ORGANIC CHEMISTRY 1 Cycloalkanes Synthesis and Properties Cycloalkanes




- Slides: 29

ORGANIC CHEMISTRY- 1 Cycloalkanes Synthesis and Properties

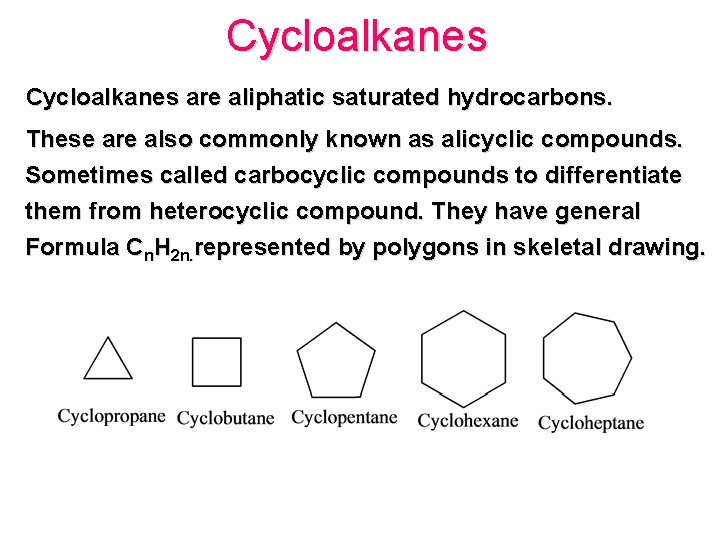
Cycloalkanes are aliphatic saturated hydrocarbons. These are also commonly known as alicyclic compounds. Sometimes called carbocyclic compounds to differentiate them from heterocyclic compound. They have general Formula Cn. H 2 n. represented by polygons in skeletal drawing.

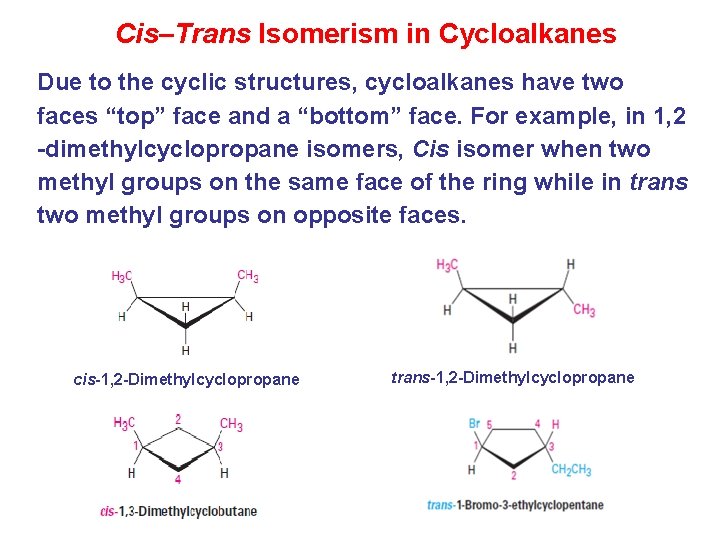
Cis–Trans Isomerism in Cycloalkanes Due to the cyclic structures, cycloalkanes have two faces “top” face and a “bottom” face. For example, in 1, 2 -dimethylcyclopropane isomers, Cis isomer when two methyl groups on the same face of the ring while in trans two methyl groups on opposite faces. cis-1, 2 -Dimethylcyclopropane trans-1, 2 -Dimethylcyclopropane

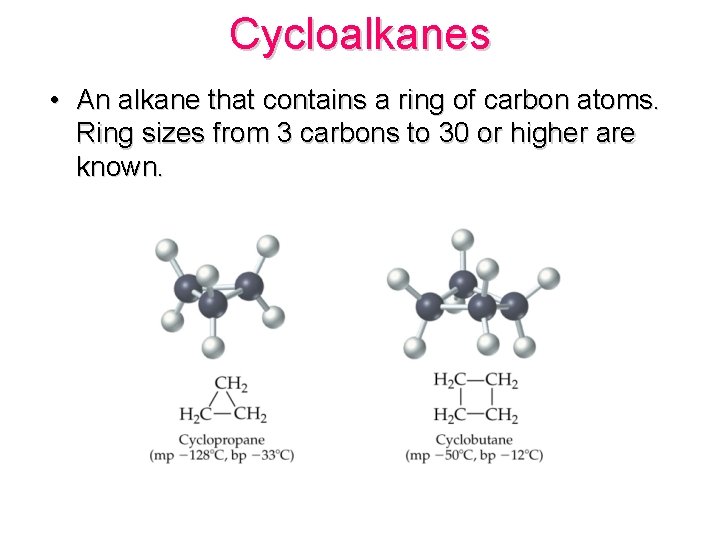
Cycloalkanes • An alkane that contains a ring of carbon atoms. Ring sizes from 3 carbons to 30 or higher are known.

• Line structure: A shorthand way of drawing structures in which atoms aren’t shown; instead a carbon atom is understood to be at each corner and hydrogens are “understood”.

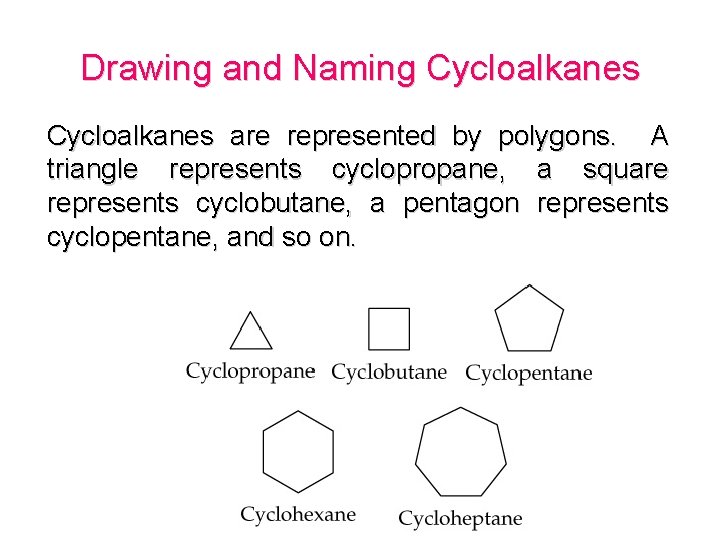
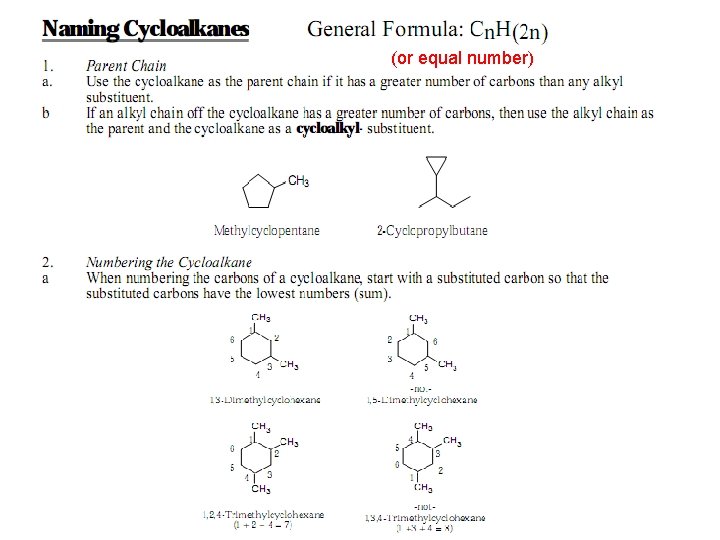
Drawing and Naming Cycloalkanes are represented by polygons. A triangle represents cyclopropane, a square represents cyclobutane, a pentagon represents cyclopentane, and so on.

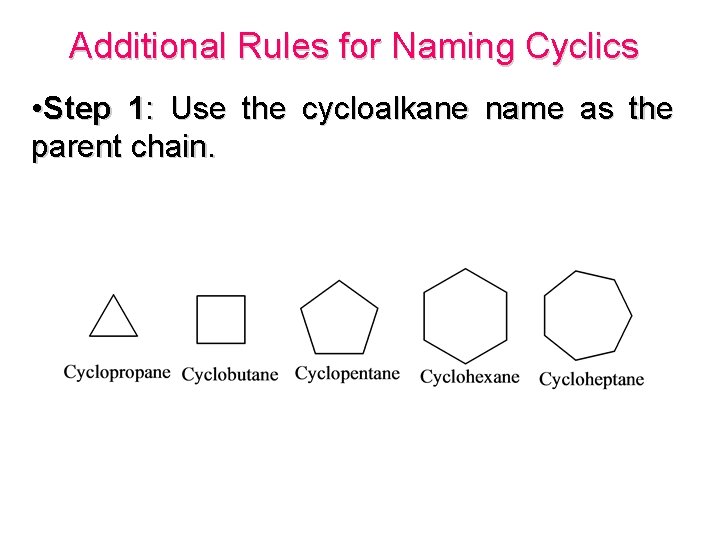
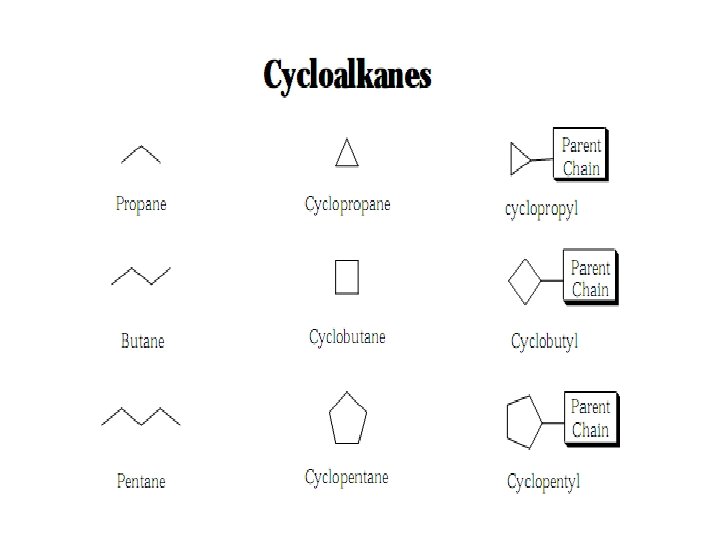
Additional Rules for Naming Cyclics • Step 1: Use the cycloalkane name as the parent chain.


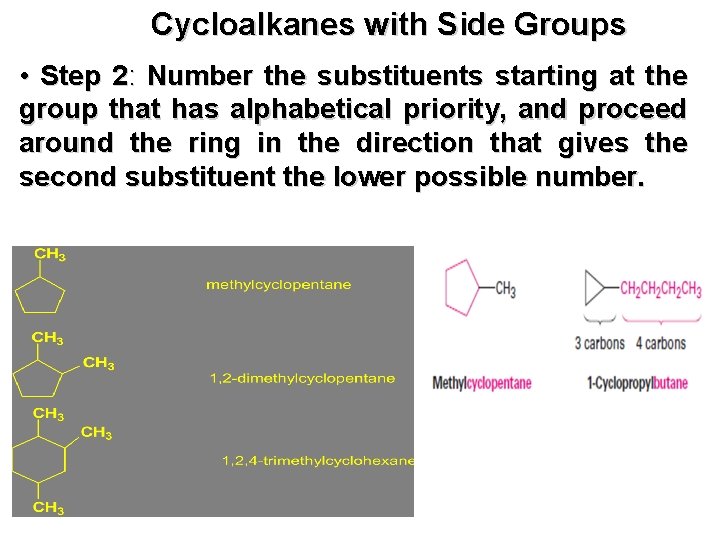
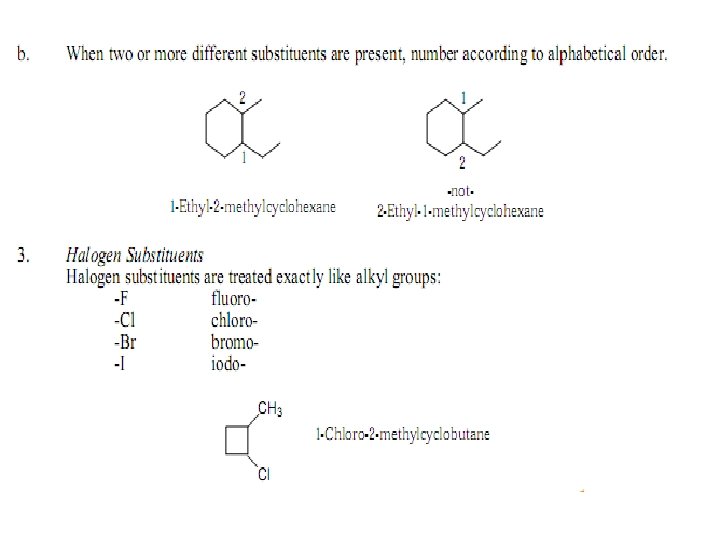
Cycloalkanes with Side Groups • Step 2: Number the substituents starting at the group that has alphabetical priority, and proceed around the ring in the direction that gives the second substituent the lower possible number.

(or equal number)


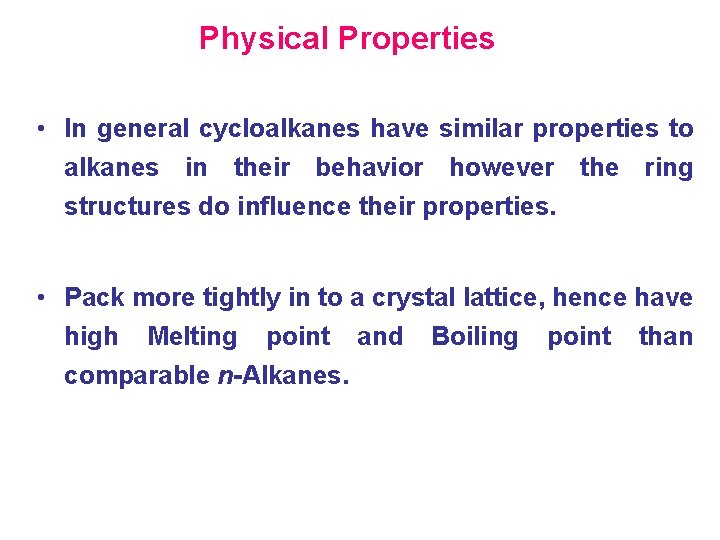
Physical Properties • In general cycloalkanes have similar properties to alkanes in their behavior however the ring structures do influence their properties. • Pack more tightly in to a crystal lattice, hence have high Melting point and Boiling point than comparable n-Alkanes.

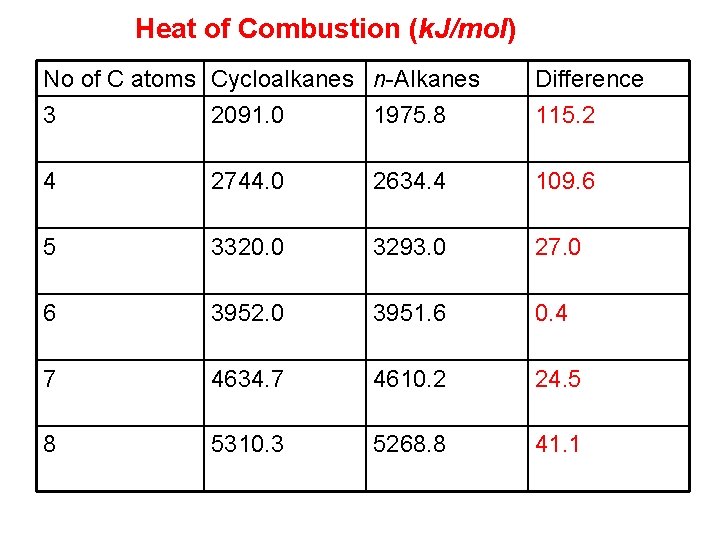
Stability of Cyclo. Alkanes Heat of combustion It is the amount of heat evolved when one mole of the substance is completely burned to carbon dioxide and water. Higher heat of combustion of a compound means its higher energy content and higher the energy of a compound, the less its stability, since different cycloalkanes cant be compared directly with each other thus they can be compared with corresponding alkanes.

Heat of Combustion (k. J/mol) No of C atoms Cycloalkanes n-Alkanes 3 2091. 0 1975. 8 Difference 115. 2 4 2744. 0 2634. 4 109. 6 5 3320. 0 3293. 0 27. 0 6 3952. 0 3951. 6 0. 4 7 4634. 7 4610. 2 24. 5 8 5310. 3 5268. 8 41. 1

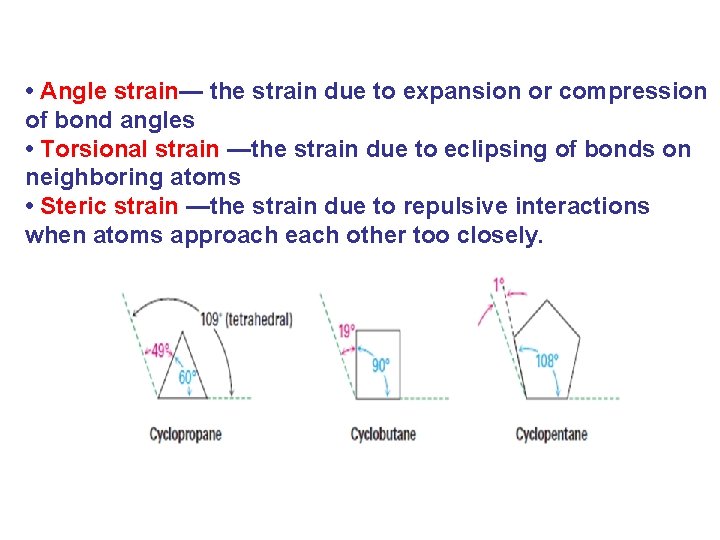
Beyer Strain Theory (Angle strain) According to this theory (Adolf von Baeyer in 1885), any deviation from the normal bond angle cause strain, called angle strain in the molecule, and greater the deviation form the normal angle, the greater the strain and the less stability. In cycloalkanes, since each carbon atom is sp 3 hybridization, the C-C-C bond Angle should be tetrahedral (109. 5 o),

• Angle strain— the strain due to expansion or compression of bond angles • Torsional strain —the strain due to eclipsing of bonds on neighboring atoms • Steric strain —the strain due to repulsive interactions when atoms approach each other too closely.

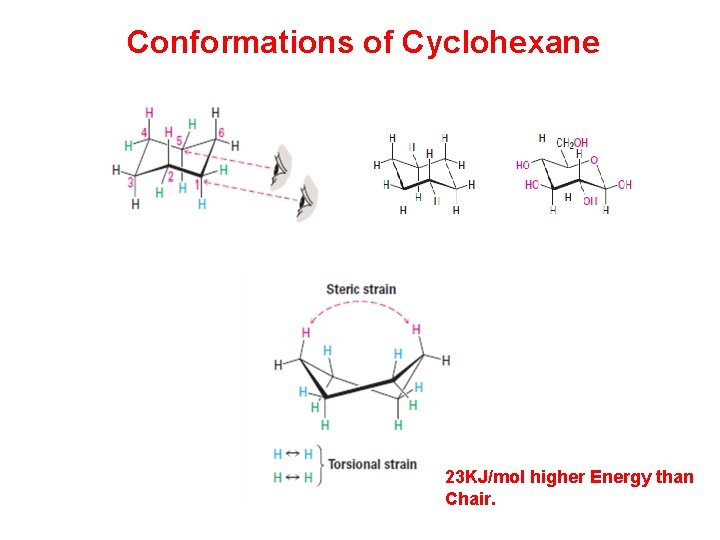
Conformations of Cyclohexane 23 KJ/mol higher Energy than Chair.

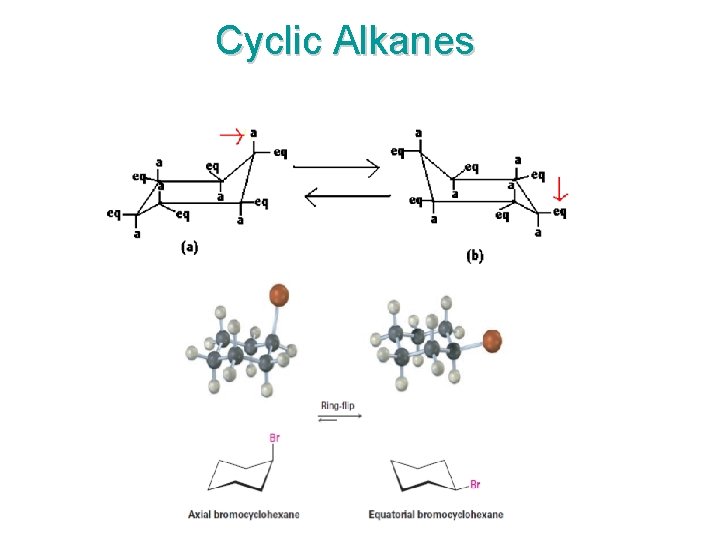
Cyclic Alkanes

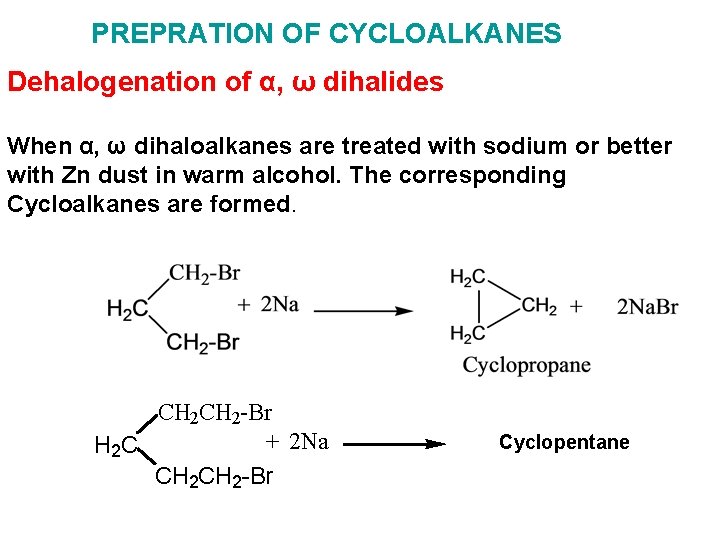
PREPRATION OF CYCLOALKANES Dehalogenation of α, ω dihalides When α, ω dihaloalkanes are treated with sodium or better with Zn dust in warm alcohol. The corresponding Cycloalkanes are formed. CH 2 -Br + 2 Na H 2 C CH 2 -Br Cyclopentane

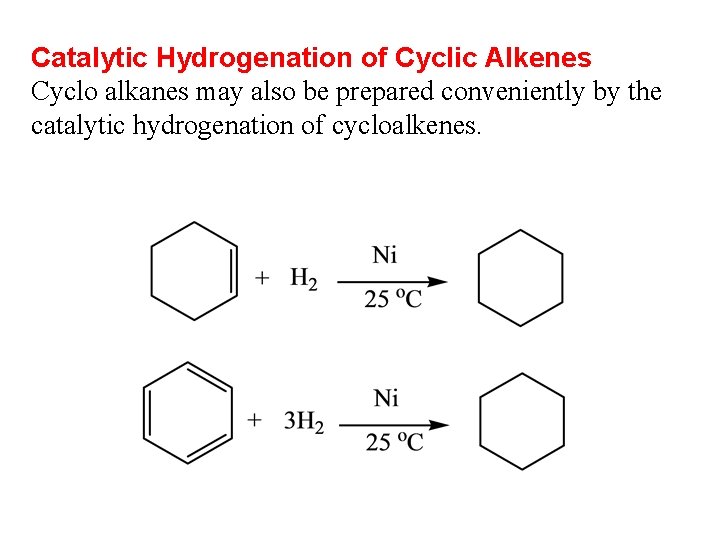
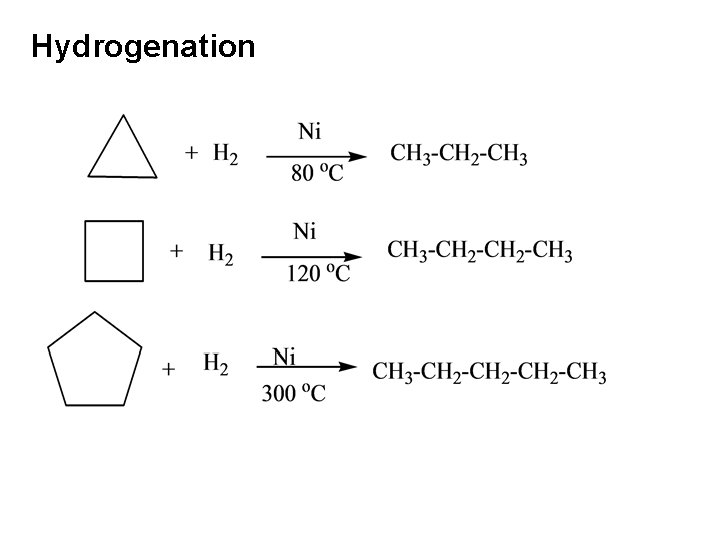
Catalytic Hydrogenation of Cyclic Alkenes Cyclo alkanes may also be prepared conveniently by the catalytic hydrogenation of cycloalkenes.

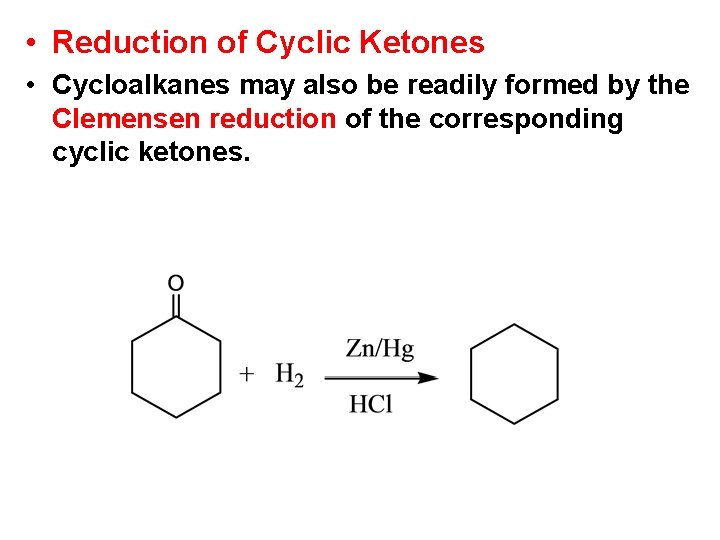
• Reduction of Cyclic Ketones • Cycloalkanes may also be readily formed by the Clemensen reduction of the corresponding cyclic ketones.

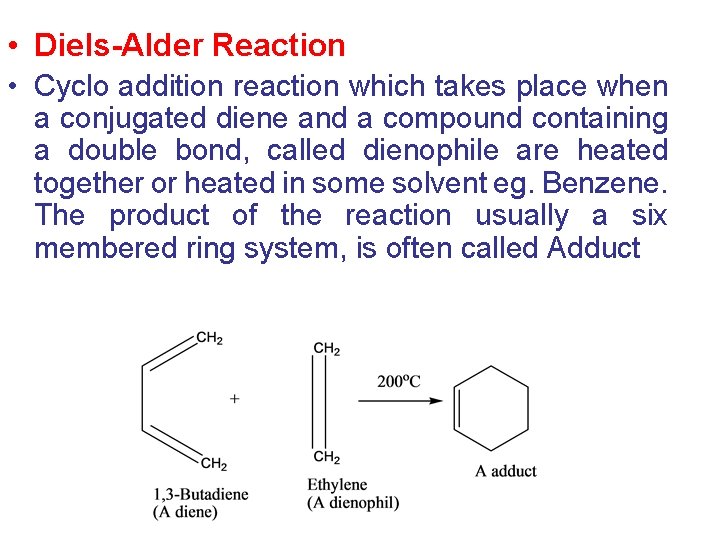
• Diels-Alder Reaction • Cyclo addition reaction which takes place when a conjugated diene and a compound containing a double bond, called dienophile are heated together or heated in some solvent eg. Benzene. The product of the reaction usually a six membered ring system, is often called Adduct

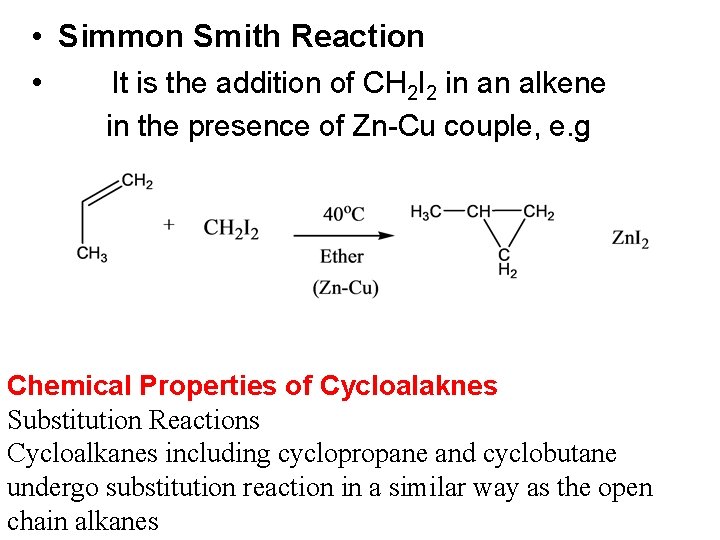
• Simmon Smith Reaction • It is the addition of CH 2 I 2 in an alkene in the presence of Zn-Cu couple, e. g Chemical Properties of Cycloalaknes Substitution Reactions Cycloalkanes including cyclopropane and cyclobutane undergo substitution reaction in a similar way as the open chain alkanes

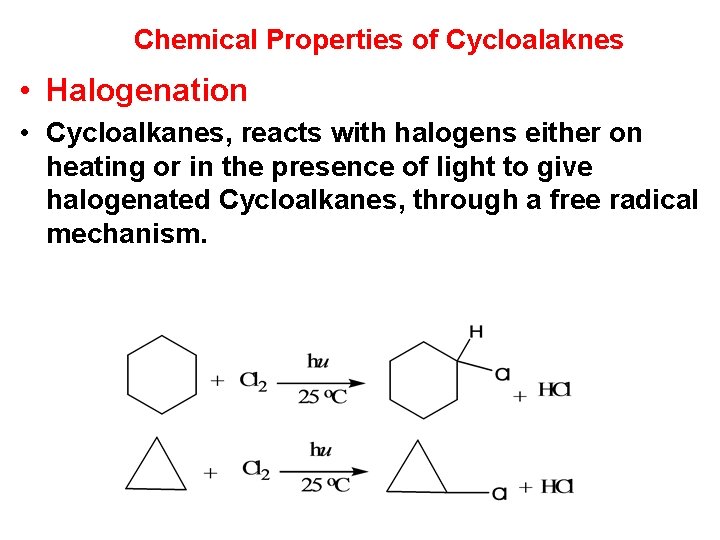
Chemical Properties of Cycloalaknes • Halogenation • Cycloalkanes, reacts with halogens either on heating or in the presence of light to give halogenated Cycloalkanes, through a free radical mechanism.

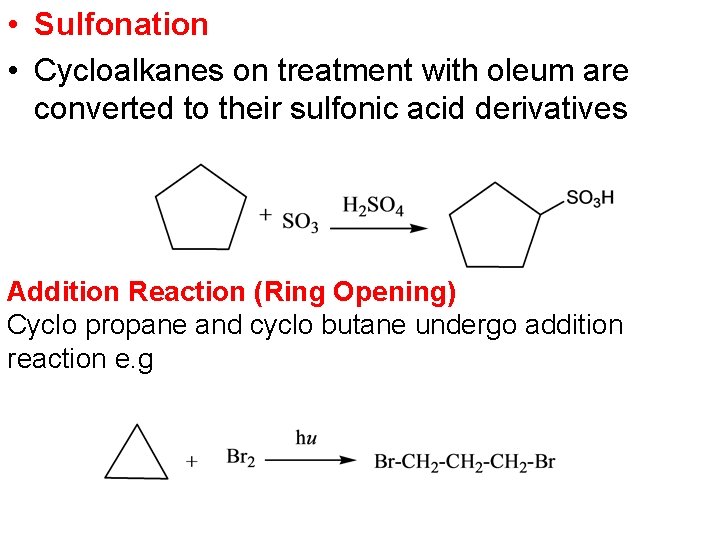
• Sulfonation • Cycloalkanes on treatment with oleum are converted to their sulfonic acid derivatives Addition Reaction (Ring Opening) Cyclo propane and cyclo butane undergo addition reaction e. g

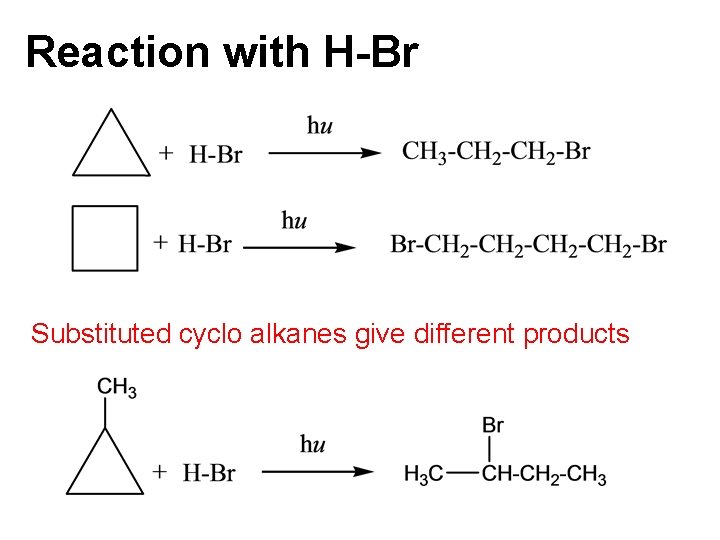
Reaction with H-Br Substituted cyclo alkanes give different products

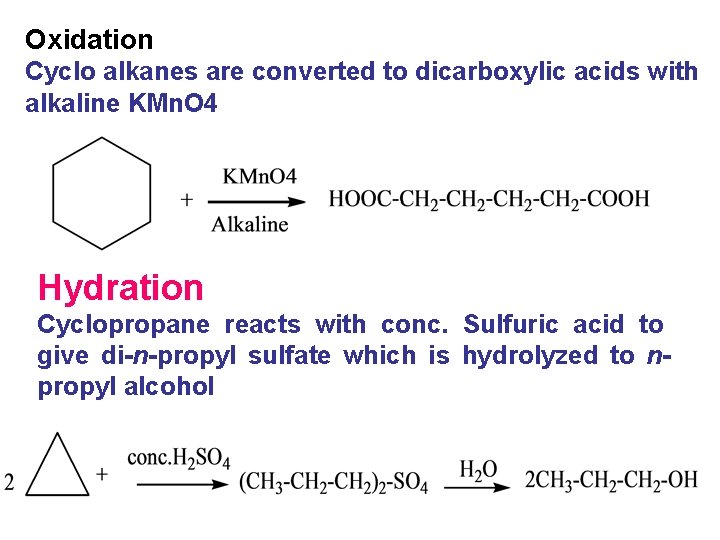
Oxidation Cyclo alkanes are converted to dicarboxylic acids with alkaline KMn. O 4 Hydration Cyclopropane reacts with conc. Sulfuric acid to give di-n-propyl sulfate which is hydrolyzed to npropyl alcohol

Hydrogenation

THE END