Organic Chemistry 1 Dr Shatha AlAqeel Course Number

Organic Chemistry (1) Dr. Shatha Al-Aqeel

• Course Number and Symbol: Chem. 240 • Credit hours: (2)


Hydrocarbons
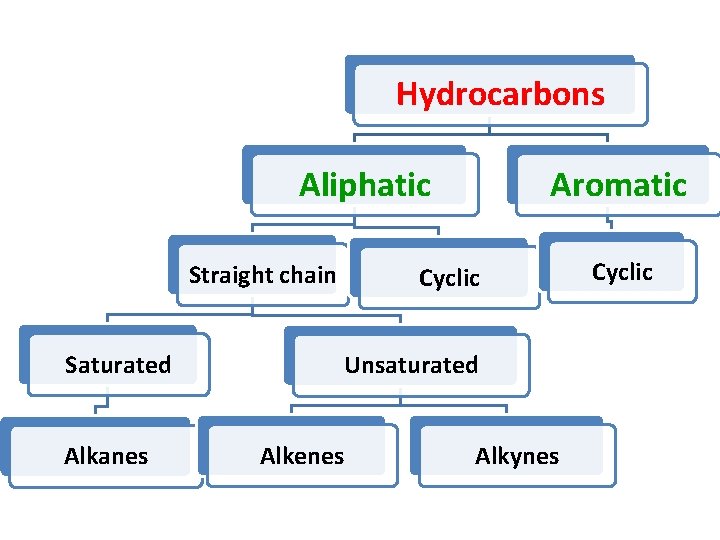
Hydrocarbons Aromatic Aliphatic Straight chain Saturated Alkanes Cyclic Unsaturated Alkenes Alkynes Cyclic
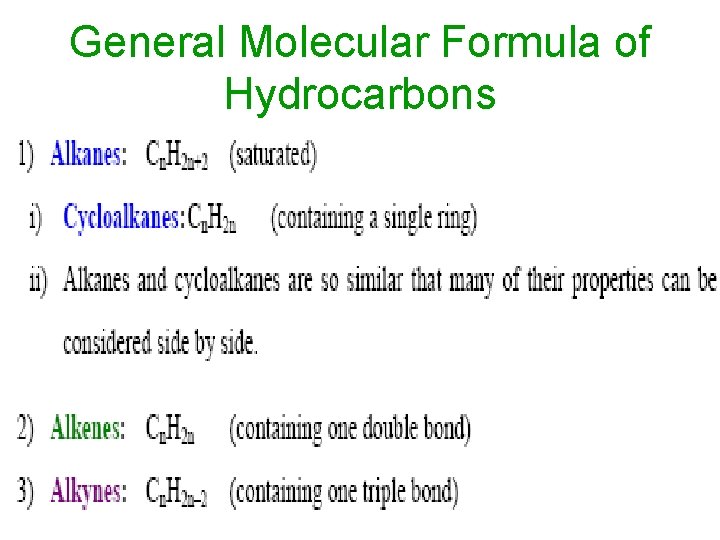
General Molecular Formula of Hydrocarbons
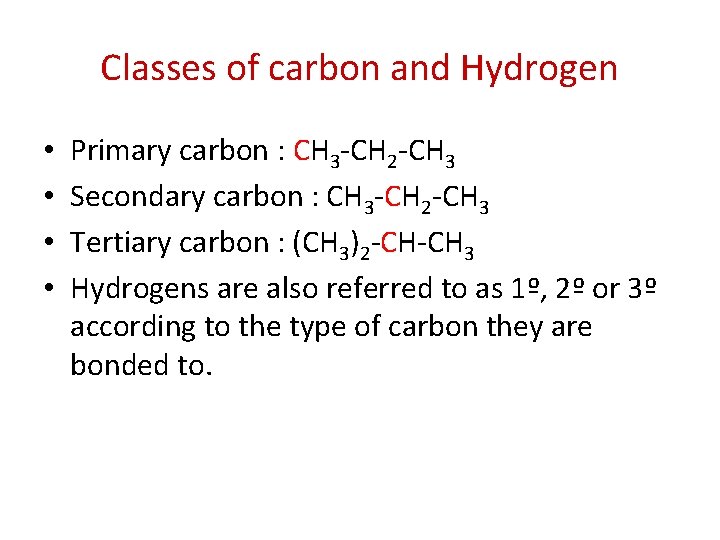
Classes of carbon and Hydrogen • • Primary carbon : CH 3 -CH 2 -CH 3 Secondary carbon : CH 3 -CH 2 -CH 3 Tertiary carbon : (CH 3)2 -CH-CH 3 Hydrogens are also referred to as 1º, 2º or 3º according to the type of carbon they are bonded to.
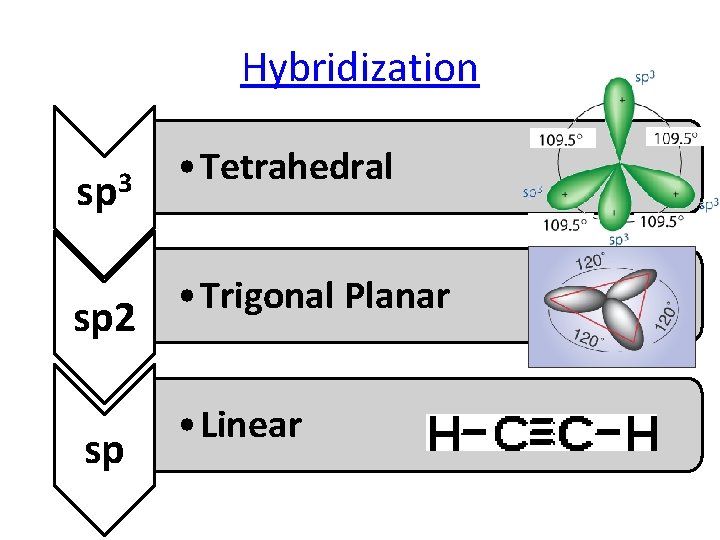
Hybridization sp 3 sp 2 sp • Tetrahedral • Trigonal Planar • Linear
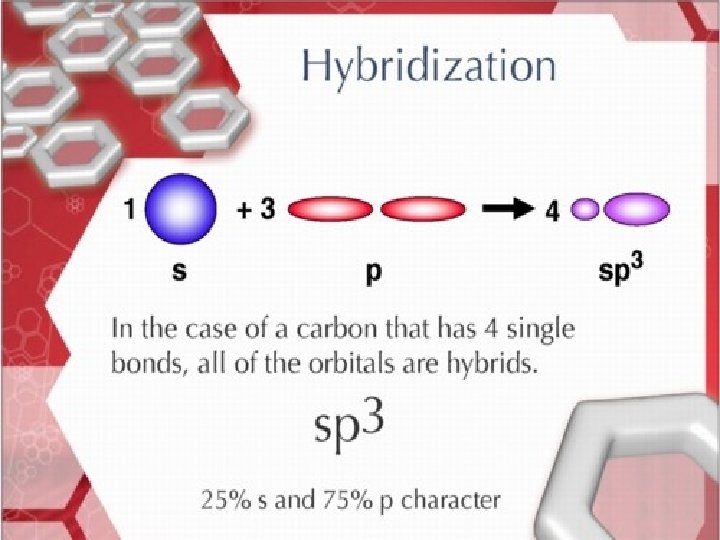
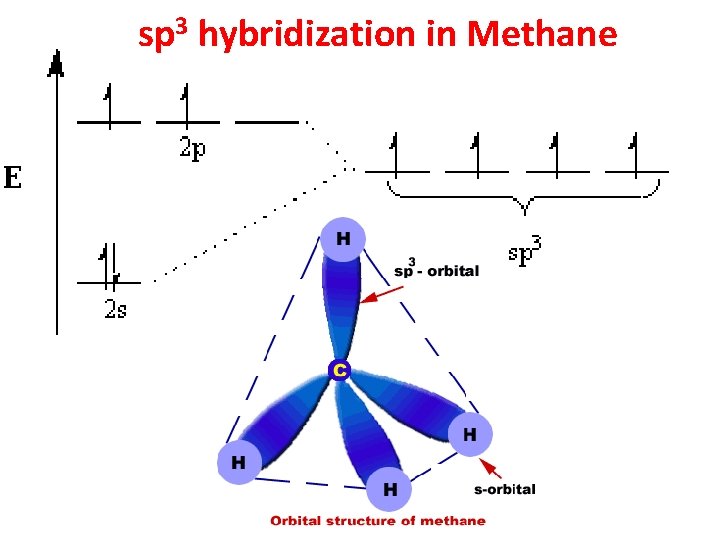
sp 3 hybridization in Methane
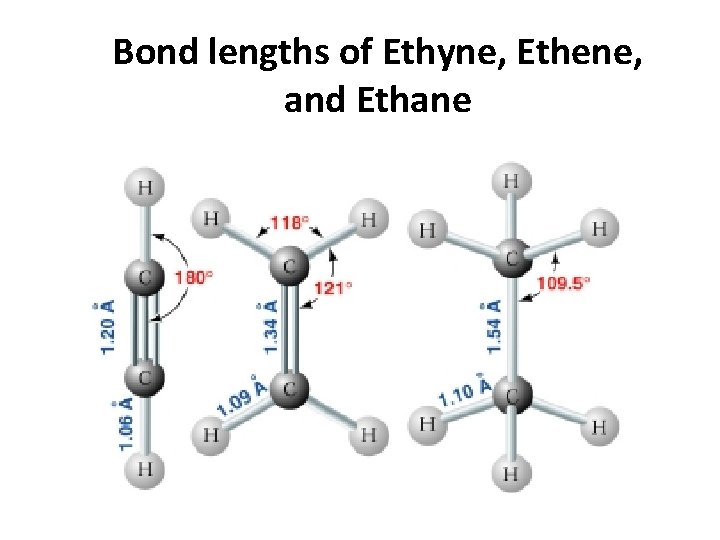
Bond lengths of Ethyne, Ethene, and Ethane
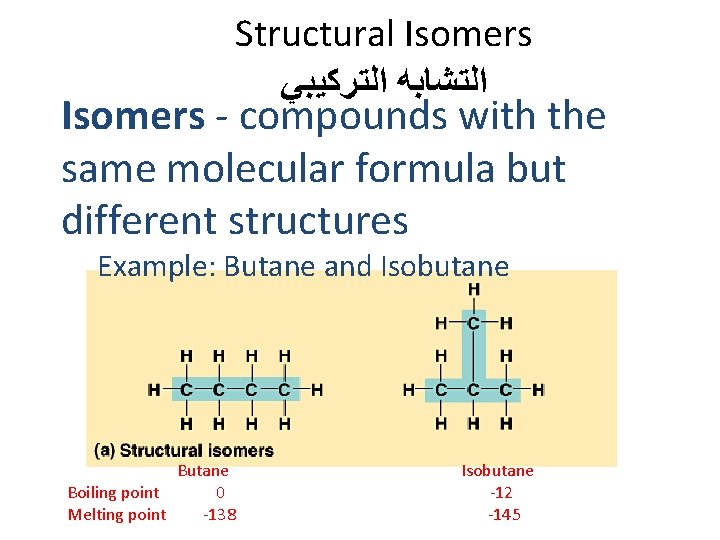
Structural Isomers ﺍﻟﺘﺸﺎﺑﻪ ﺍﻟﺘﺮﻛﻴﺒﻲ Isomers - compounds with the same molecular formula but different structures Example: Butane and Isobutane Boiling point 0 Melting point -138 Isobutane -12 -145
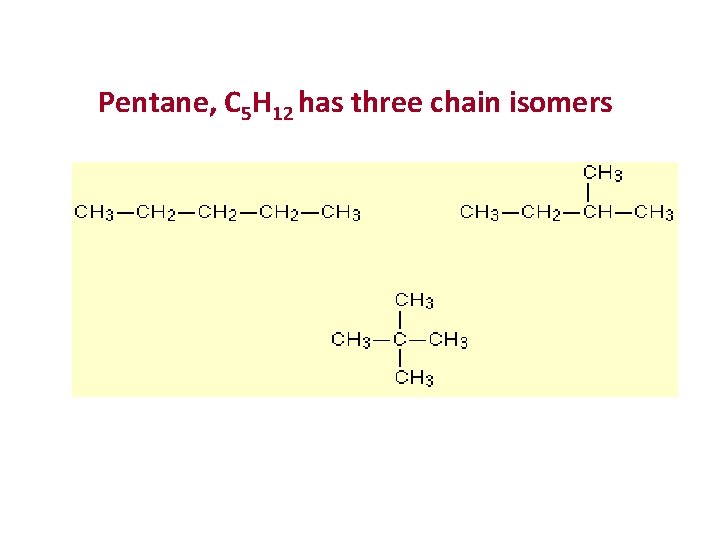
Pentane, C 5 H 12 has three chain isomers.
IUPAC Nomenclature • IUPAC (International Union of Pure and Applied Chemistry) names: • 1 - The unbranched alkanes (homologous series) • 2 - Branched alkanes
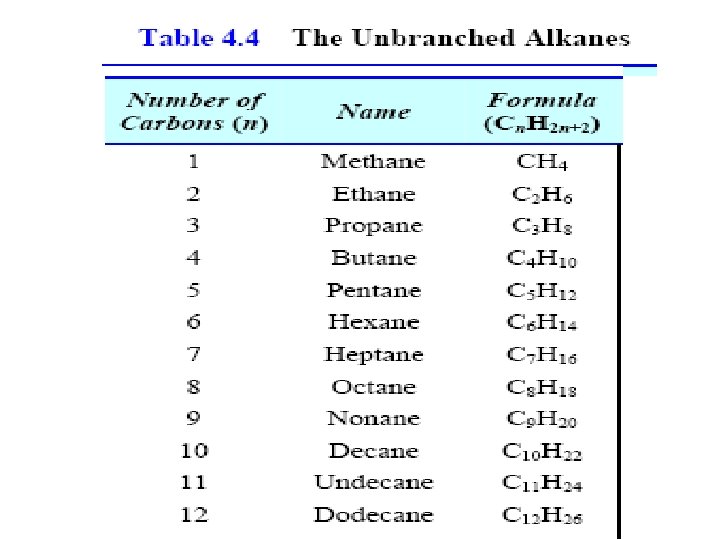
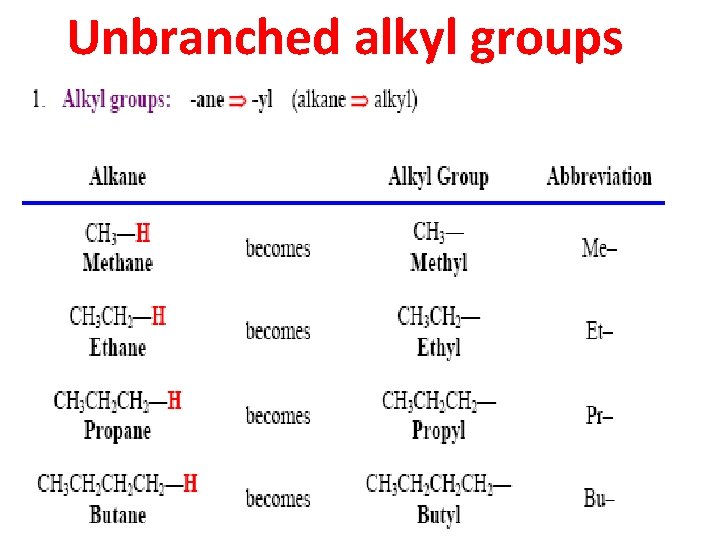
Unbranched alkyl groups
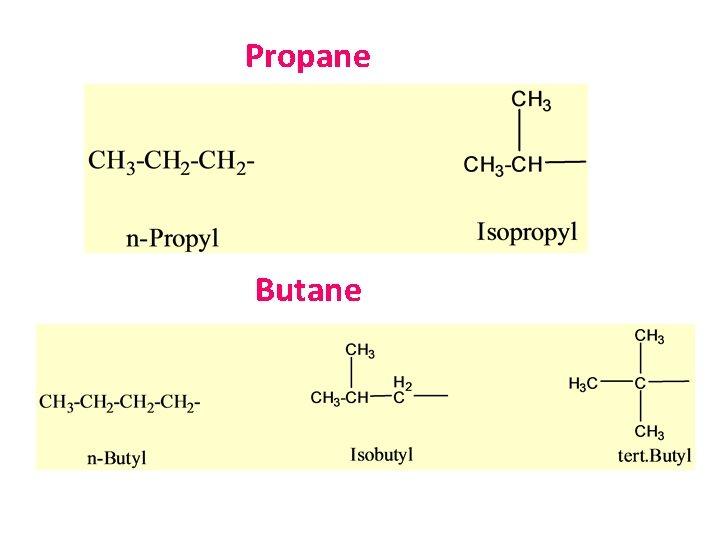
Propane Butane
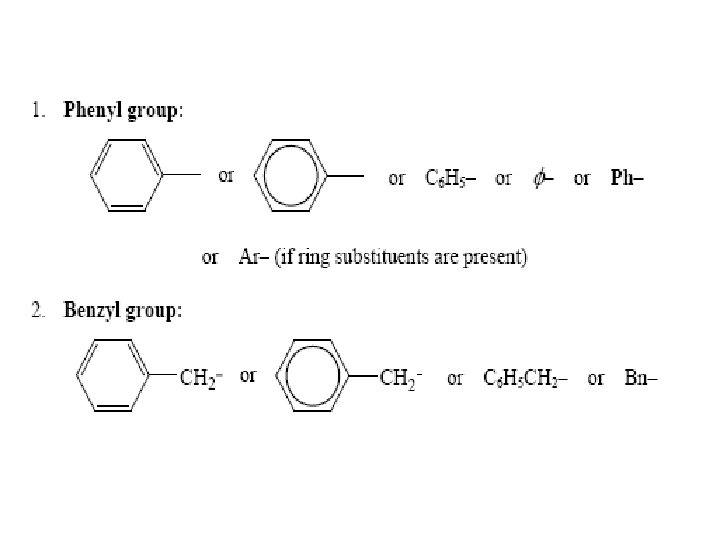
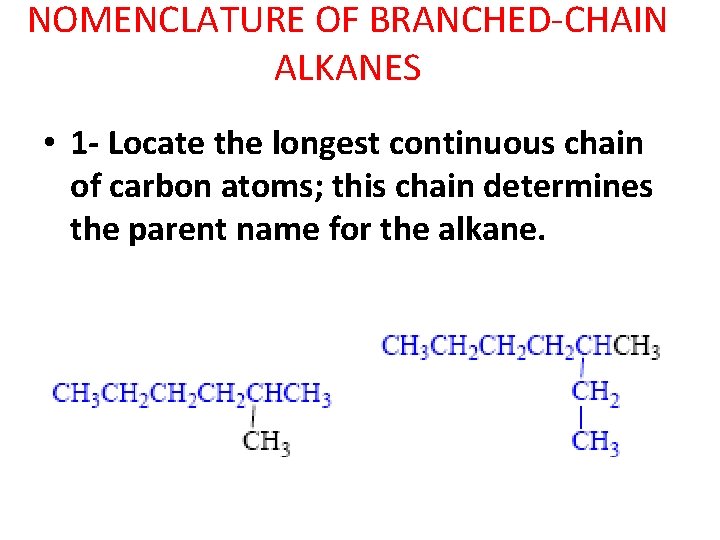
NOMENCLATURE OF BRANCHED-CHAIN ALKANES • 1 - Locate the longest continuous chain of carbon atoms; this chain determines the parent name for the alkane.
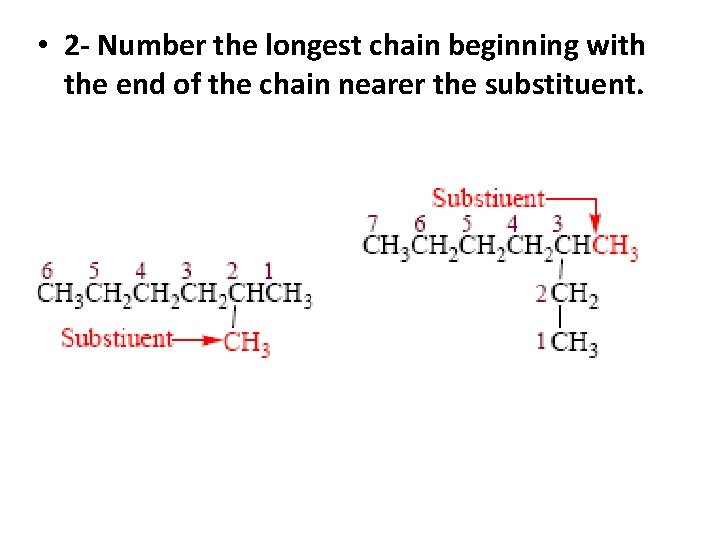
• 2 - Number the longest chain beginning with the end of the chain nearer the substituent.
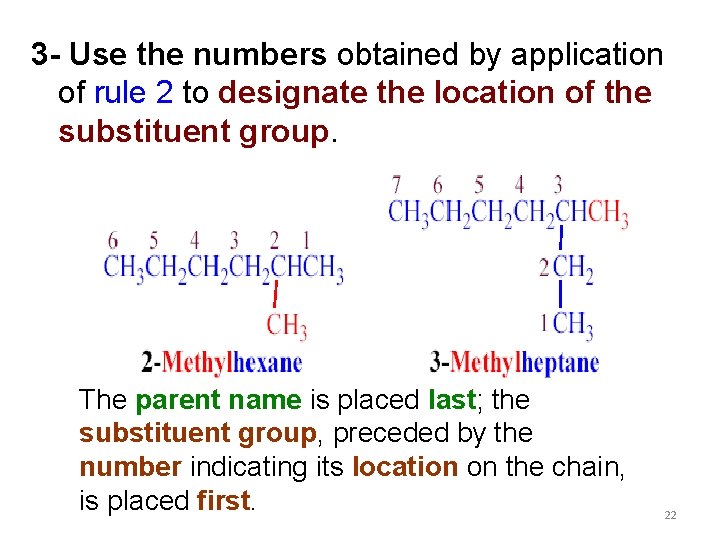
3 - Use the numbers obtained by application of rule 2 to designate the location of the substituent group. The parent name is placed last; the substituent group, preceded by the number indicating its location on the chain, is placed first. 22
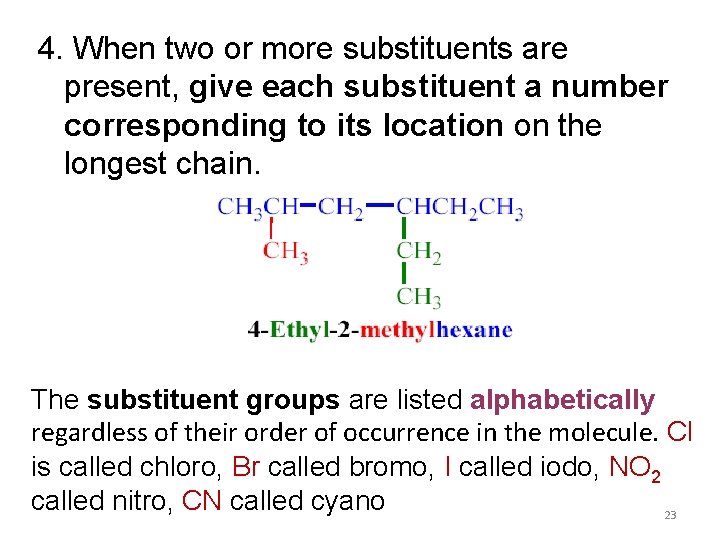
4. When two or more substituents are present, give each substituent a number corresponding to its location on the longest chain. The substituent groups are listed alphabetically regardless of their order of occurrence in the molecule. Cl is called chloro, Br called bromo, I called iodo, NO 2 called nitro, CN called cyano 23
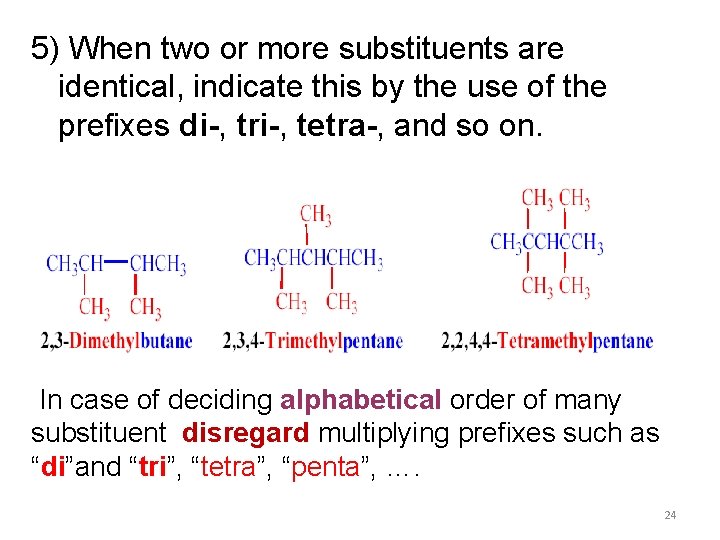
5) When two or more substituents are identical, indicate this by the use of the prefixes di-, tri-, tetra-, and so on. In case of deciding alphabetical order of many substituent disregard multiplying prefixes such as “di”and “tri”, “tetra”, “penta”, …. 24
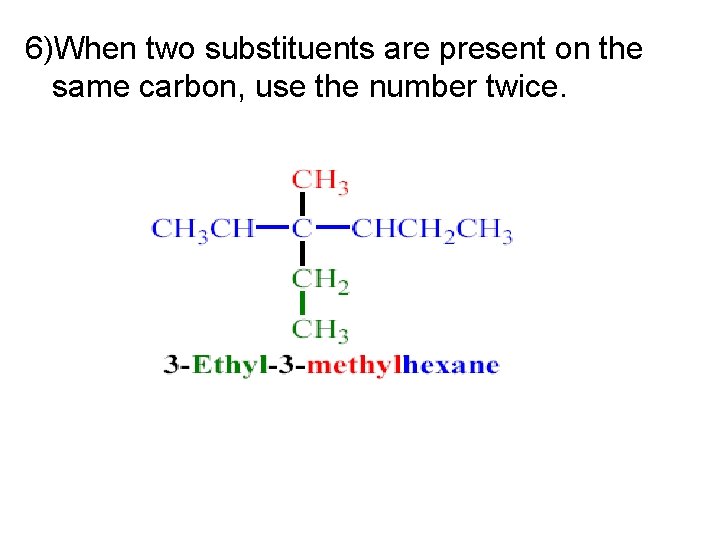
6)When two substituents are present on the same carbon, use the number twice.
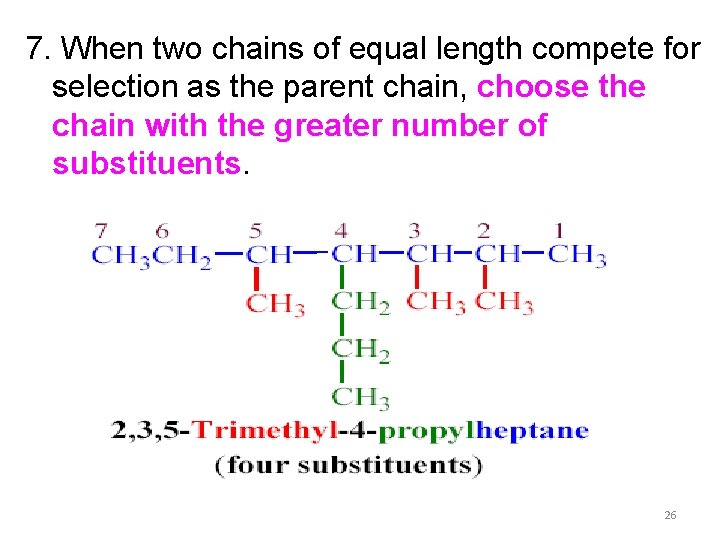
7. When two chains of equal length compete for selection as the parent chain, choose the chain with the greater number of substituents. 26
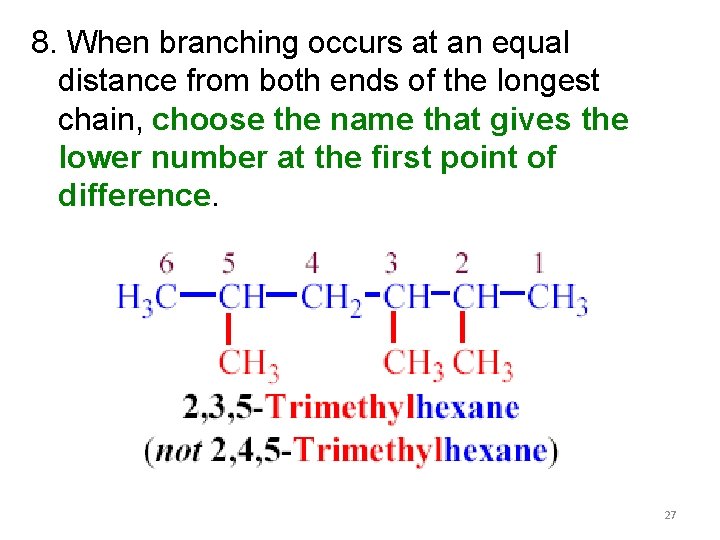
8. When branching occurs at an equal distance from both ends of the longest chain, choose the name that gives the lower number at the first point of difference. 27

• If any other substituents are found on the parent chain, all these substituents are arranged alphabetically. • • • -NO 2 nitro - NH 2 amino -CN cyano - Cl Chloro -Br bromo - I iodo
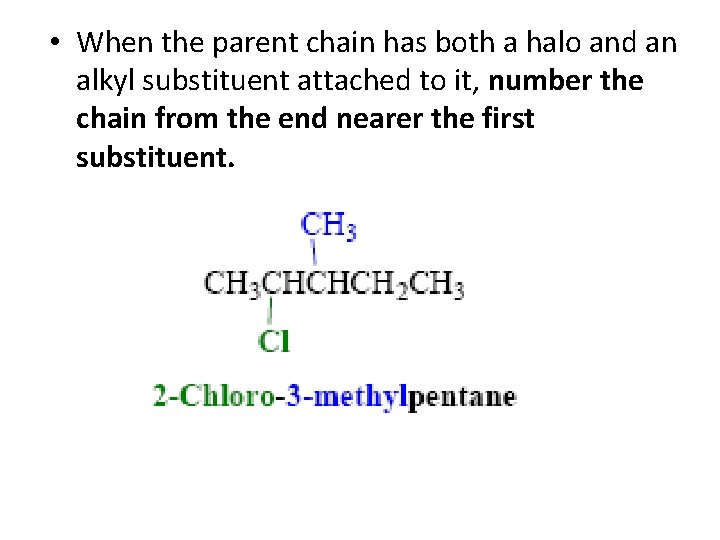
• When the parent chain has both a halo and an alkyl substituent attached to it, number the chain from the end nearer the first substituent.
Important note • The common names isopropyl, isobutyl, secbutyl, tert-butyl are approved by the IUPAC for the unsubstituted groups. • In deciding on alphabetically order disregard structure-defining prefixes that are written in italics and separated from the name by a hyphen. Thus “tert-butyl” precedes “ethyl”, but “ethyl” precedes “isobutyl”.

PHYSICAL PROPERTIES OF ALKANES • 1) At room temperature (rt, 25 °C) and 1 atm pressure, the C 1 -C 4 unbranched alkanes are gases; the C 5 -C 17 unbranched alkanes are liquids; the unbranched alkanes with 18 or more carbon atoms are solids.

BOILING POINTS • The boiling points of alkanes increase with molecular weight. • Branching reduces the boiling point, the more branching the lower the boiling point. • Alkanes are almost completely insoluble in water.
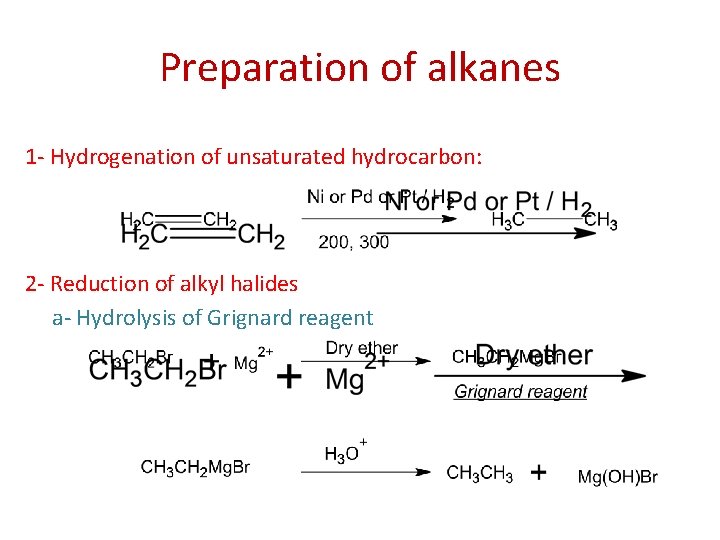
Preparation of alkanes 1 - Hydrogenation of unsaturated hydrocarbon: 2 - Reduction of alkyl halides a- Hydrolysis of Grignard reagent
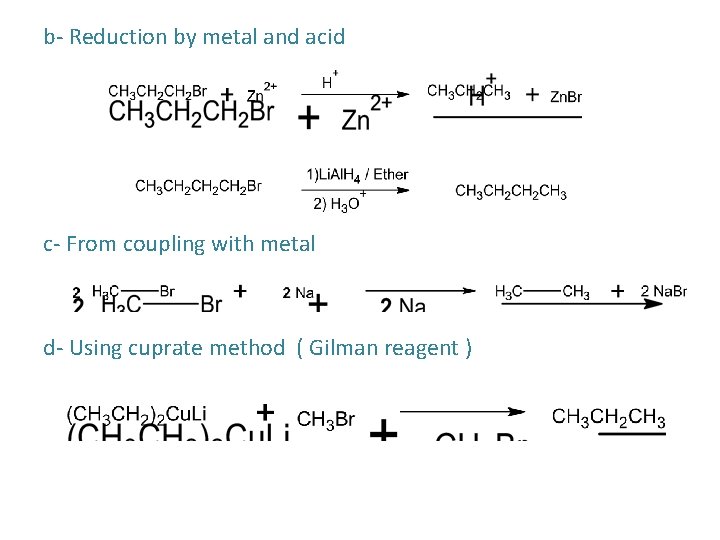
b- Reduction by metal and acid c- From coupling with metal d- Using cuprate method ( Gilman reagent )
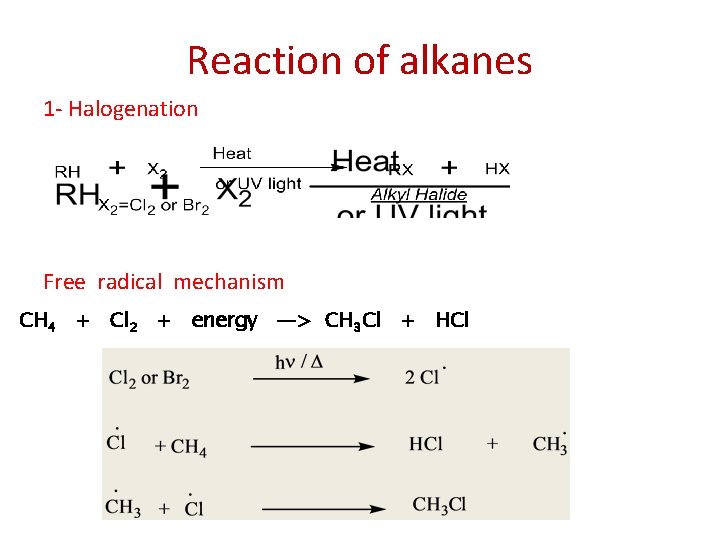
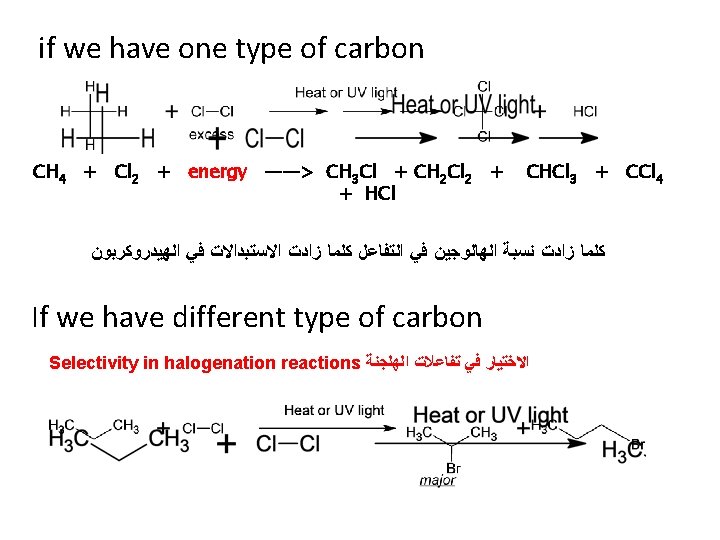
Reaction of alkanes 1 - Halogenation Free radical mechanism CH 4 + Cl 2 + energy —> CH 3 Cl + HCl
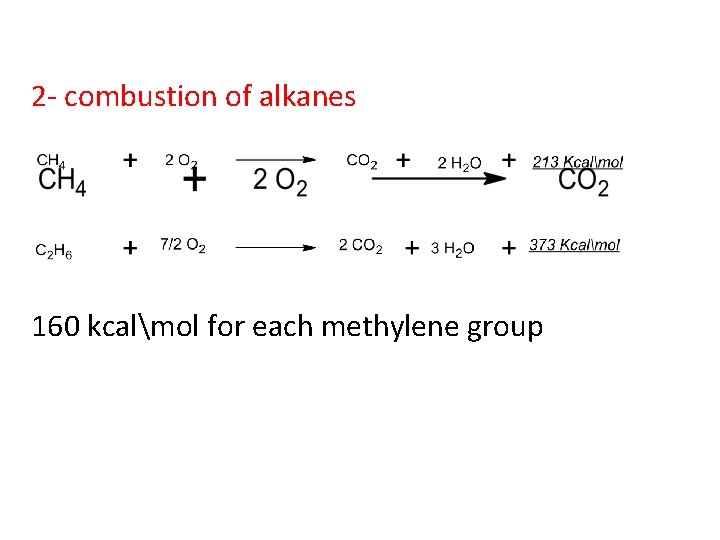
2 - combustion of alkanes 160 kcalmol for each methylene group
- Slides: 37