Organic Chemical Analysis of organic compounds How to



![Polarimetery Why Measure optical activity ([a]) and/or optical purity (and %ee) for chiral compounds Polarimetery Why Measure optical activity ([a]) and/or optical purity (and %ee) for chiral compounds](https://slidetodoc.com/presentation_image_h2/411d6a14b3f8d957ed8c28d016fe4f6d/image-8.jpg)





- Slides: 16

Organic Chemical Analysis (of organic compounds)

How to Find Identity! • • Melting point (or boiling point) Chromatography IR spectrum Polarimetry Refractometry MS spectrum NMR spectrum Then, • Identity is certain!

Melting Point Determination (or boiling point) Why Verify product identity, characterize new products, measure purity, possibly identify material from a list of compounds. How Variety of apparatuses – all measure exactly the same thing – phase transition, Mixed melting point useful in identification Can’t Tell structure or identity, except by comparison

Liquid Chromatography Why To analyze and/or separate mixtures of compounds, identify by comparison to known compounds How Column (gravity, flash), thin-layer (TLC), Chromatotron (radial chromatography), High Pressure (Performance) Liquid Chromatography (HPLC) Can’t Tell structure or identity, except by comparison – more certain than MP or BP

Automated HPLC Shimadzu-2010 HT

Infrared Spectroscopy (FT-IR) Why Identify major functional groups in an organic molecule, check purity, gauge minor electronic effects (conjugation or lack thereof), provide a fingerprint of a compound (reproducible) How Thin Film, Na. Cl plates, KBr pellet, Attenuated Total Reflectance (ATR) FT-IR Can’t Tell exact structure (except with rigorous decade-long training), though fingerprint comparison can confirm known structure

FT-IR (x 2) PE Spectrum RX
![Polarimetery Why Measure optical activity a andor optical purity and ee for chiral compounds Polarimetery Why Measure optical activity ([a]) and/or optical purity (and %ee) for chiral compounds](https://slidetodoc.com/presentation_image_h2/411d6a14b3f8d957ed8c28d016fe4f6d/image-8.jpg)
Polarimetery Why Measure optical activity ([a]) and/or optical purity (and %ee) for chiral compounds How Polarimeter (also indirect methods based on diastereomeric mixture using NMR or GC) Can’t Tell structure or absolute configuration (R or S)

Refractometery Why Measure refractive index (n. D) – another physical property for a chemical, indicates purity, useful for binary liquid mixtures (gives accurate ratios), also used in wine and beer making industries (fermentable sugar content) How Refractometer Can’t Only useful for liquids

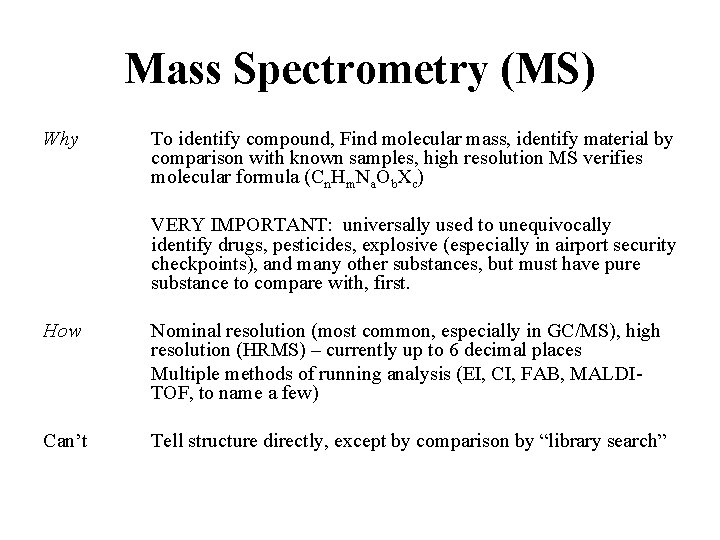
Mass Spectrometry (MS) Why To identify compound, Find molecular mass, identify material by comparison with known samples, high resolution MS verifies molecular formula (Cn. Hm. Na. Ob. Xc) VERY IMPORTANT: universally used to unequivocally identify drugs, pesticides, explosive (especially in airport security checkpoints), and many other substances, but must have pure substance to compare with, first. How Nominal resolution (most common, especially in GC/MS), high resolution (HRMS) – currently up to 6 decimal places Multiple methods of running analysis (EI, CI, FAB, MALDITOF, to name a few) Can’t Tell structure directly, except by comparison by “library search”

Automated GC/MS HP 5890 -II

X-ray Crystallography Why To determine 3 -D structure of chemical compounds. Ultimate proof of exact structure. How Using an X-ray diffractometer. Must have high quality crystals. Requires extensive experience in interpretation of data. Can’t Find structure of liquids. Absolute configuration is possible, but not guaranteed (mirror image problem)

Nuclear Magnetic Resonance (NMR) Why To determine different types of hydrogens or carbons (or other atoms) within a molecule and possibly the structure (connectedness) and geometry. How 1 H Can do almost anything! Really!!! NMR, 13 C NMR (other nuclei are possible – 19 F, 31 P) 1 D and 2 D NMR techniques

NMR Spectrometer

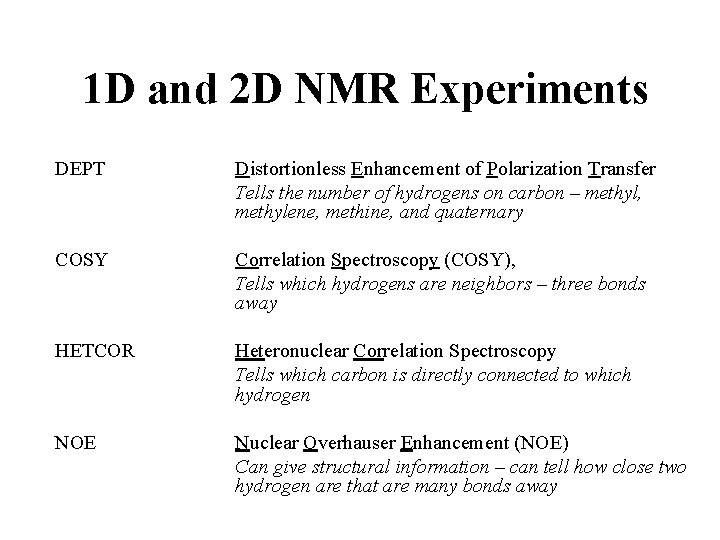
1 D and 2 D NMR Experiments DEPT Distortionless Enhancement of Polarization Transfer Tells the number of hydrogens on carbon – methyl, methylene, methine, and quaternary COSY Correlation Spectroscopy (COSY), Tells which hydrogens are neighbors – three bonds away HETCOR Heteronuclear Correlation Spectroscopy Tells which carbon is directly connected to which hydrogen NOE Nuclear Overhauser Enhancement (NOE) Can give structural information – can tell how close two hydrogen are that are many bonds away

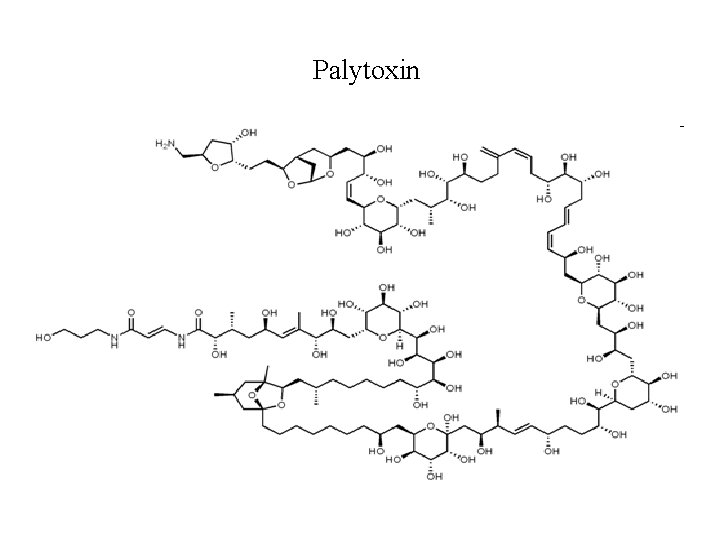
Palytoxin