Order in which subshells are filled with electrons
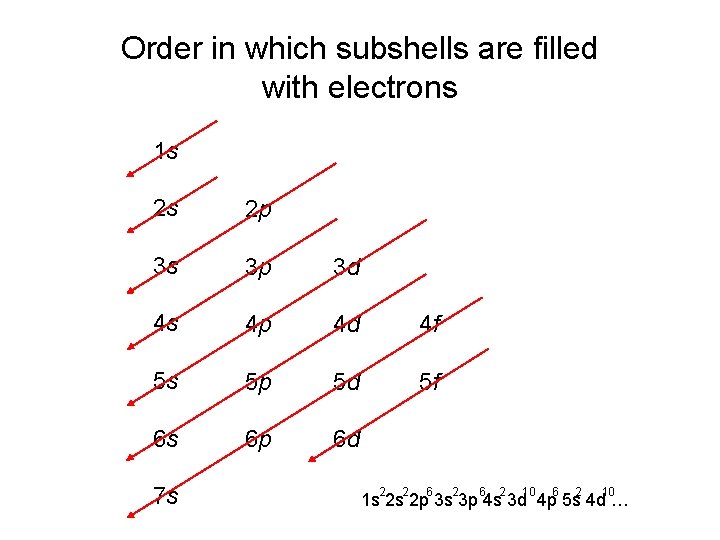
Order in which subshells are filled with electrons 1 s 2 s 2 p 3 s 3 p 3 d 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 6 s 6 p 6 d 7 s 2 2 6 2 10 1 s 2 s 2 p 3 s 3 p 4 s 3 d 4 p 5 s 4 d …
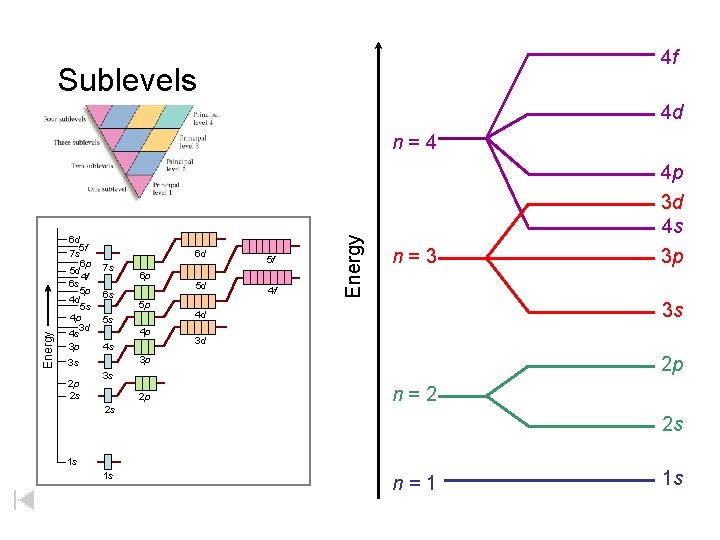
4 f Sublevels 4 d Energy 6 d 5 f 7 s 6 p 5 d 4 f 6 s 5 p 4 d 5 s 4 p 3 d 4 s 3 p 6 d 7 s 6 s 5 p 5 s 4 p 4 s 5 d 4 f n=3 3 d 2 p 3 s 2 p 2 s 4 p 3 d 4 s 3 p 3 s 4 d 3 p 3 s 2 p 2 s 6 p 5 f Energy n=4 n=2 2 s 1 s 1 s n=1 1 s
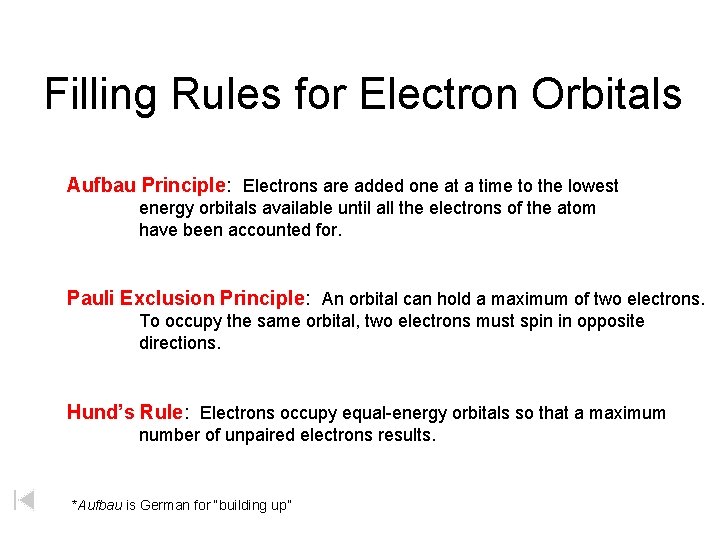
Filling Rules for Electron Orbitals Aufbau Principle: Electrons are added one at a time to the lowest energy orbitals available until all the electrons of the atom have been accounted for. Pauli Exclusion Principle: An orbital can hold a maximum of two electrons. To occupy the same orbital, two electrons must spin in opposite directions. Hund’s Rule: Electrons occupy equal-energy orbitals so that a maximum number of unpaired electrons results. *Aufbau is German for “building up”
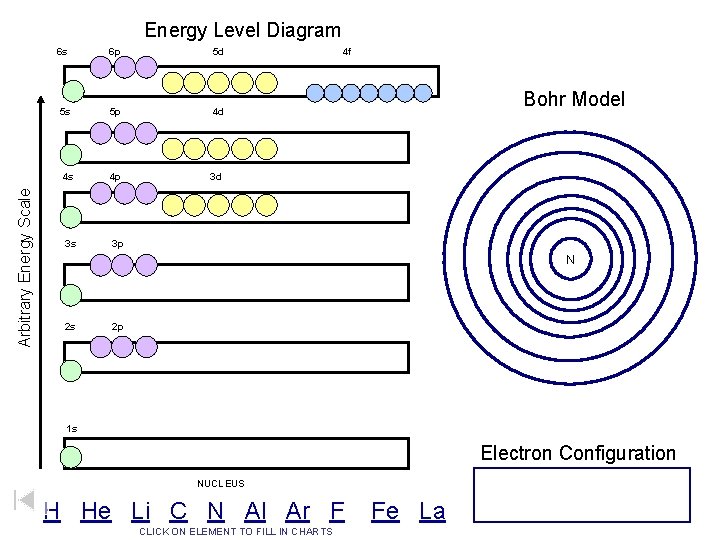
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La
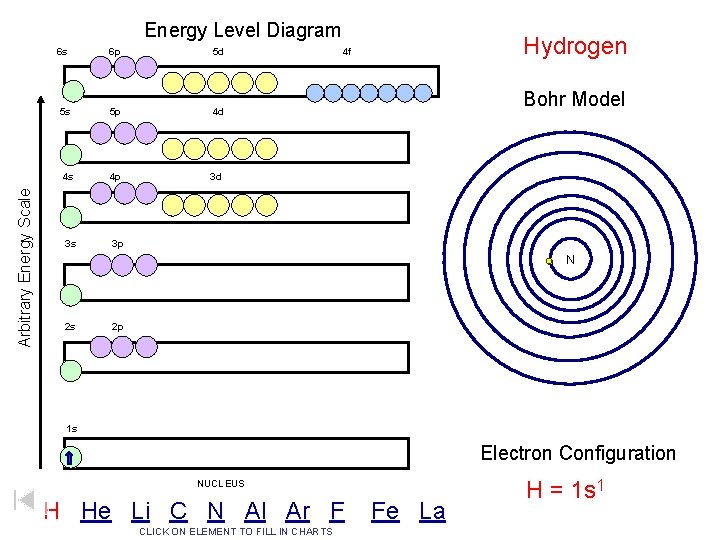
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Hydrogen 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La H = 1 s 1
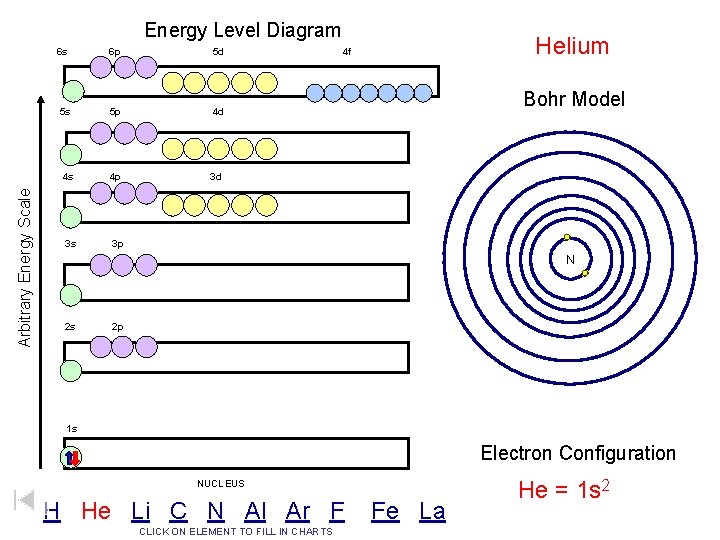
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Helium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La He = 1 s 2
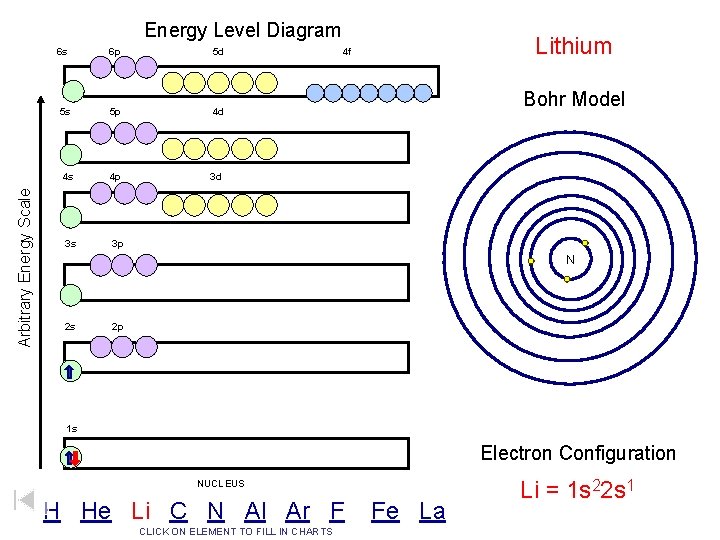
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lithium 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Li = 1 s 22 s 1
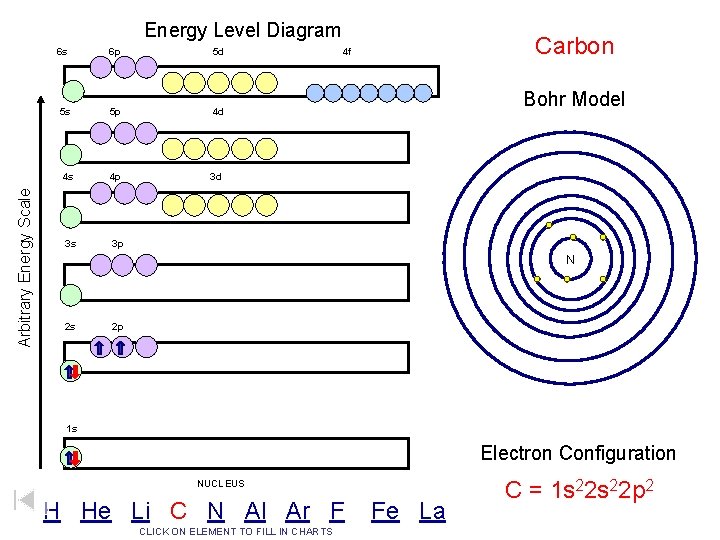
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Carbon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La C = 1 s 22 p 2
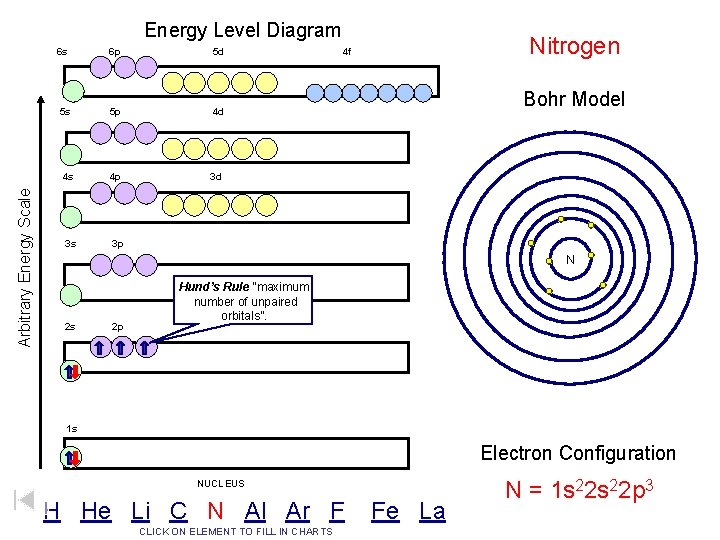
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Nitrogen 4 f Bohr Model N 2 s 2 p Hund’s Rule “maximum number of unpaired orbitals”. 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La N = 1 s 22 p 3
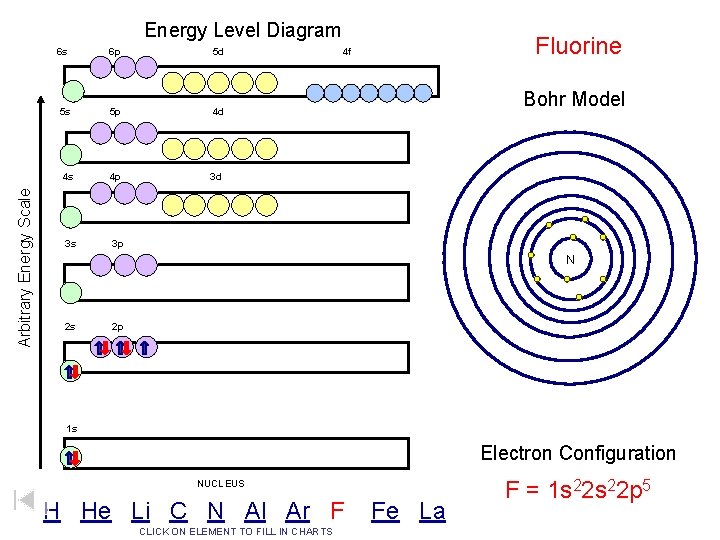
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Fluorine 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La F = 1 s 22 p 5
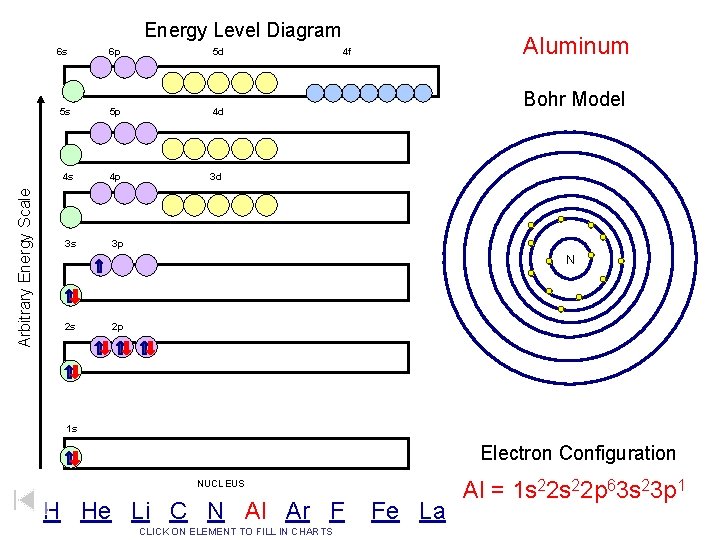
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Aluminum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Al = 1 s 22 p 63 s 23 p 1
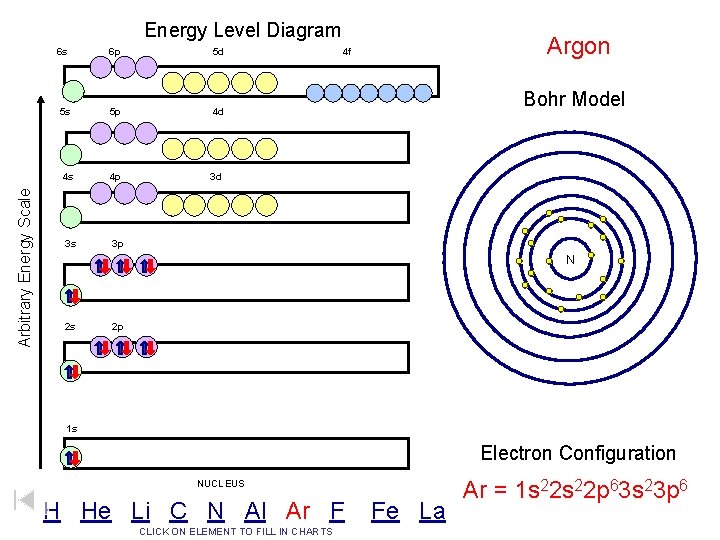
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Argon 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La Ar = 1 s 22 p 63 s 23 p 6
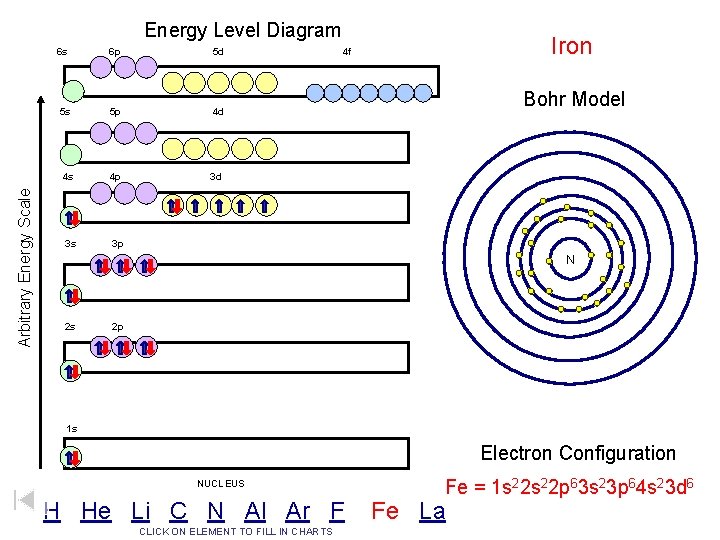
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Iron 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe = 1 s 22 p 63 s 23 p 64 s 23 d 6 Fe La
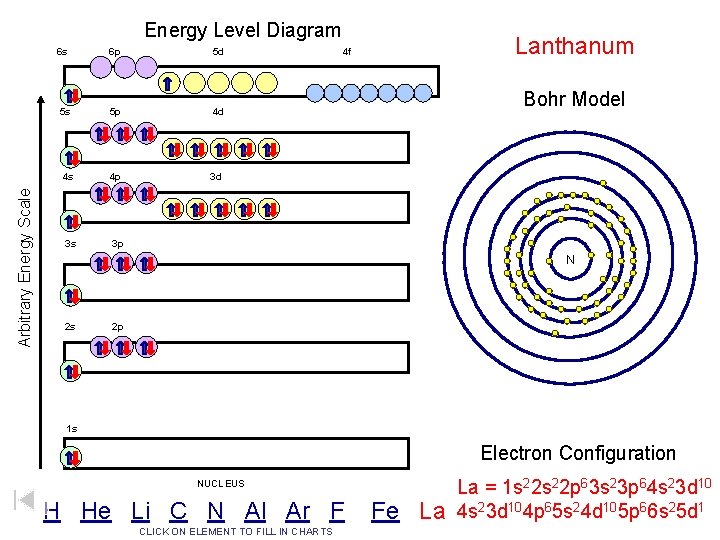
Arbitrary Energy Scale Energy Level Diagram 6 s 6 p 5 d 5 s 5 p 4 d 4 s 4 p 3 d 3 s 3 p Lanthanum 4 f Bohr Model N 2 s 2 p 1 s Electron Configuration NUCLEUS H He Li C N Al Ar F CLICK ON ELEMENT TO FILL IN CHARTS Fe La La = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 25 d 1
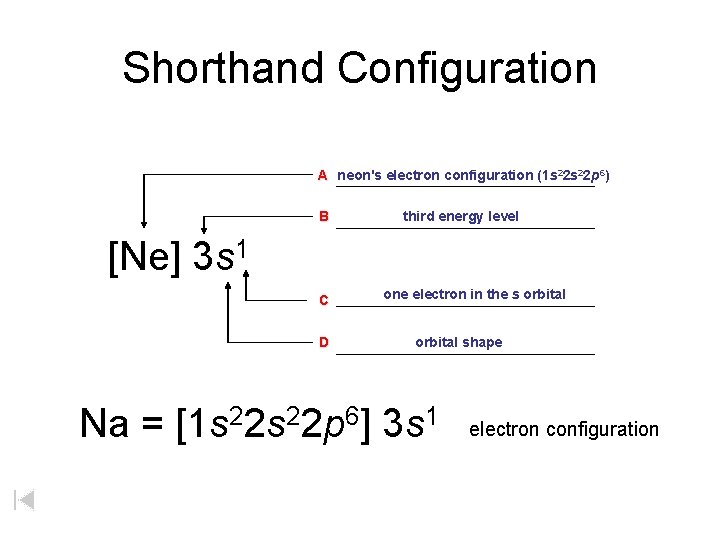
Shorthand Configuration A neon's electron configuration (1 s 22 p 6) B third energy level [Ne] 3 s 1 C D one electron in the s orbital shape Na = [1 s 22 p 6] 3 s 1 electron configuration
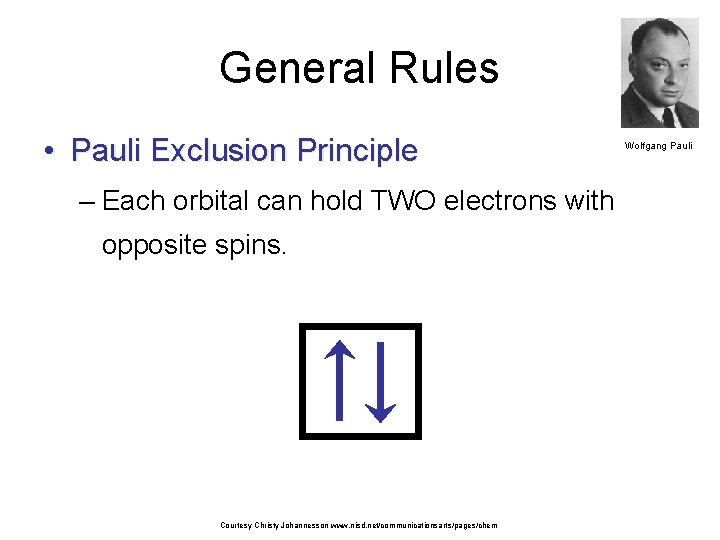
General Rules • Pauli Exclusion Principle – Each orbital can hold TWO electrons with opposite spins. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem Wolfgang Pauli
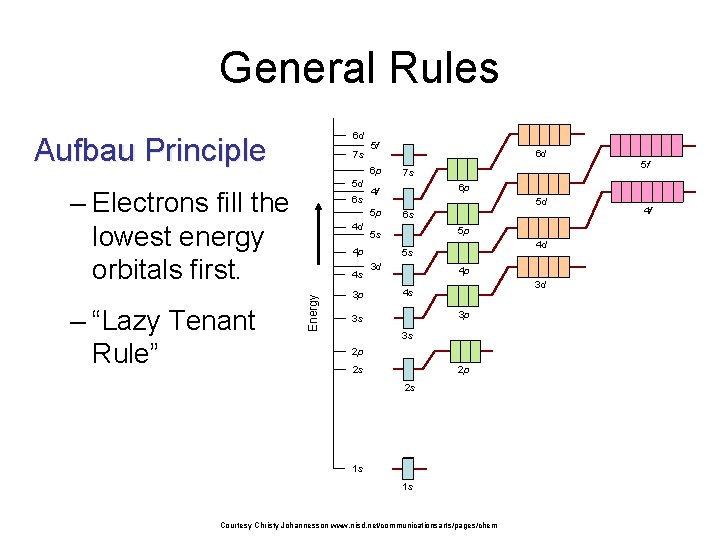
General Rules 6 d Aufbau Principle 7 s 6 p 5 d – Electrons fill the lowest energy orbitals first. 6 s 4 d 3 p 7 s 5 f 6 p 5 d 6 s 5 p 5 s 4 p 4 s 6 d 4 f 5 p Energy – “Lazy Tenant Rule” 5 f 4 d 5 s 3 d 4 p 3 d 4 s 3 p 3 s 3 s 2 p 2 p 2 s 2 s 1 s 1 s Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 4 f
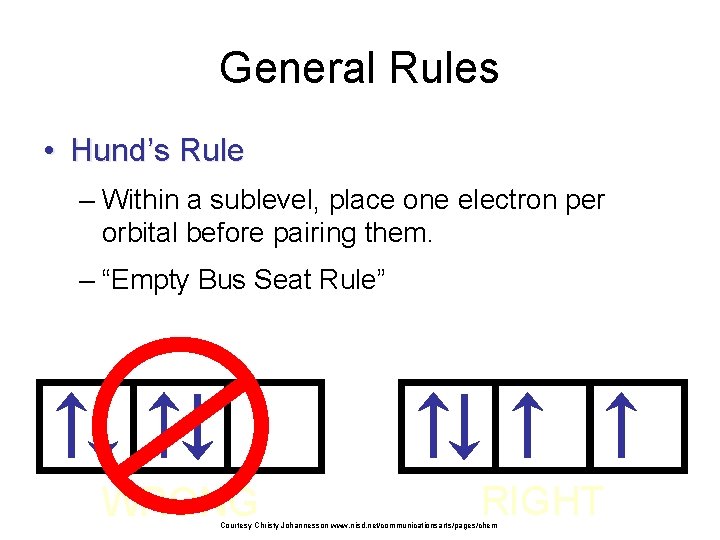
General Rules • Hund’s Rule – Within a sublevel, place one electron per orbital before pairing them. – “Empty Bus Seat Rule” WRONG RIGHT Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
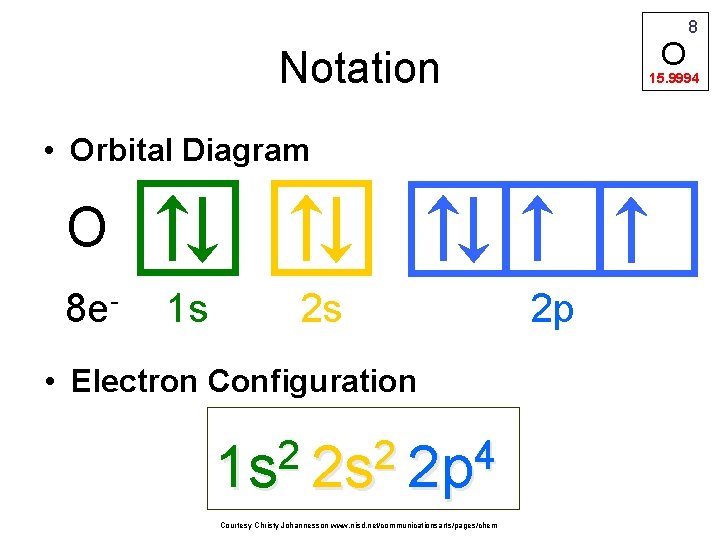
O Notation 15. 9994 • Orbital Diagram O 8 e- 1 s 2 s • Electron Configuration 2 2 4 1 s 2 s 2 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 8 2 p
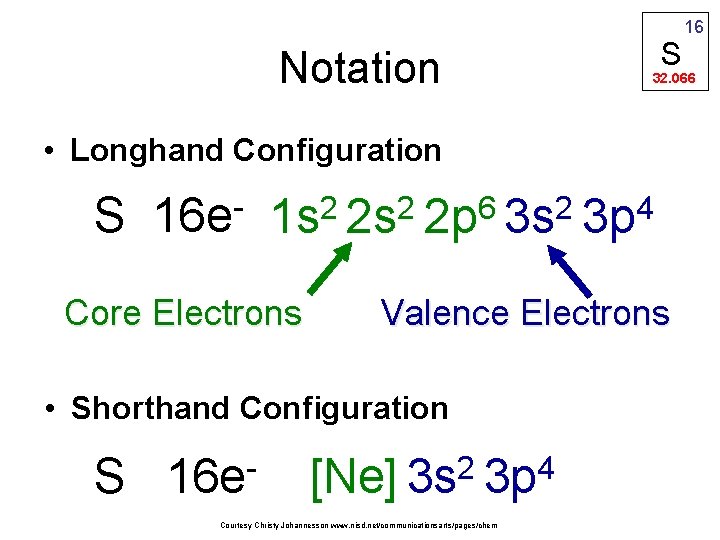
Notation S 32. 066 • Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons S 16 e 4 3 p Valence Electrons • Shorthand Configuration 2 4 [Ne] 3 s 3 p Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 16
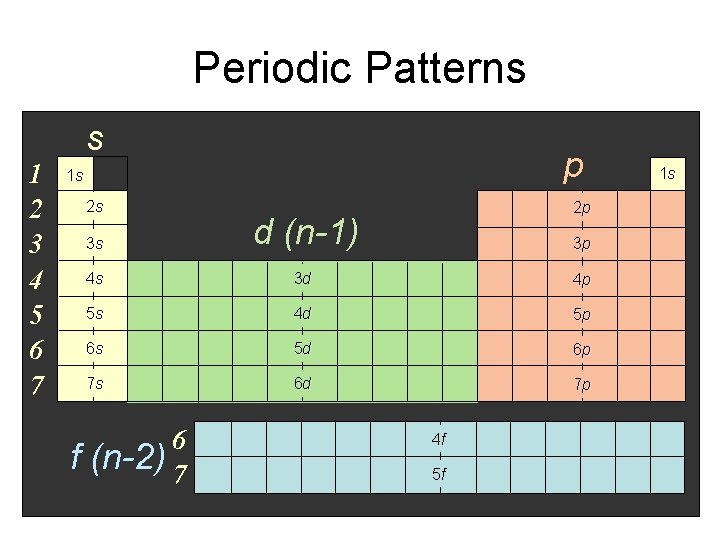
Periodic Patterns s 1 2 3 4 5 6 7 p 1 s 2 s f 2 p 3 s d (n-1) 4 s 3 d 4 p 5 s 4 d 5 p 6 s 5 d 6 p 7 s 6 d 7 p 6 (n-2) 7 3 p 4 f 5 f 1 s
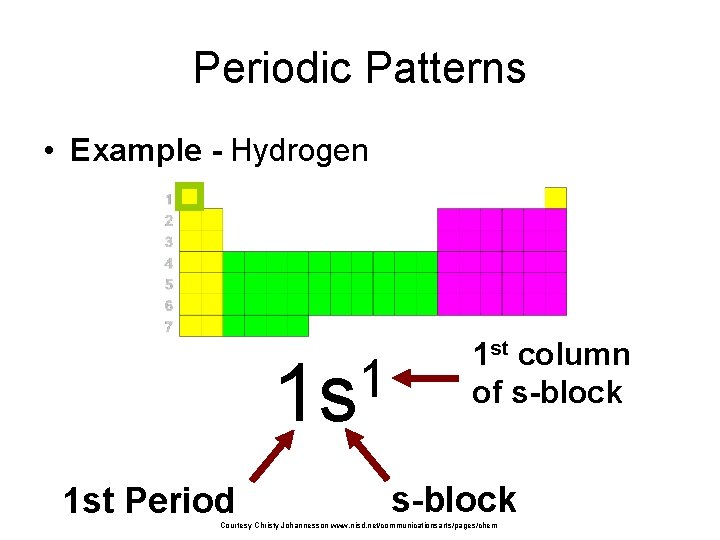
Periodic Patterns • Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
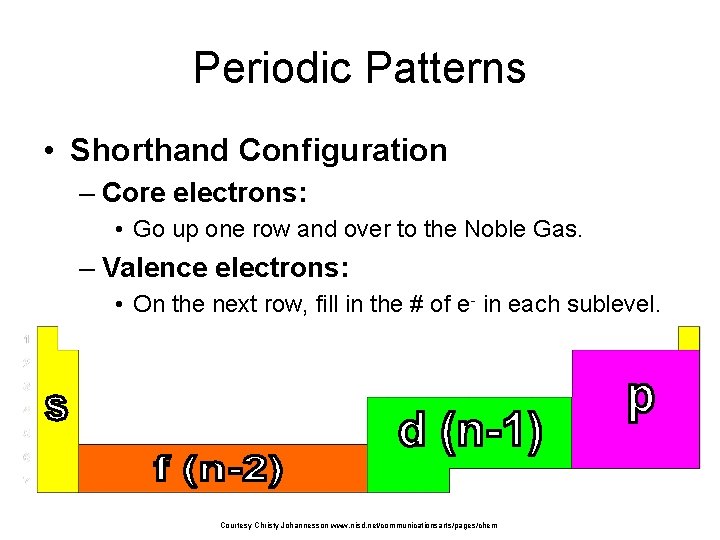
Periodic Patterns • Shorthand Configuration – Core electrons: • Go up one row and over to the Noble Gas. – Valence electrons: • On the next row, fill in the # of e- in each sublevel. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
![32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d 32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d](http://slidetodoc.com/presentation_image_h2/cb4c02f08015d3beb384c0e917dc5948/image-24.jpg)
32 Periodic Patterns • Example - Germanium [Ar] 2 4 s 10 3 d Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem 2 4 p Ge 72. 61
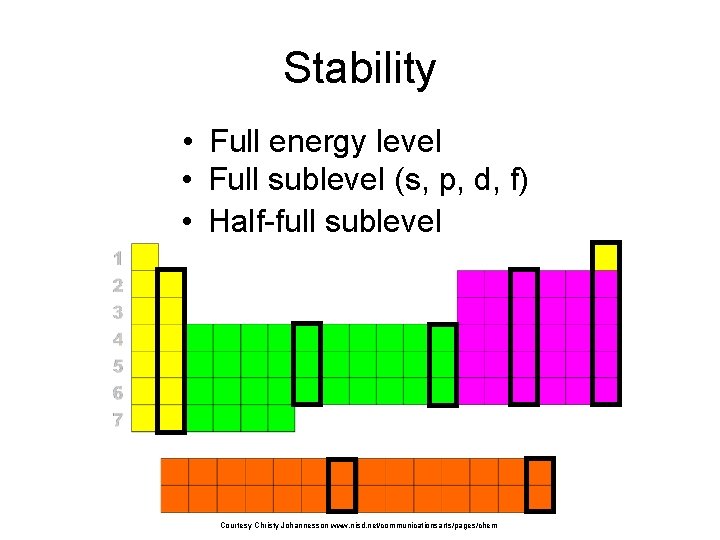
Stability • Full energy level • Full sublevel (s, p, d, f) • Half-full sublevel Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

The Octet Rule Atoms tend to gain, lose, or share electrons until they have eight valence electrons. This fills the valence shell and tends to give the atom the stability of the inert gasses. 8 ONLY s- and p-orbitals are valence electrons.
![Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d](http://slidetodoc.com/presentation_image_h2/cb4c02f08015d3beb384c0e917dc5948/image-27.jpg)
Stability • Electron Configuration Exceptions – Copper EXPECT: [Ar] 4 s 2 3 d 9 ACTUALLY: [Ar] 4 s 1 3 d 10 – Copper gains stability with a full d-sublevel. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
![Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d](http://slidetodoc.com/presentation_image_h2/cb4c02f08015d3beb384c0e917dc5948/image-28.jpg)
Stability • Electron Configuration Exceptions – Chromium EXPECT: [Ar] 4 s 2 3 d 4 ACTUALLY: [Ar] 4 s 1 3 d 5 – Chromium gains stability with a half-full d -sublevel. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
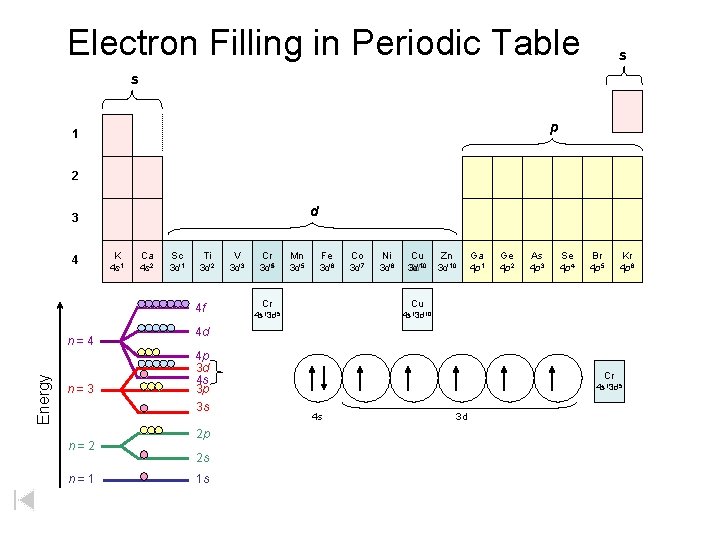
Electron Filling in Periodic Table s s p 1 2 d 3 4 K 4 s 1 Ca 4 s 2 Sc 3 d 1 Ti 3 d 2 4 f Energy n=4 n=3 n=2 n=1 V 3 d 3 Cr 3 d 54 Mn 3 d 5 Fe 3 d 6 Co 3 d 7 Ni 3 d 8 Cu 9 3 d 3 d 10 Cr Cu 4 s 13 d 5 4 s 13 d 10 Zn 3 d 10 Ga 4 p 1 Ge 4 p 2 As 4 p 3 Se 4 p 4 Br 4 p 5 Kr 4 p 6 4 d 4 p 3 d 4 s 3 p 3 s Cr 4 s 13 d 5 4 s 3 d 2 p 2 s Cu 1 s 4 s 13 d 10 4 s 3 d
Stability • Ion Formation – Atoms gain or lose electrons to become more stable. – Isoelectronic with the Noble Gases. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
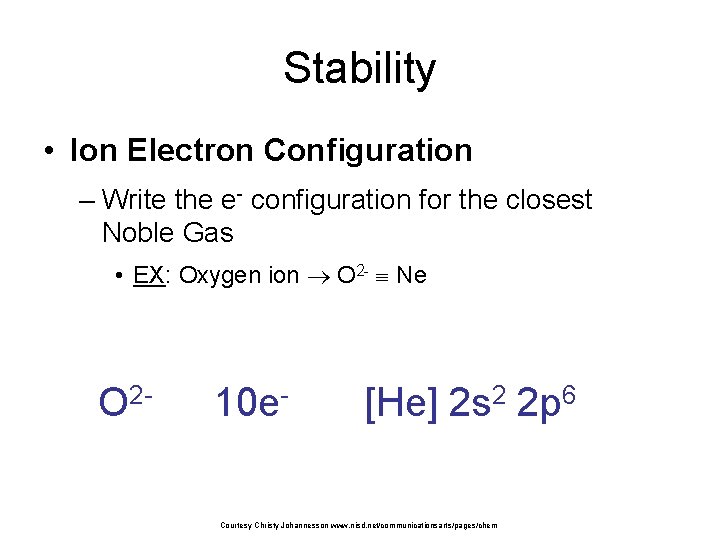
Stability • Ion Electron Configuration – Write the e- configuration for the closest Noble Gas • EX: Oxygen ion O 2 - Ne O 2 - 10 e- [He] 2 s 2 2 p 6 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
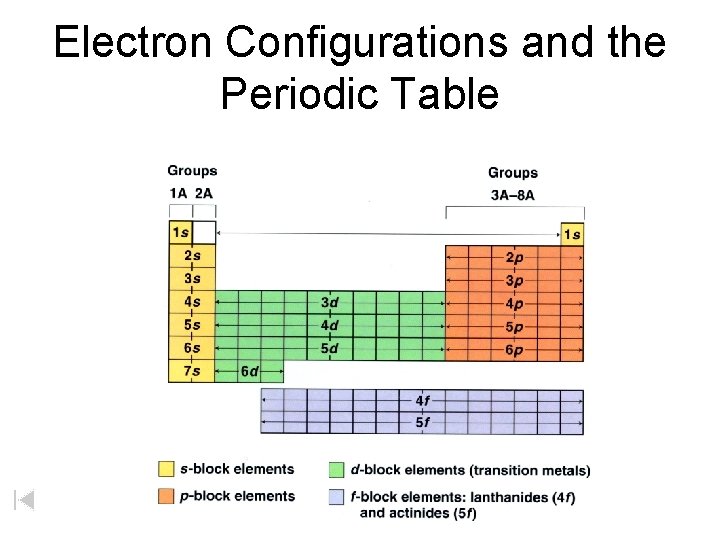
Electron Configurations and the Periodic Table
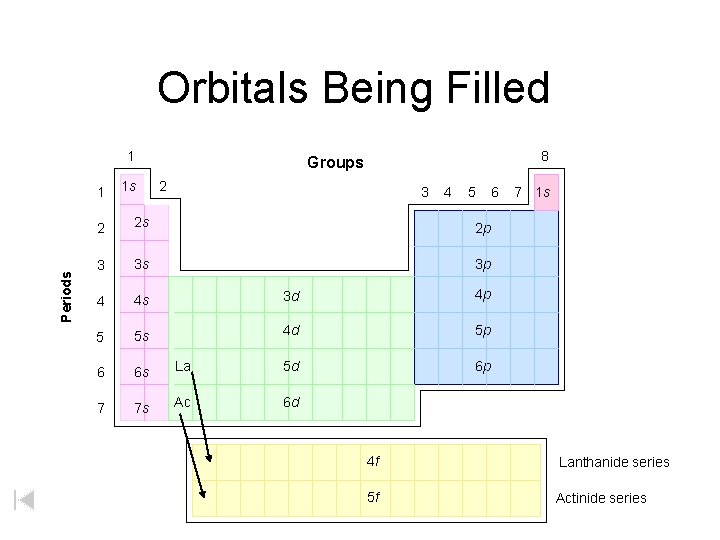
Orbitals Being Filled 1 Periods 1 1 s 8 Groups 2 3 4 5 2 2 s 2 p 3 3 s 3 p 4 4 s 3 d 4 p 5 5 s 4 d 5 p 6 6 s La 5 d 6 p 7 7 s Ac 6 d 6 7 1 s 4 f Lanthanide series 5 f Actinide series
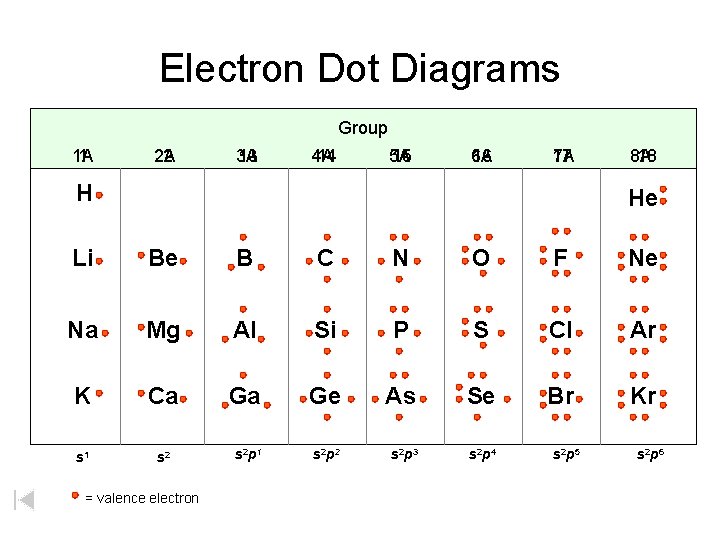
Electron Dot Diagrams Group 1 A 1 2 A 2 3 A 13 4 A 14 5 A 15 6 A 16 17 7 A H 8 A 18 He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Ga Ge As Se Br Kr s 1 s 2 p 2 s 2 p 3 s 2 p 4 = valence electron s 2 p 5 s 2 p 6
- Slides: 34