Structure and bonding in organic compounds. Covalent and ionic bonds. Hybridization of carbon (sp 3, sp 2, sp). Basic conceptions and definitions – acidity, basicity, polarizability, nucleophilicity, electrophilicity. Structure and molecular properties. • Isomerism of organic compounds: constitutional and stereoisomerism (cis-trans isomers, chirality of carbon, optical rotation, absolute configuration of chiral carbon, racemate, enantiomers, diastereomers, meso compounds). Writing and naming of stereoisomers. • Mechanisms of organic reactions – parameters describing the reaction, energy profiles. General types of organic reactions. Writing balanced reaction equations. • Hydrocarbons: alkanes, cycloalkanes (radical halogenation); alkenes, alkynes (electrophilic addition to multiple bonds); arenes (electrophilic aromatic substitution).

Lectures • Methods of isolation and purification of organic compounds (crystallization, distillation, chromatography). • Structure determination by spectroscopic methods: general principles of mass spectrometry (MS), infrared spectroscopy (IR), nuclear magnetic resonance spectroscopy (NMR), ultraviolet spectroscopy (UV). • Alkyl halides – nucleophilic substitution and elimination (SN 1, SN 2, E 1, E 2 mechanisms) • Alcohols, phenols – preparation and chemical reactivity. • Ethers and epoxides - preparation and chemical reactivity. • Overview of carbonyl group chemistry • Aldehydes and ketones – preparation and chemical reactions: nucleophilic addition to carbonyl group; -substitution reactions, carbonyl condensation reactions (aldol condensation). • Carboxylic acids and their derivatives (acyl chlorides, anhydrides, esters, amides, nitriles) – preparation and chemical behaviour (nucleophilic acyl substitution, -substitution reactions).

Tutorials • Different manners of writing and drawing structural formulas of organic molecules. • Systematic (IUPAC) and common names of hydrocarbons. • Systematic (IUPAC) and common names of compounds with single functional group. • Isomerism of organic compounds. a) Constitutional isomerism b) Stereoisomerism. Writing and drawing stereoisomers on the plane. Cis-trans isomers of alkenes and cycloalkanes. Chirality of organic molecules – enantiomers. Absolute and relative configuration of chiral centre. • Equation of chemical reaction – its meaning and application. Material balance in chemical equation. • Classifying of organic reactions into general categories: addition, elimination, substitution, rearrangement. • Chemical reactivity of hydrocarbons: alkanes, alkenes, alkynes, arenes.

Textbooks • In English: John Mc. Murry, “Organic Chemistry”, 8 th ed. Brooks/Cole Publishing Co. , Pacific Grove, California (or earlier editions) K. P. C. Vollhardt, N. E. Schore, “Organic Chemistry, Structure and Function”, 3 rd ed. W. H. Freeman and Co. , New York 1999 F. A. Bettelheim, J. March, “General, Organic and Biochemistry”, 5 th ed. Harcourt Brace College Publishers 1998 J. Clayden, N. Greeves, S. Warren, P. Wothers, “Organic Chemistry”, Oxford University Press 2000 • In Polish: John Mc. Murry, “Chemia organiczna”, t 1/2, translation from 4 th ed. , PWN Warszawa 2000 (or later editions) H. Hart, L. E. Craine, D. J. Hart, “Chemia organiczna. Krótki kurs”, Wyd. I, PZWL Warszawa 1999

Textbooks Organic Chemistry with Biological Applications Organic Chemistry Fundamentals of Organic Chemistry


ORGANIC CHEMISTRY 1 Lecture 1 Different manners of writing and drawing structural formulas of organic molecules Systematic (IUPAC) and common names of hydrocarbons Systematic (IUPAC) and common names of compounds with single functional group.

Language of organic chemists

Types of organic compounds HYDROCARBON FRAMEWORK FUNCTIONAL GROUP

Drawing and naming of organic structures

Rules for drawing skeletal structures • Carbon atoms are not shown. They assumed to be at each intersection of two lines (bonds) and at the end of each line. Occasionally, a carbon atom might be indicated for emphasis or clarity. • Hydrogen atoms bonded to carbon are not shown. Since carbon has always valence of 4, we mentally supply the correct number of hydrogen atoms to fill the valence of each carbon. • All atoms other than carbon and hydrogen (heteroatoms) are indicated.

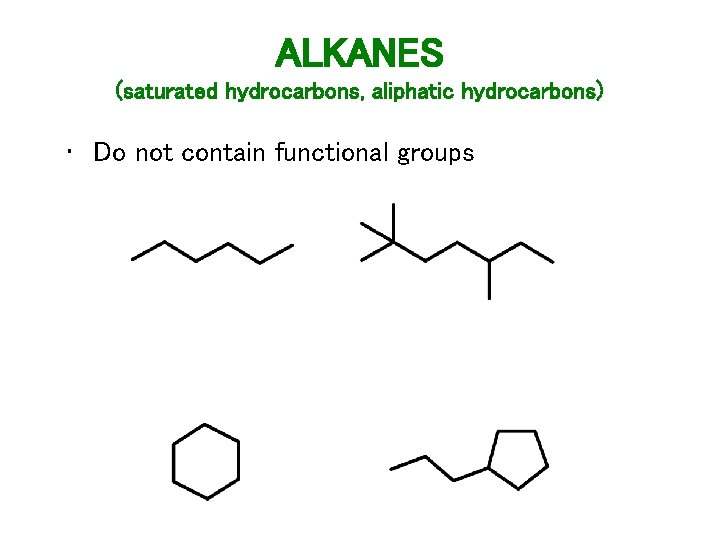
ALKANES (saturated hydrocarbons, aliphatic hydrocarbons) • Do not contain functional groups

Functional groups. • With carbon-carbon multiple bonds Alkene Alkyne Arene (aromatic ring)

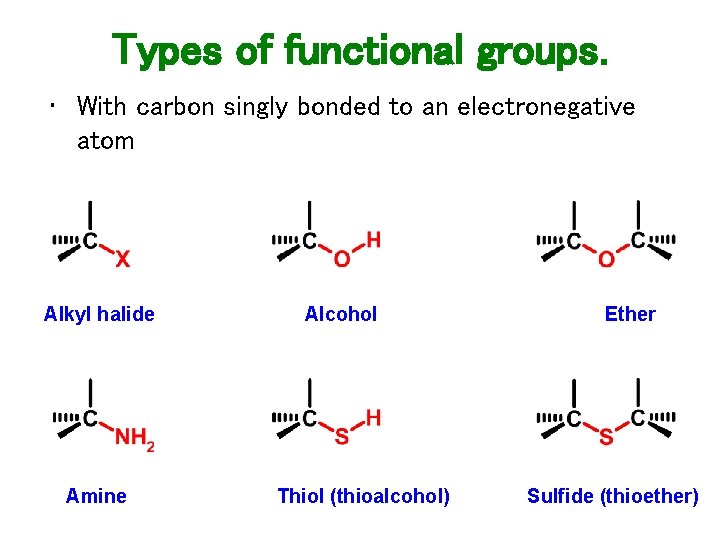
Types of functional groups. • With carbon singly bonded to an electronegative atom Alkyl halide Amine Alcohol Thiol (thioalcohol) Ether Sulfide (thioether)

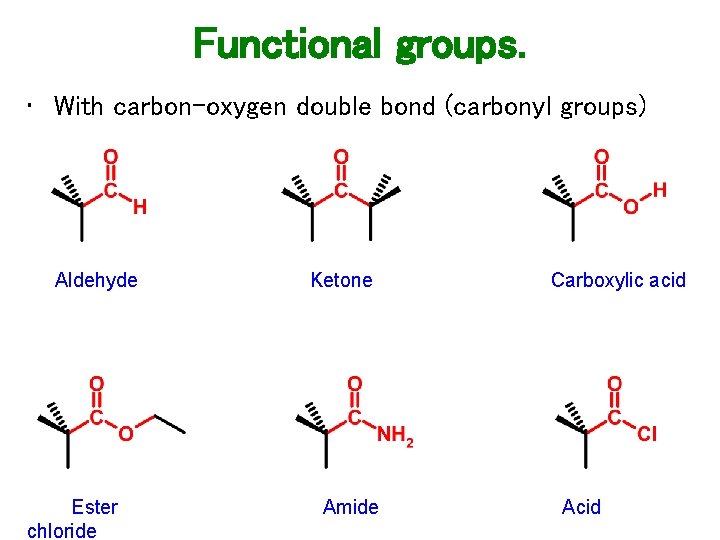
Functional groups. • With carbon-oxygen double bond (carbonyl groups) Aldehyde Ester chloride Ketone Amide Carboxylic acid Acid

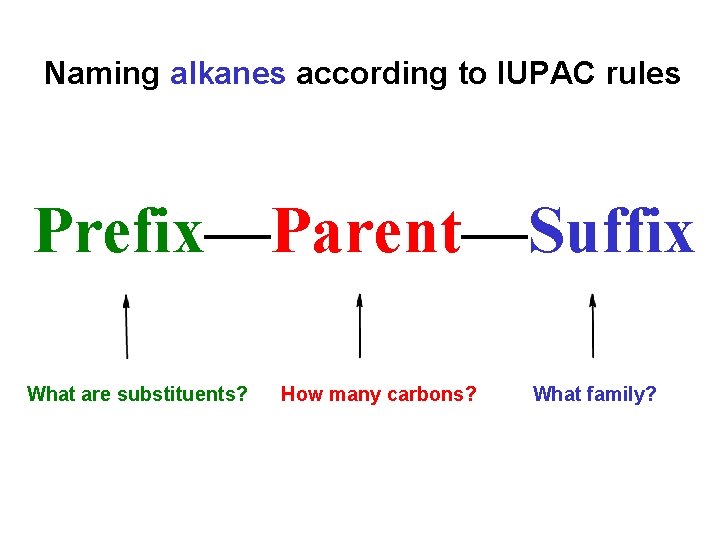
Naming alkanes according to IUPAC rules Prefix—Parent—Suffix What are substituents? How many carbons? What family?

Parent names of chain alkanes from C 1 to C 20 C 1 C 2 C 3 C 4 C 5 C 6 C 7 C 8 C 9 C 10 methane propane butane pentane hexane heptane octane nonane decane C 11 C 12 C 13 C 14 C 15 C 16 C 17 C 18 C 19 C 20 undecane dodecane tridecane tetradecane pentadecane hexadecane heptadecane octadecane nonadecane eicosane

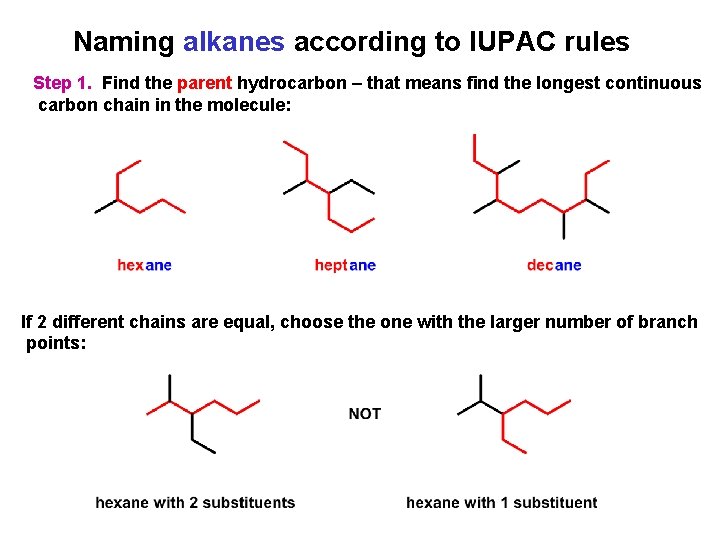
Naming alkanes according to IUPAC rules Step 1. Find the parent hydrocarbon – that means find the longest continuous carbon chain in the molecule: If 2 different chains are equal, choose the one with the larger number of branch points:

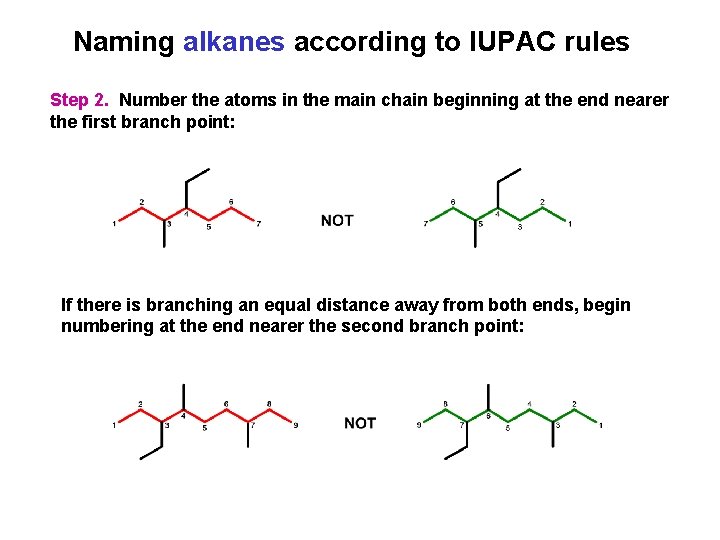
Naming alkanes according to IUPAC rules Step 2. Number the atoms in the main chain beginning at the end nearer the first branch point: If there is branching an equal distance away from both ends, begin numbering at the end nearer the second branch point:

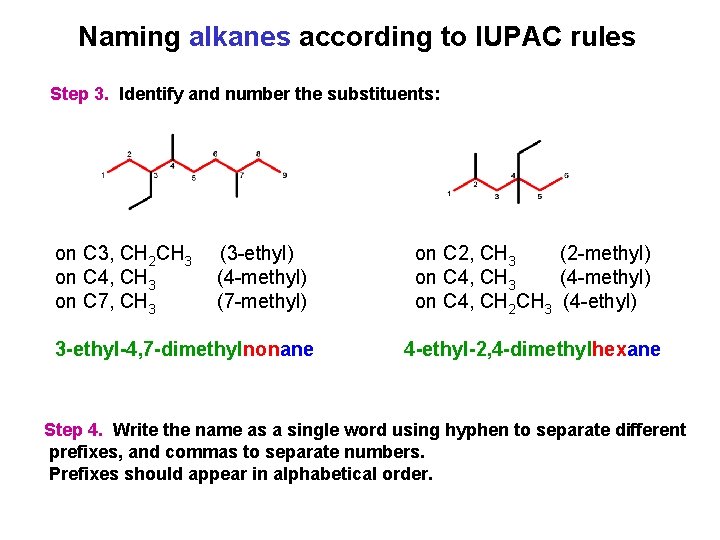
Naming alkanes according to IUPAC rules Step 3. Identify and number the substituents: on C 3, CH 2 CH 3 on C 4, CH 3 on C 7, CH 3 (3 -ethyl) (4 -methyl) (7 -methyl) 3 -ethyl-4, 7 -dimethylnonane on C 2, CH 3 (2 -methyl) on C 4, CH 3 (4 -methyl) on C 4, CH 2 CH 3 (4 -ethyl) 4 -ethyl-2, 4 -dimethylhexane Step 4. Write the name as a single word using hyphen to separate different prefixes, and commas to separate numbers. Prefixes should appear in alphabetical order.

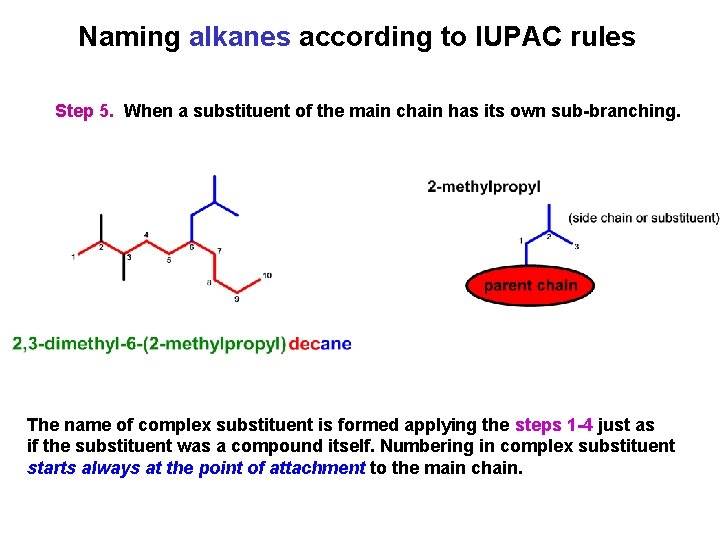
Naming alkanes according to IUPAC rules Step 5. When a substituent of the main chain has its own sub-branching. The name of complex substituent is formed applying the steps 1 -4 just as if the substituent was a compound itself. Numbering in complex substituent starts always at the point of attachment to the main chain.

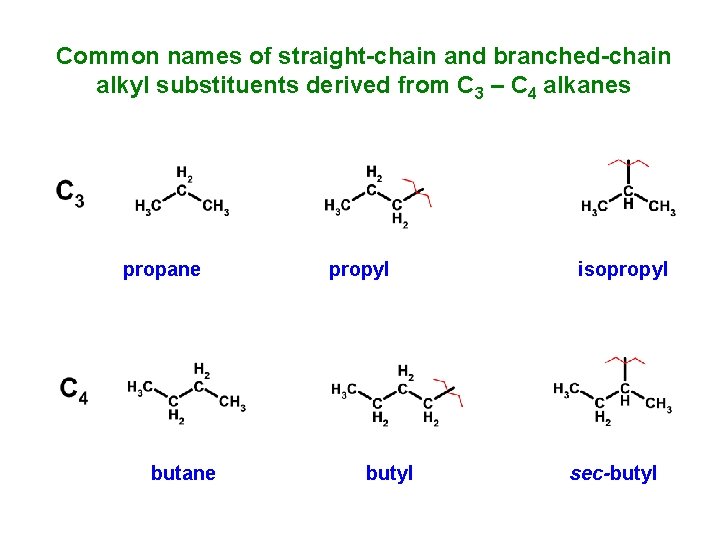
Common names of straight-chain and branched-chain alkyl substituents derived from C 3 – C 4 alkanes propane butane propyl butyl isopropyl sec-butyl

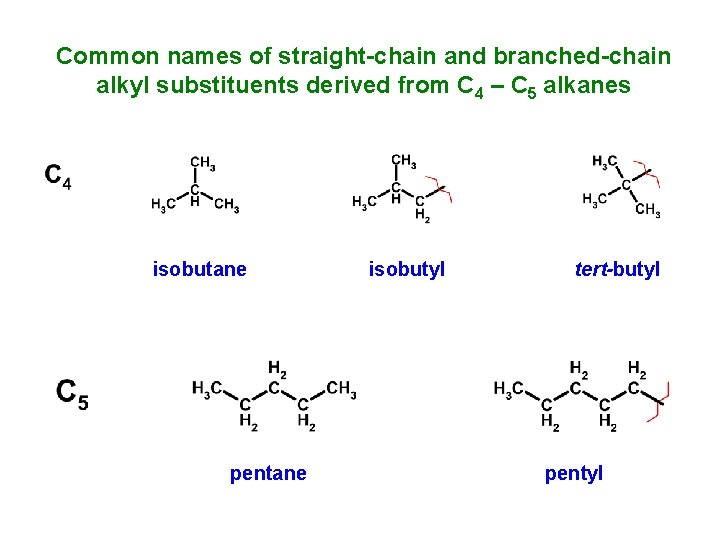
Common names of straight-chain and branched-chain alkyl substituents derived from C 4 – C 5 alkanes isobutane pentane isobutyl tert-butyl pentyl

Common names of straight-chain and branched-chain alkyl substituents derived from C 5 alkanes isopentane isopentyl neopentane tert-pentyl neopentyl

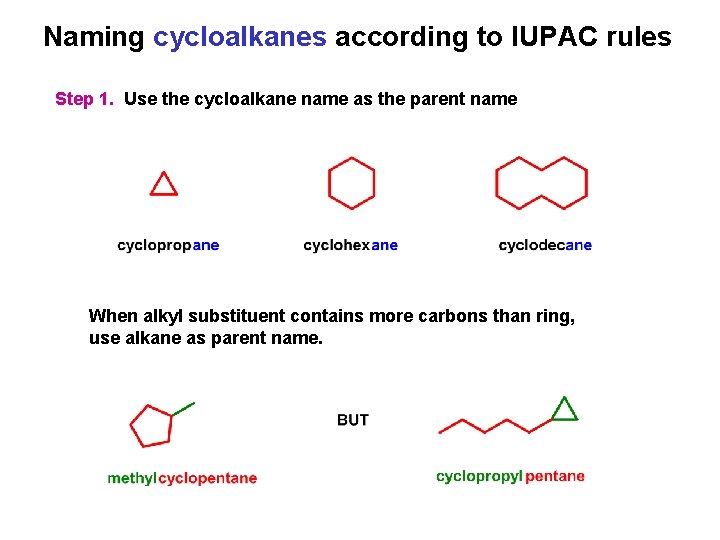
Naming cycloalkanes according to IUPAC rules Step 1. Use the cycloalkane name as the parent name When alkyl substituent contains more carbons than ring, use alkane as parent name.

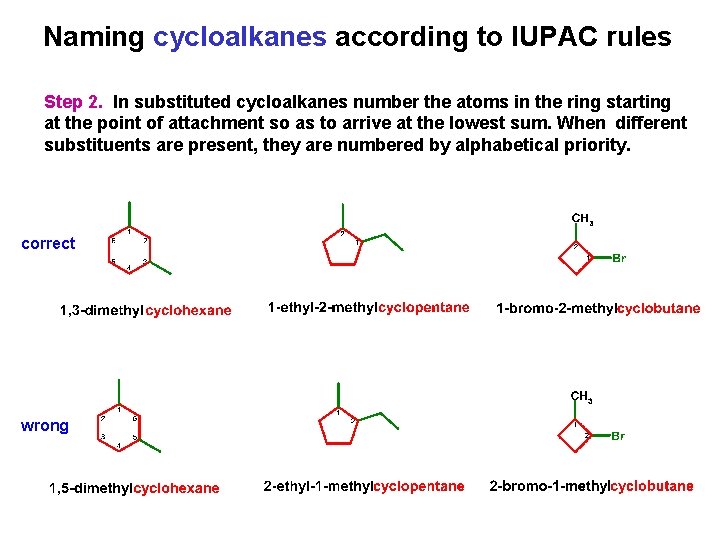
Naming cycloalkanes according to IUPAC rules Step 2. In substituted cycloalkanes number the atoms in the ring starting at the point of attachment so as to arrive at the lowest sum. When different substituents are present, they are numbered by alphabetical priority. correct wrong

Nomenclature of alkenes according to IUPAC rules Alkenes are named according to a series of rules similar to those developed for alkanes, with the suffix –ene used instead of -ane to identify the family. Step 1. Find the parent hydrocarbon – that means find the longest carbon chain containing the double bond:

Step 2. Number the atoms in the chain beginning at the end nearer the double bond. If the double bond is equidistant from the two ends, begin at the end nearer the first branch point. This rule assures that the double bond carbons receive the lowest possible numbers: 2 -hexene 2 -methyl-3 -hexene Step 3. Write the full name numbering the substituents according to their position in the chain and listing them alphabetically. Indicate the position of the double bond by giving the number of the first alkene carbon. If more than one double bond is present indicate the position of each and use the suffixes diene, -triene, -tetraene, and so on. 2 -ethyl-1 -pentene 2 -methyl-1, 3 -butadiene

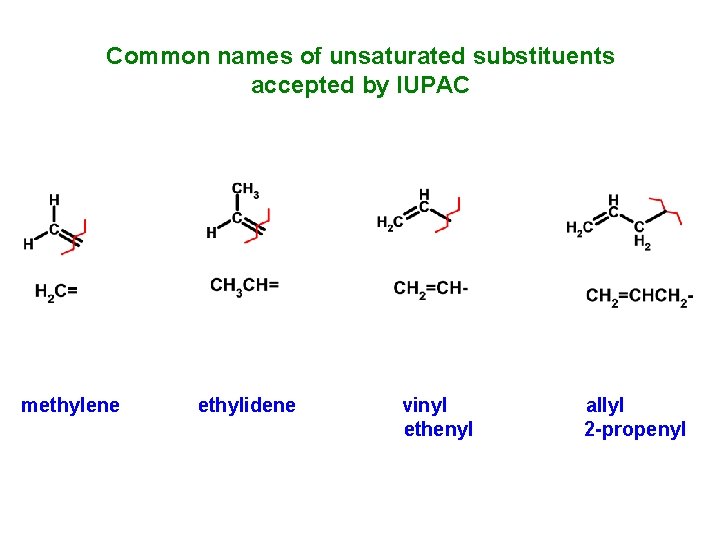
Common names of unsaturated substituents accepted by IUPAC methylene ethylidene vinyl ethenyl allyl 2 -propenyl

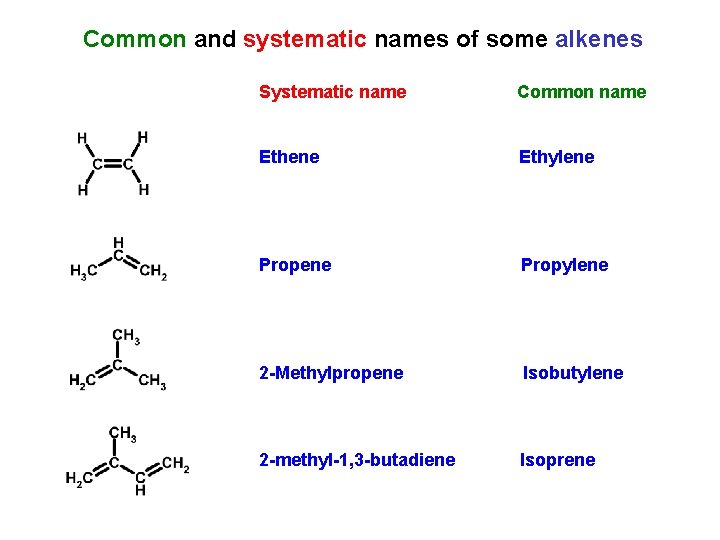
Common and systematic names of some alkenes Systematic name Common name Ethene Ethylene Propylene 2 -Methylpropene Isobutylene 2 -methyl-1, 3 -butadiene Isoprene

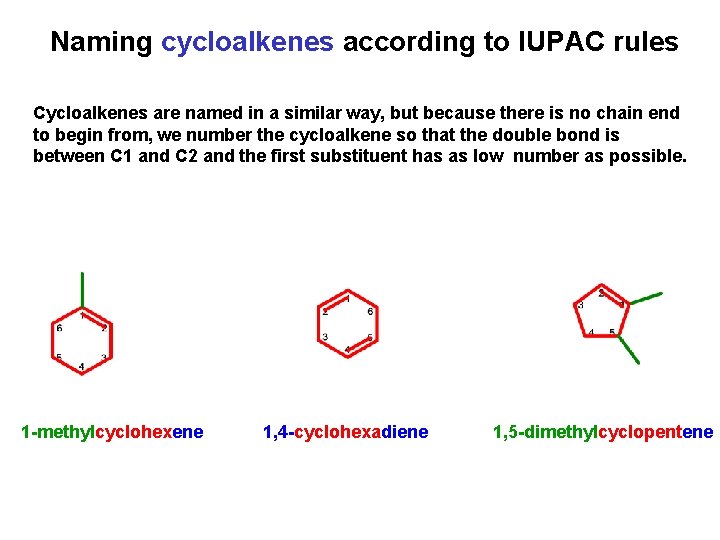
Naming cycloalkenes according to IUPAC rules Cycloalkenes are named in a similar way, but because there is no chain end to begin from, we number the cycloalkene so that the double bond is between C 1 and C 2 and the first substituent has as low number as possible. 1 -methylcyclohexene 1, 4 -cyclohexadiene 1, 5 -dimethylcyclopentene

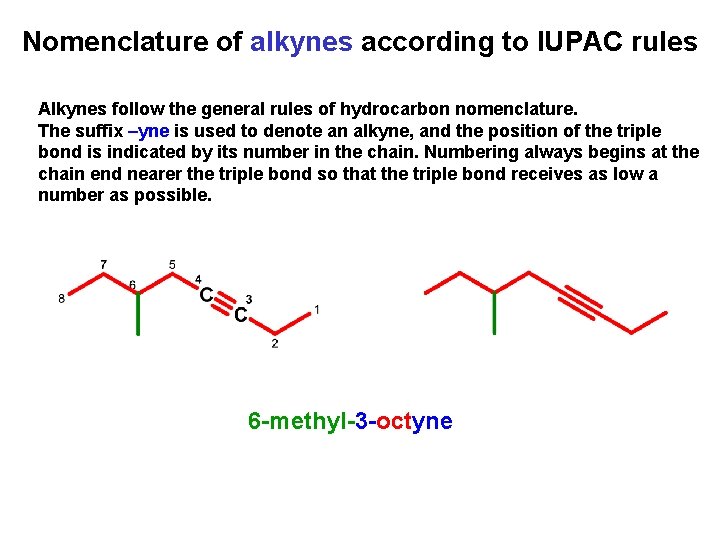
Nomenclature of alkynes according to IUPAC rules Alkynes follow the general rules of hydrocarbon nomenclature. The suffix –yne is used to denote an alkyne, and the position of the triple bond is indicated by its number in the chain. Numbering always begins at the chain end nearer the triple bond so that the triple bond receives as low a number as possible. 6 -methyl-3 -octyne

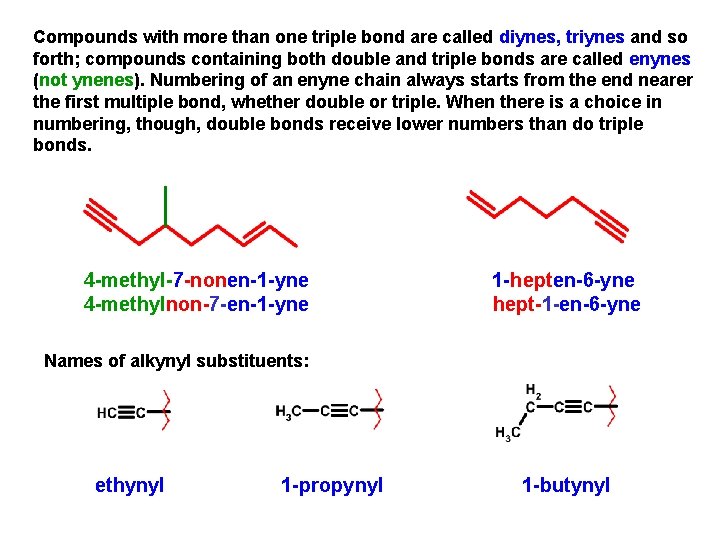
Compounds with more than one triple bond are called diynes, triynes and so forth; compounds containing both double and triple bonds are called enynes (not ynenes). Numbering of an enyne chain always starts from the end nearer the first multiple bond, whether double or triple. When there is a choice in numbering, though, double bonds receive lower numbers than do triple bonds. 4 -methyl-7 -nonen-1 -yne 4 -methylnon-7 -en-1 -yne 1 -hepten-6 -yne hept-1 -en-6 -yne Names of alkynyl substituents: ethynyl 1 -propynyl 1 -butynyl

Nomenclature of arenes Benzene Ethylbenzene Bromobenzene Disubstituted benzene derivatives: ortho-dibromobenzene meta-dibromobenzene m-dibromobenzene para-dibromobenzene p-dibromobenzene 1, 2 -dibromobenzene 1, 3 -dibromobenzene 1, 4 -dibromobenzene

In tri- and more substituted benzenes the lowest possible numbers are used, and substituents are listed alphabetically 1, 2 -dibromo-4 -methylbenzene 1, 3, 5 -trimethylbenzene 1, 4 -dibromo-2, 5 -dimethylbenzene

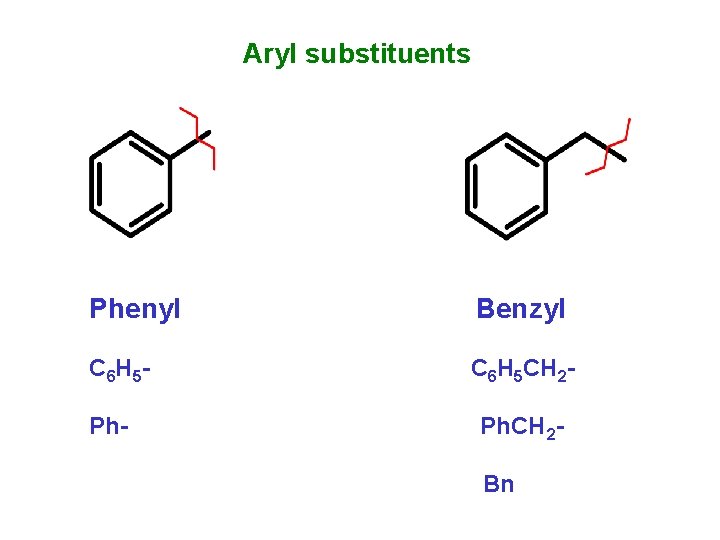
Aryl substituents Phenyl Benzyl C 6 H 5 - C 6 H 5 CH 2 - Ph. CH 2 Bn

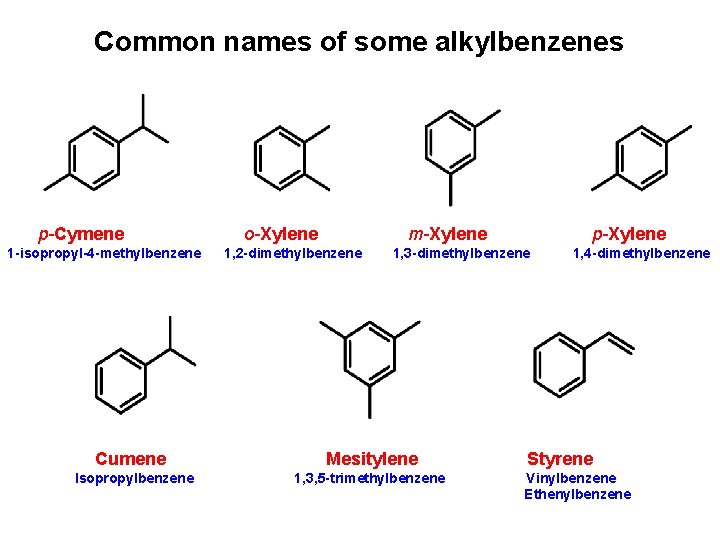
Common names of some alkylbenzenes p-Cymene 1 -isopropyl-4 -methylbenzene o-Xylene m-Xylene 1, 2 -dimethylbenzene p-Xylene 1, 3 -dimethylbenzene Cumene Mesitylene Isopropylbenzene 1, 3, 5 -trimethylbenzene 1, 4 -dimethylbenzene Styrene Vinylbenzene Ethenylbenzene

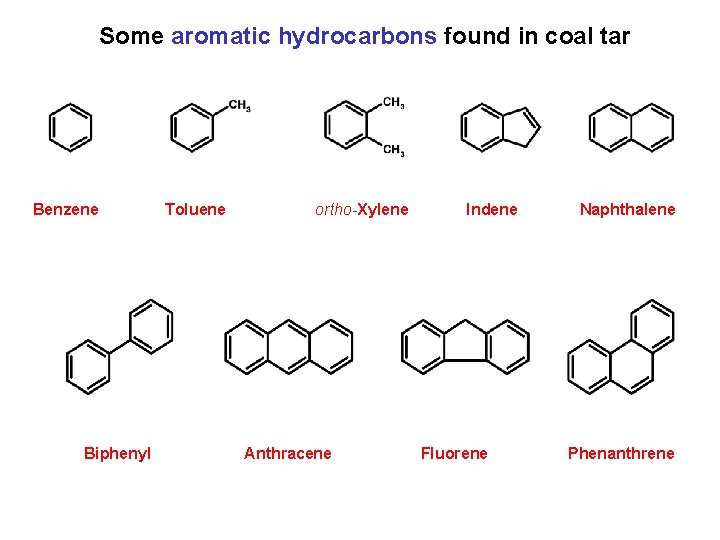
Some aromatic hydrocarbons found in coal tar Benzene Biphenyl Toluene ortho-Xylene Anthracene Indene Fluorene Naphthalene Phenanthrene

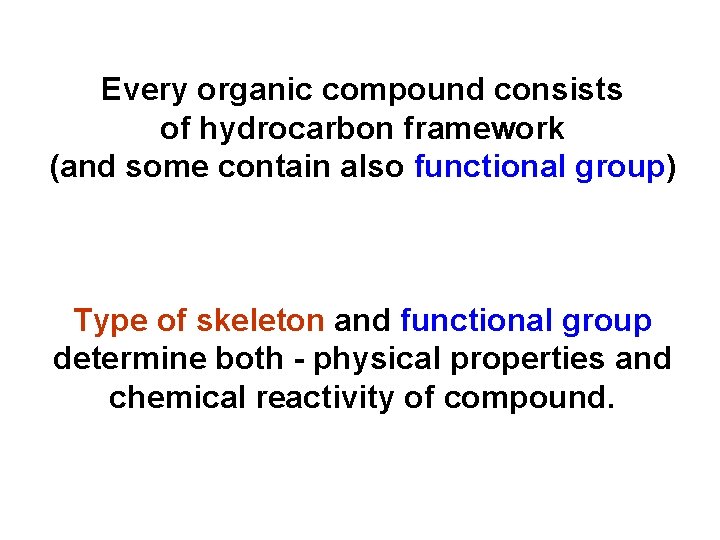
Every organic compound consists of hydrocarbon framework (and some contain also functional group) Type of skeleton and functional group determine both - physical properties and chemical reactivity of compound.

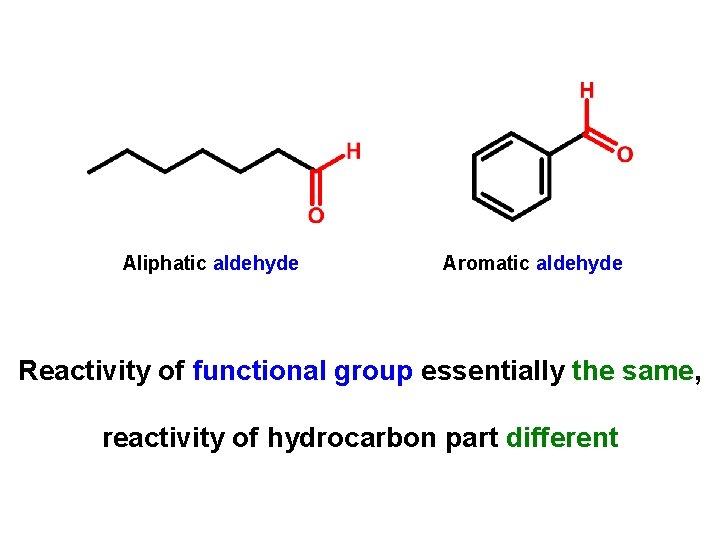
Aliphatic aldehyde Aromatic aldehyde Reactivity of functional group essentially the same, reactivity of hydrocarbon part different

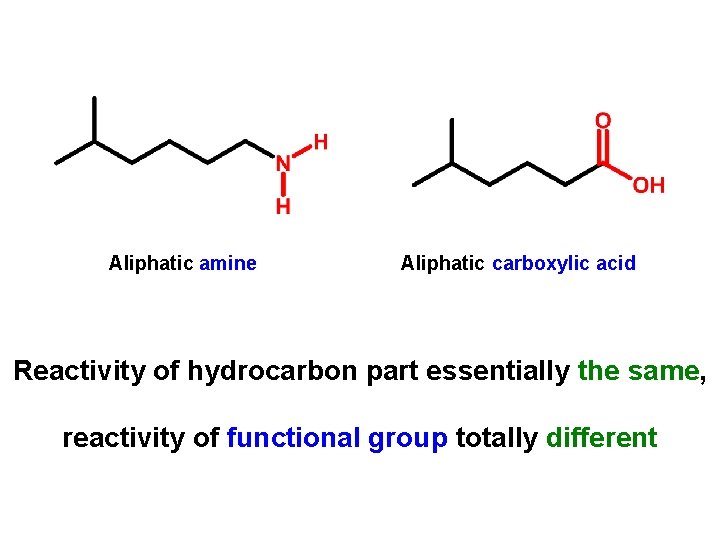
Aliphatic amine Aliphatic carboxylic acid Reactivity of hydrocarbon part essentially the same, reactivity of functional group totally different

Overview of functional groups in organic molecules

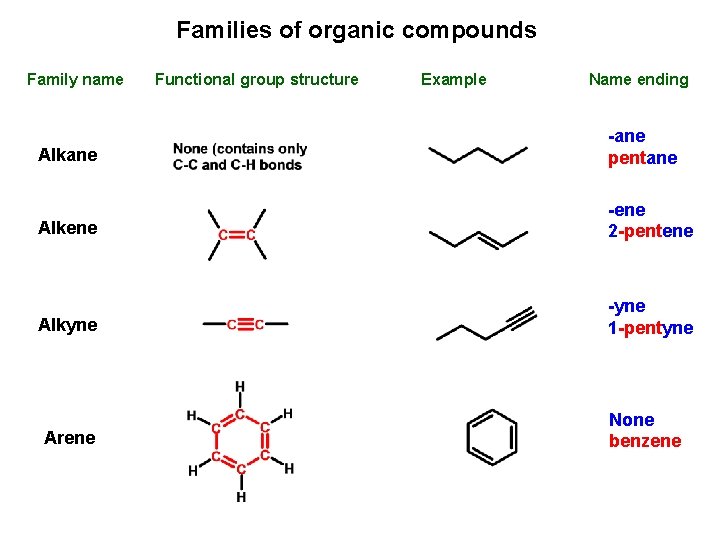
Families of organic compounds Family name Functional group structure Example Name ending Alkane -ane pentane Alkene -ene 2 -pentene Alkyne -yne 1 -pentyne Arene None benzene

Halide Alcohol Ether None 3 -chloropentane -ol 2 -pentanol ether dipentyl ether

Amine -amine pentylamine Nitrile -nitrile pentanenitrile Nitro None 3 -nitropentane

Thiol -thiol 2 -pentanthiol Sulfide sulfide dipentyl sulfide Sulfoxide sulfoxide dipentyl sulfoxide Sulfone sulfone dipentyl sulfone

Aldehyde Ketone -al pentanal -one 2 -pentanone

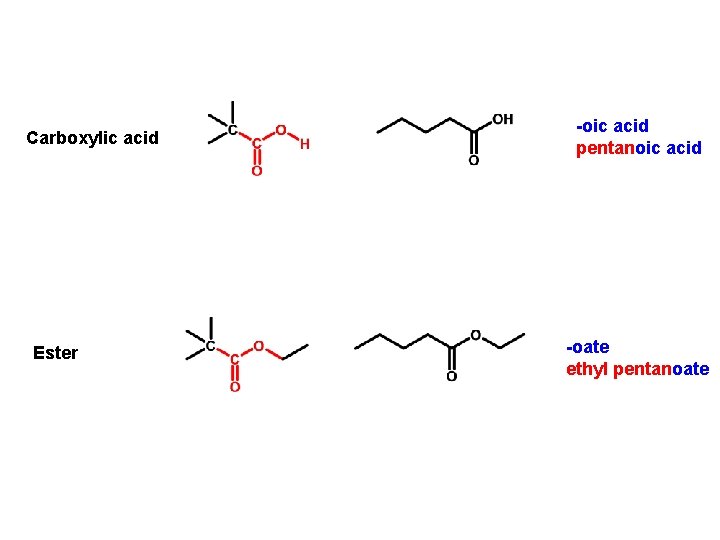
Carboxylic acid Ester -oic acid pentanoic acid -oate ethyl pentanoate

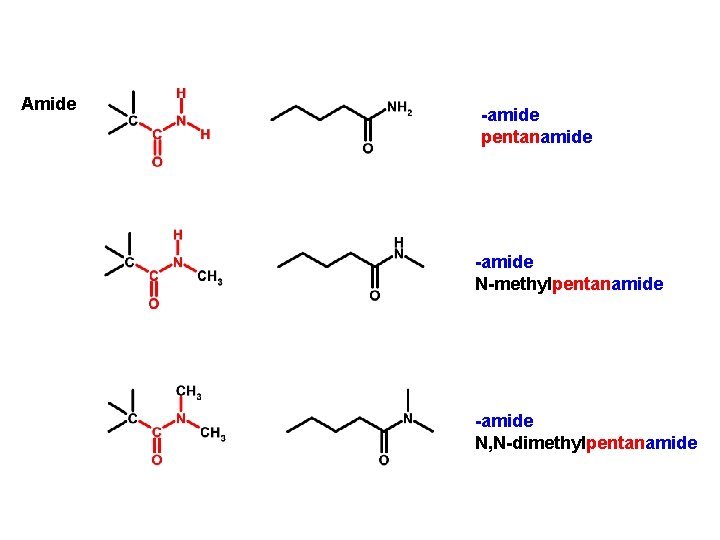
Amide -amide pentanamide -amide N-methylpentanamide -amide N, N-dimethylpentanamide

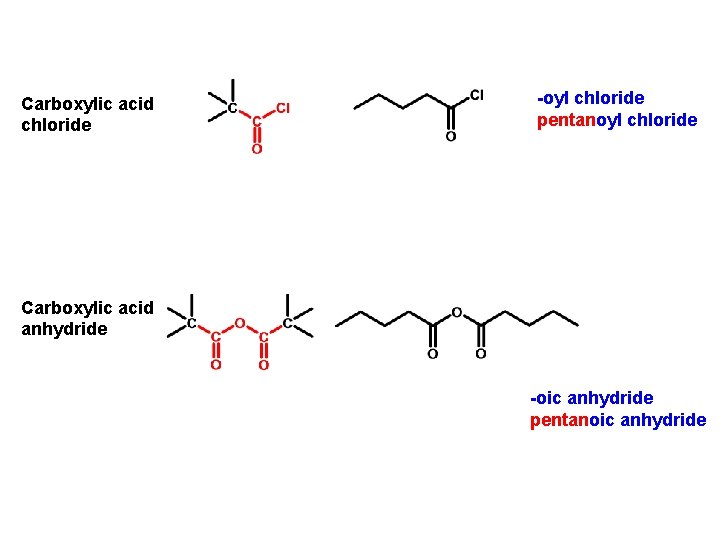
Carboxylic acid chloride -oyl chloride pentanoyl chloride Carboxylic acid anhydride -oic anhydride pentanoic anhydride

Naming of compounds containing single functional group

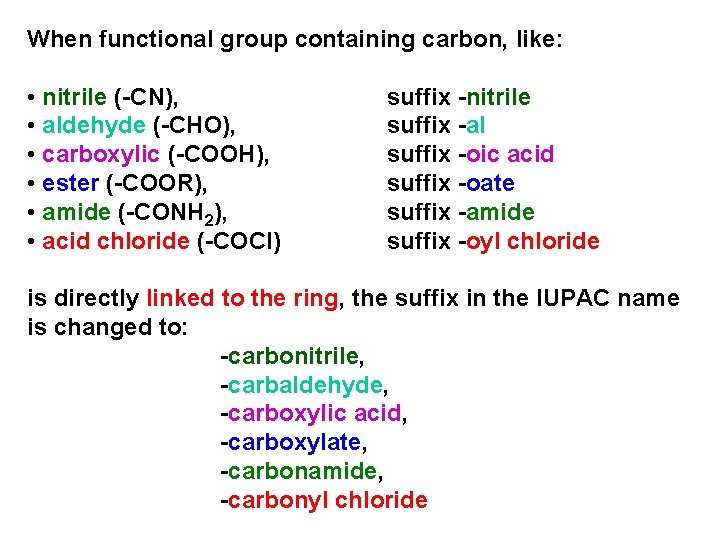
In chain structures with functional group containing carbon, like: • nitrile (-CN), • aldehyde (-CHO), • carboxylic acid (-COOH), • ester (-COOR), • amide (-CONH 2), • acid chloride (-COCl) carbon atom of functional group has to be number 1

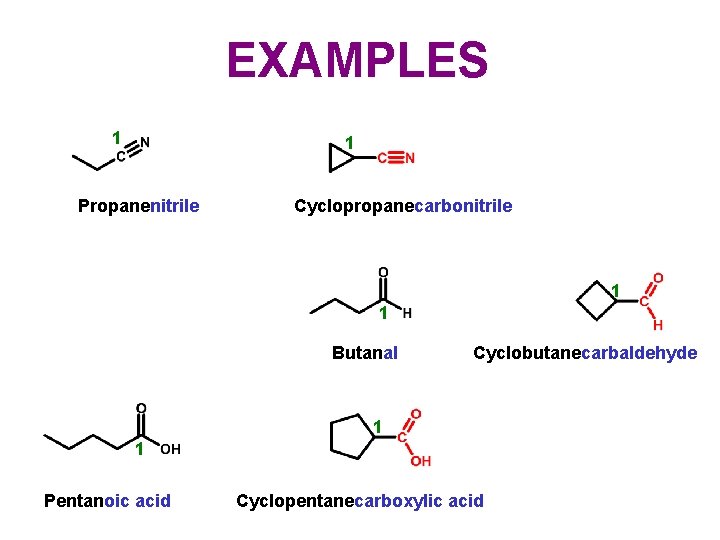
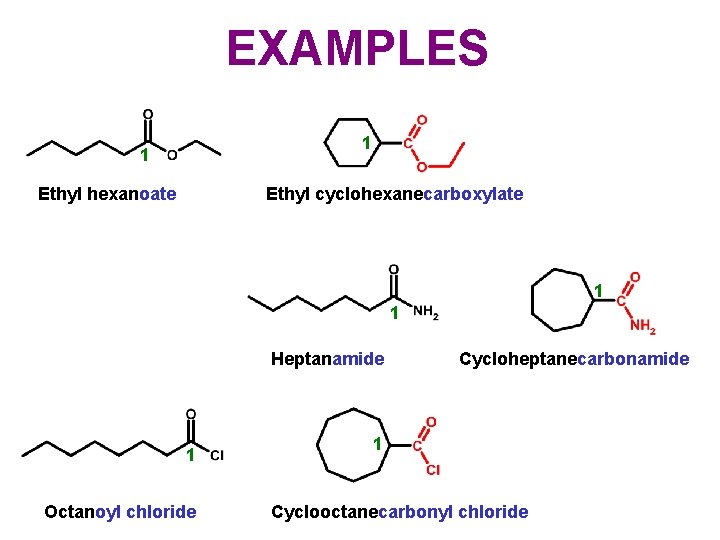
When functional group containing carbon, like: • nitrile (-CN), • aldehyde (-CHO), • carboxylic (-COOH), • ester (-COOR), • amide (-CONH 2), • acid chloride (-COCl) suffix -nitrile suffix -al suffix -oic acid suffix -oate suffix -amide suffix -oyl chloride is directly linked to the ring, the suffix in the IUPAC name is changed to: -carbonitrile, -carbaldehyde, -carboxylic acid, -carboxylate, -carbonamide, -carbonyl chloride

and ring carbon bonded to functional group is assign as number 1 !!!

EXAMPLES 1 1 Propanenitrile Cyclopropanecarbonitrile 1 1 Butanal Cyclobutanecarbaldehyde 1 1 Pentanoic acid Cyclopentanecarboxylic acid

EXAMPLES 1 1 Ethyl hexanoate Ethyl cyclohexanecarboxylate 1 1 Heptanamide 1 Octanoyl chloride Cycloheptanecarbonamide 1 Cyclooctanecarbonyl chloride

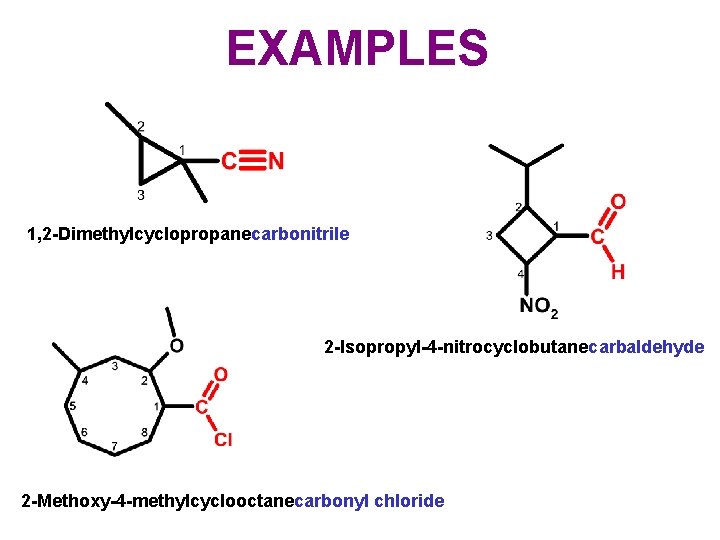
EXAMPLES 1, 2 -Dimethylcyclopropanecarbonitrile 2 -Isopropyl-4 -nitrocyclobutanecarbaldehyde 2 -Methoxy-4 -methylcyclooctanecarbonyl chloride

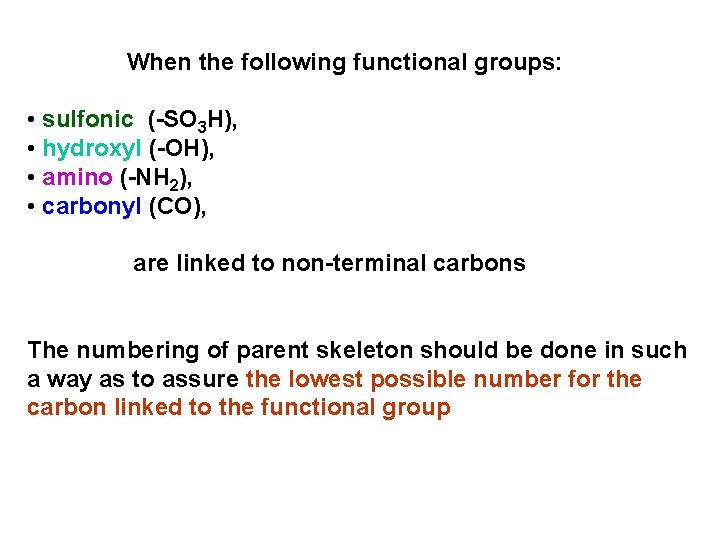
When the following functional groups: • sulfonic (-SO 3 H), • hydroxyl (-OH), • amino (-NH 2), • carbonyl (CO), are linked to non-terminal carbons The numbering of parent skeleton should be done in such a way as to assure the lowest possible number for the carbon linked to the functional group

EXAMPLES 5 -Methylheptane-3 -sulfonic acid 3 -Chloro-4 -methylbenzenesulfonic acid

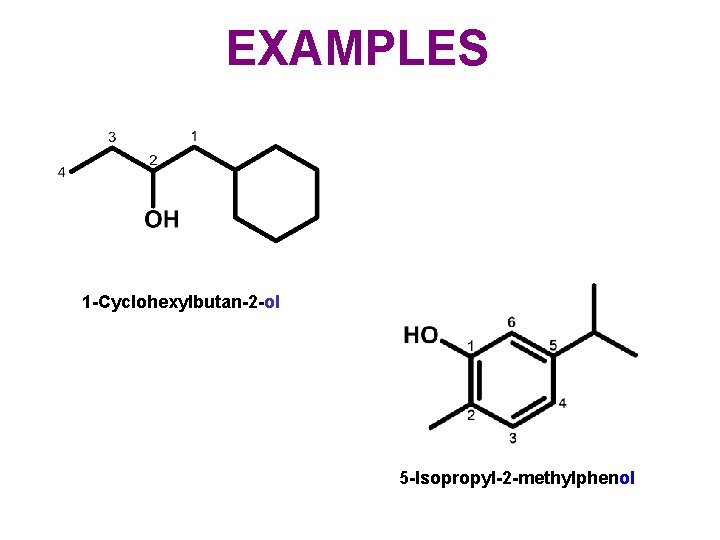
EXAMPLES 1 -Cyclohexylbutan-2 -ol 5 -Isopropyl-2 -methylphenol

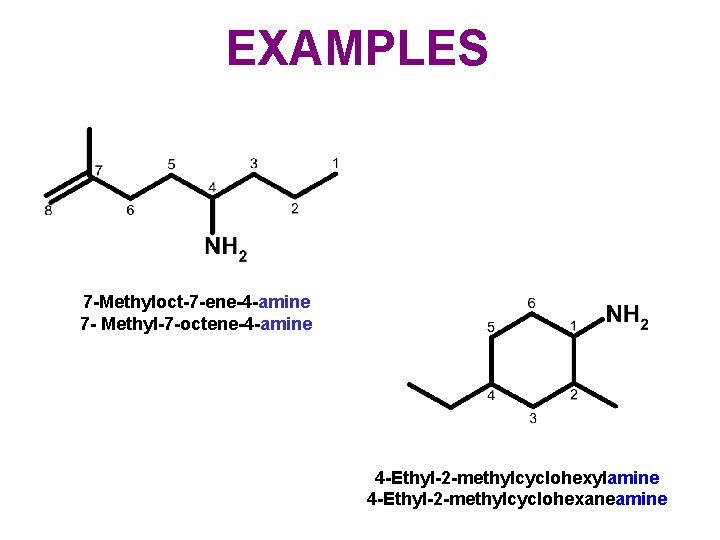
EXAMPLES 7 -Methyloct-7 -ene-4 -amine 7 - Methyl-7 -octene-4 -amine 4 -Ethyl-2 -methylcyclohexylamine 4 -Ethyl-2 -methylcyclohexaneamine

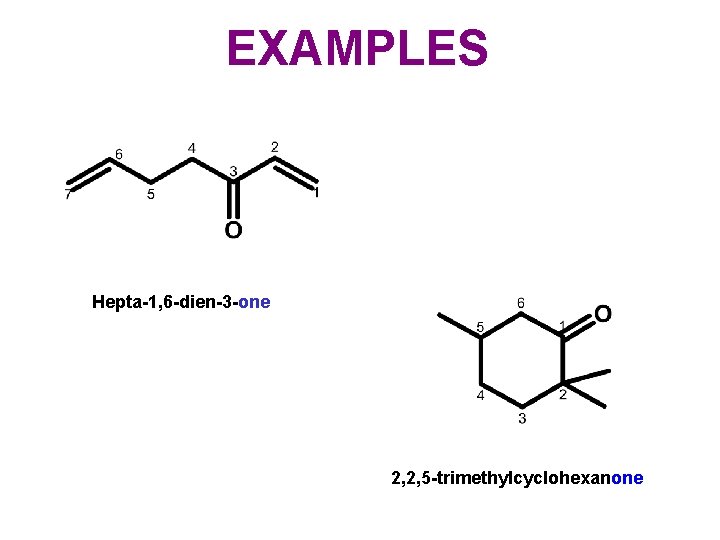
EXAMPLES Hepta-1, 6 -dien-3 -one 2, 2, 5 -trimethylcyclohexanone

All principal functional groups are of higher priority than C=C or C≡C bonds!!!

SUBORDINATE FUNCTIONAL GROUPS – EXCLUSIVELY AS PREFIXES -F fluoro- -Cl chloro- -Br bromo- -I iodo- -NO 2 nitro- -R alkyl- (methyl-, cyclopentyl-…. etc. ) -OR alkyloxy- or alkoxy (methoxy-, cyclopentyloxy-…etc. ) -Ar aryl- (phenyl-, naphthyl-, anthryl-…etc. ) -OAr aryloxy- or aroxy- (phenoxy-, naphthyloxy-…etc. )

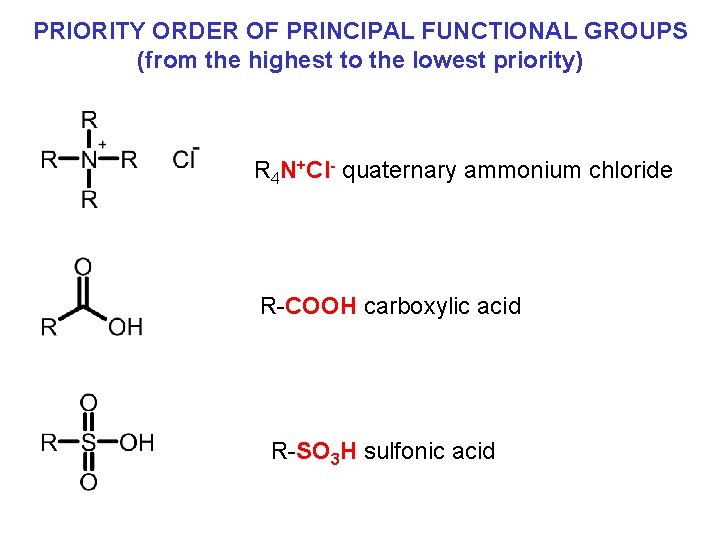
PRIORITY ORDER OF PRINCIPAL FUNCTIONAL GROUPS (from the highest to the lowest priority) R 4 N+Cl- quaternary ammonium chloride R-COOH carboxylic acid R-SO 3 H sulfonic acid

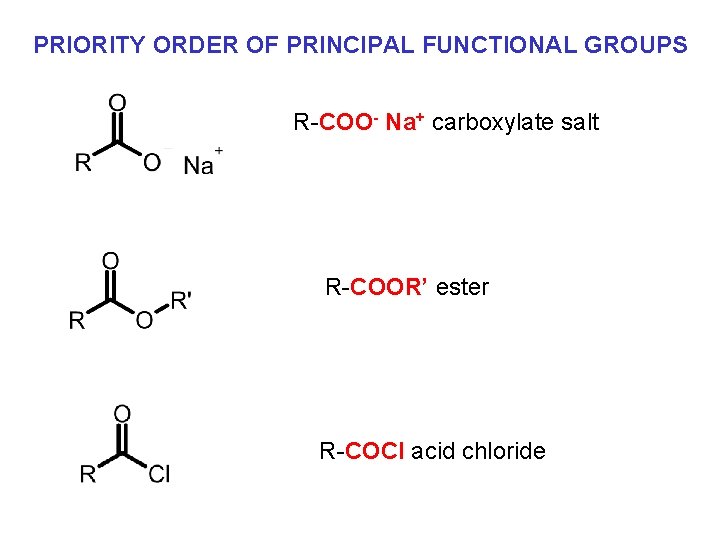
PRIORITY ORDER OF PRINCIPAL FUNCTIONAL GROUPS R-COO- Na+ carboxylate salt R-COOR’ ester R-COCl acid chloride

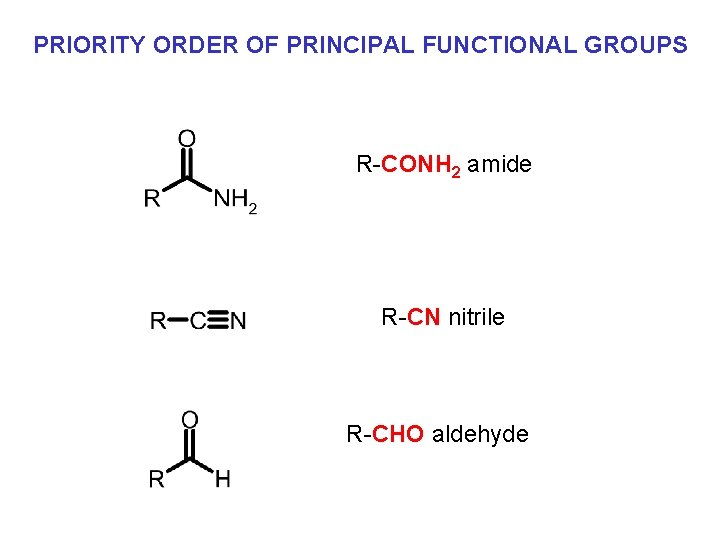
PRIORITY ORDER OF PRINCIPAL FUNCTIONAL GROUPS R-CONH 2 amide R-CN nitrile R-CHO aldehyde

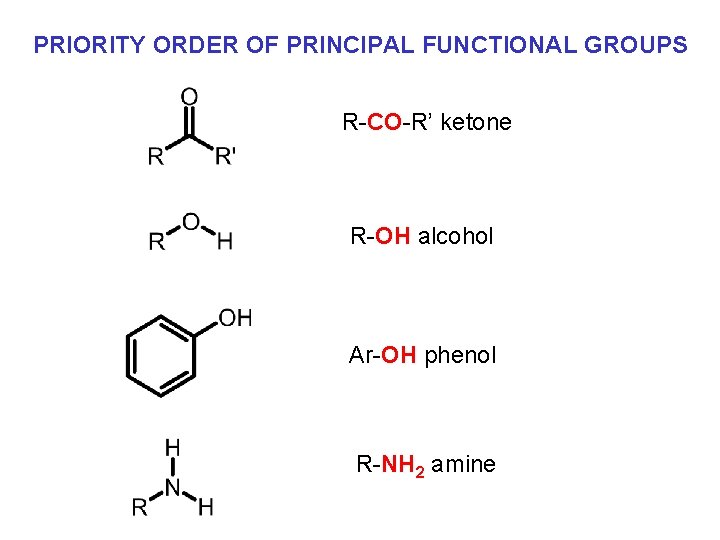
PRIORITY ORDER OF PRINCIPAL FUNCTIONAL GROUPS R-CO-R’ ketone R-OH alcohol Ar-OH phenol R-NH 2 amine

Common names of some functional benzene derivatives Phenol Acetophenone 1 -Phenylethanone Aniline Phenylamine Benzonitrile Benzenecarbonitrile Benzoic acid Benzaldehyde Benzenecarboxylic acid Benzenecarbaldehyde Salicylic acid 2 -Hydroxybenzenecarboxylic acid Nitrobenzene
 Ib organic chemistry functional groups
Ib organic chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Kamiska
Kamiska Kamiska
Kamiska Janina pawlowski
Janina pawlowski Janina miłosz
Janina miłosz Janina bauman lydia bauman
Janina bauman lydia bauman Inkrustasi
Inkrustasi Janina miłosz
Janina miłosz Aleksandra krawczyk
Aleksandra krawczyk Janina curk
Janina curk Kosystem
Kosystem Janina curk
Janina curk Janina curk
Janina curk Where is lysine found
Where is lysine found A level chemistry ocr organic synthesis
A level chemistry ocr organic synthesis Nbs
Nbs Father of organic chemistry
Father of organic chemistry Halohydrin
Halohydrin Met et prop but pent hex hept oct
Met et prop but pent hex hept oct Organic chemistry 2nd edition klein
Organic chemistry 2nd edition klein Cyclo organic chemistry
Cyclo organic chemistry Hammond's postulate organic chemistry
Hammond's postulate organic chemistry Cis-2 3-dimethyloxirane
Cis-2 3-dimethyloxirane David klein organic chemistry
David klein organic chemistry Iodine test for starch
Iodine test for starch Soap organic chemistry
Soap organic chemistry Polarimetry organic chemistry
Polarimetry organic chemistry David klein
David klein Organic chemistry nomenclature
Organic chemistry nomenclature Organic chemistry vocabulary
Organic chemistry vocabulary Organic chemistry
Organic chemistry Cycloalkanes
Cycloalkanes Ethene hbr
Ethene hbr Ir spectroscopy
Ir spectroscopy Objective lab report example
Objective lab report example Organic chemistry
Organic chemistry Organic chemistry
Organic chemistry Ee organic chemistry
Ee organic chemistry Klein
Klein What functional group is ch3
What functional group is ch3 Organic chemistry class 11 notes
Organic chemistry class 11 notes Rancidity meaning
Rancidity meaning Organic chemistry
Organic chemistry Topic 11 organic chemistry
Topic 11 organic chemistry Organic chemistry naming practice
Organic chemistry naming practice Fractional distillation of petroleum
Fractional distillation of petroleum Organic chemistry cheat sheet
Organic chemistry cheat sheet Structure and bonding in organic chemistry
Structure and bonding in organic chemistry Enols and enolates organic chemistry
Enols and enolates organic chemistry Is alkane an organic compound
Is alkane an organic compound Organic chemistry third edition david klein
Organic chemistry third edition david klein Resonance hybrid
Resonance hybrid Ester organic chemistry
Ester organic chemistry Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry
Organic chemistry What is organic chemistry like
What is organic chemistry like Cyclopentane condensed structural formula
Cyclopentane condensed structural formula Gc organic chemistry
Gc organic chemistry Organic chemistry
Organic chemistry Organic chemistry conversion chart
Organic chemistry conversion chart Organic vs inorganic compounds
Organic vs inorganic compounds Nonene
Nonene Met et prop but pent hex hept oct
Met et prop but pent hex hept oct Leveling effect organic chemistry
Leveling effect organic chemistry Organic chemistry chapter 9
Organic chemistry chapter 9 Hybridisation
Hybridisation Ario acidity
Ario acidity Butan 2 on
Butan 2 on Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry
Organic chemistry The art of writing reasonable organic reaction mechanisms
The art of writing reasonable organic reaction mechanisms Meth eth prop but
Meth eth prop but Mindup mind map
Mindup mind map Oxidation of carbohydrates
Oxidation of carbohydrates Organic chemistry
Organic chemistry Ario practice problems
Ario practice problems Organic chemistry
Organic chemistry Iupac
Iupac Pentanoic acid structure
Pentanoic acid structure Organic chemistry laboratory ch 2540 manual
Organic chemistry laboratory ch 2540 manual Chemistry
Chemistry Organic chemistry
Organic chemistry