Orbital Diagrams Hunds Rule Since electrons repel they

Orbital Diagrams Hund’s Rule
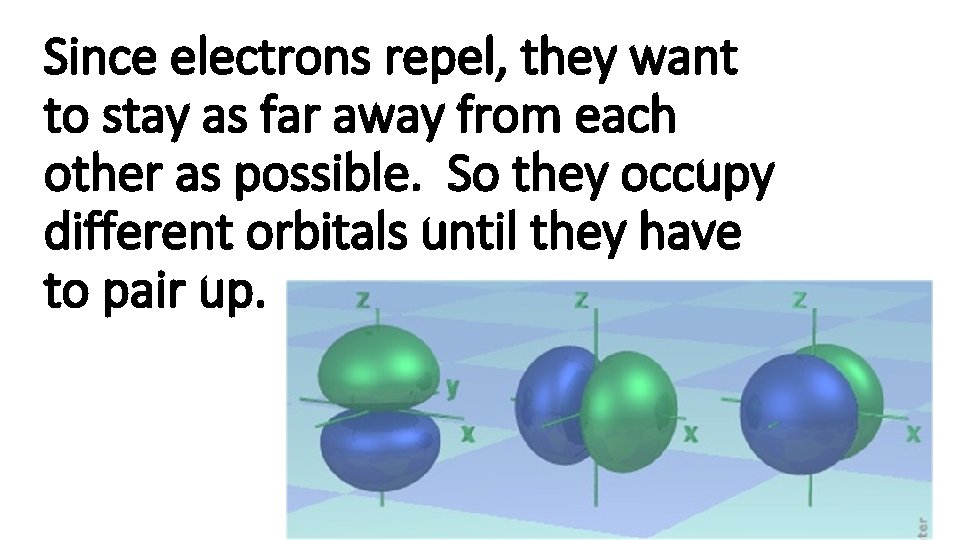
Since electrons repel, they want to stay as far away from each other as possible. So they occupy different orbitals until they have to pair up.
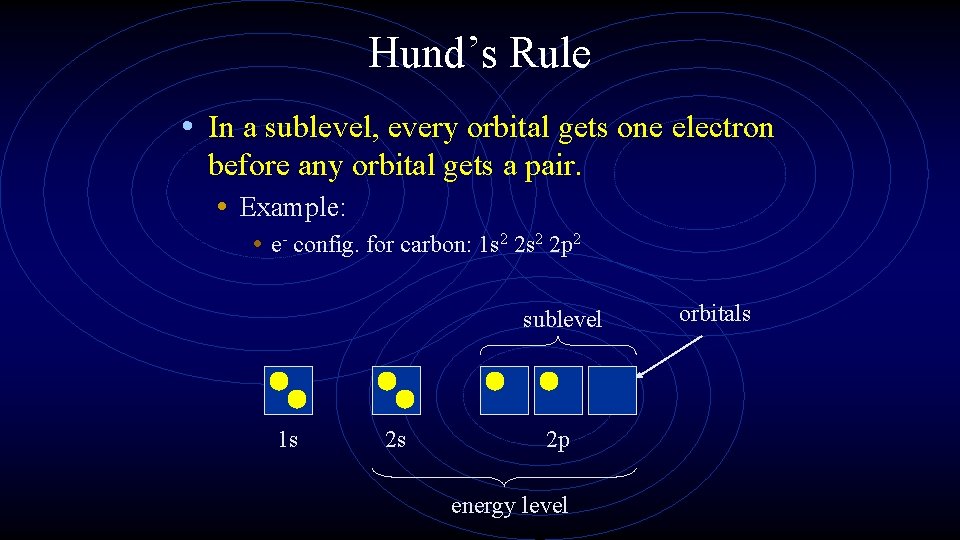
Hund’s Rule • In a sublevel, every orbital gets one electron before any orbital gets a pair. • Example: • e- config. for carbon: 1 s 2 2 p 2 sublevel 1 s 2 s 2 p energy level orbitals
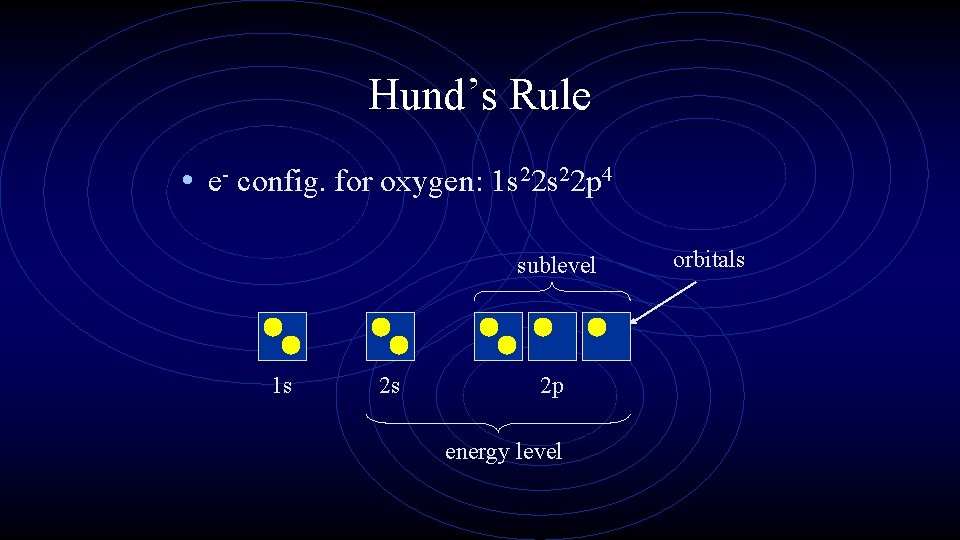
Hund’s Rule • e- config. for oxygen: 1 s 22 p 4 sublevel 1 s 2 s 2 p energy level orbitals
Orbital Diagrams • Show the arrangement of electrons in orbitals within an atom. • Use boxes to represent orbitals. • One arrow (↑) represents 1 e-. • 2 arrows (↑↓) rep. 2 e-.

Orbital Diagrams • Orbital diagram for hydrogen. • e- config: 1 s 1 1 s

Orbital Diagrams • Orbital diagram for helium. • e- config: 1 s 2 1 s

Orbital Diagrams • Orbital diagram for lithium. • e- config: 1 s 2 2 s 1 1 s 2 s

Orbital Diagrams • Orbital diagram for beryllium. • e- config: 1 s 2 2 s 2 1 s 2 s
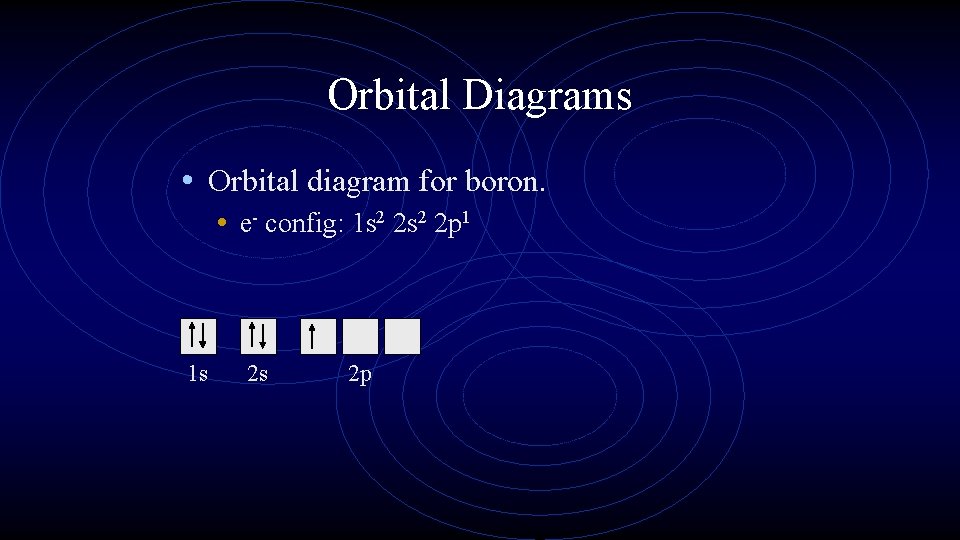
Orbital Diagrams • Orbital diagram for boron. • e- config: 1 s 2 2 p 1 1 s 2 s 2 p
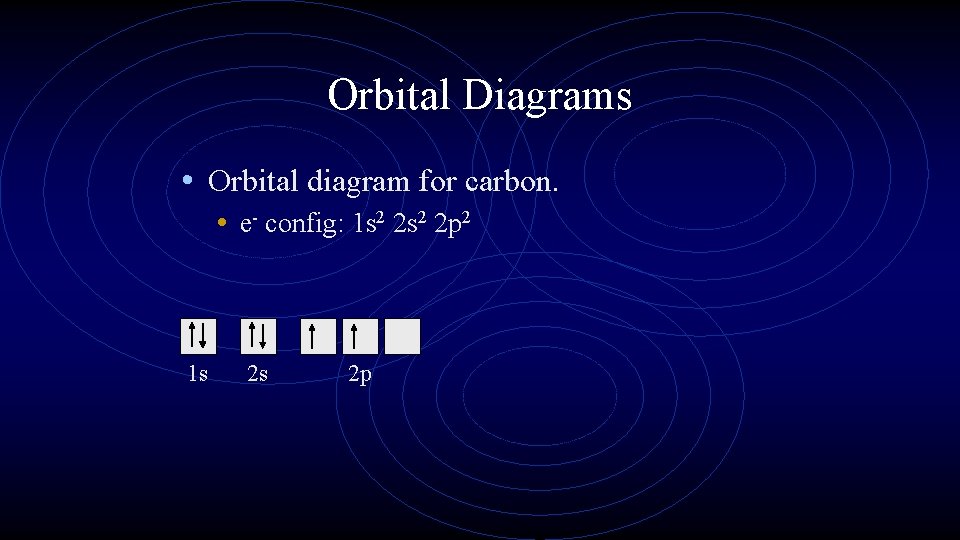
Orbital Diagrams • Orbital diagram for carbon. • e- config: 1 s 2 2 p 2 1 s 2 s 2 p
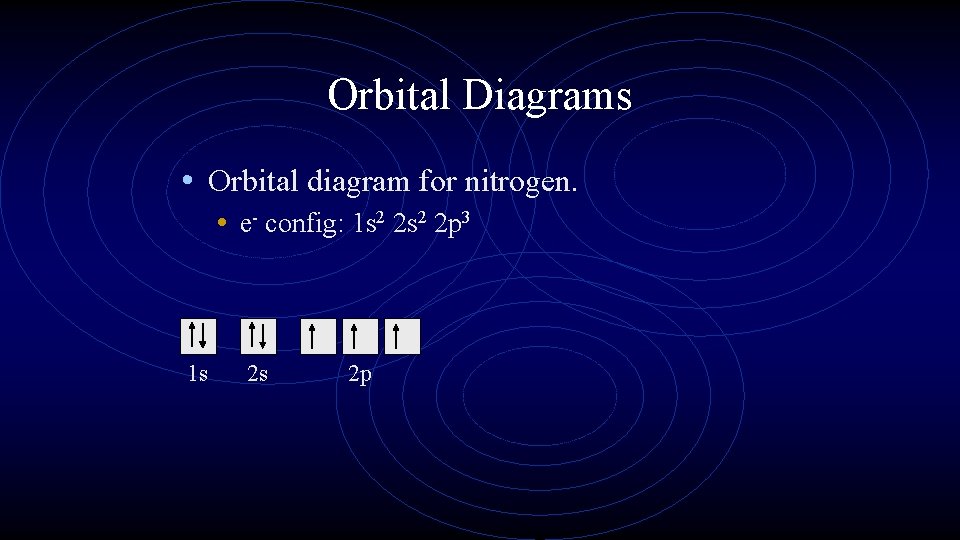
Orbital Diagrams • Orbital diagram for nitrogen. • e- config: 1 s 2 2 p 3 1 s 2 s 2 p
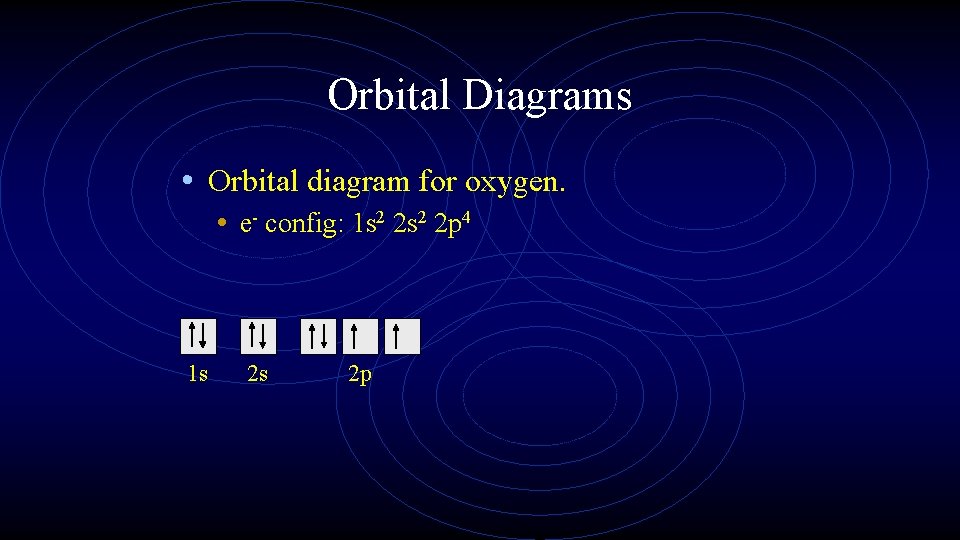
Orbital Diagrams • Orbital diagram for oxygen. • e- config: 1 s 2 2 p 4 1 s 2 s 2 p
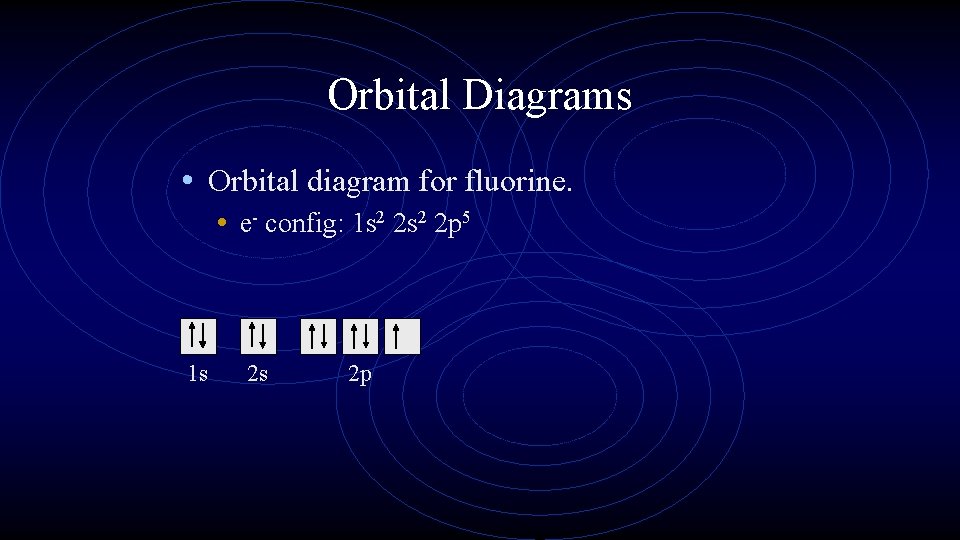
Orbital Diagrams • Orbital diagram for fluorine. • e- config: 1 s 2 2 p 5 1 s 2 s 2 p
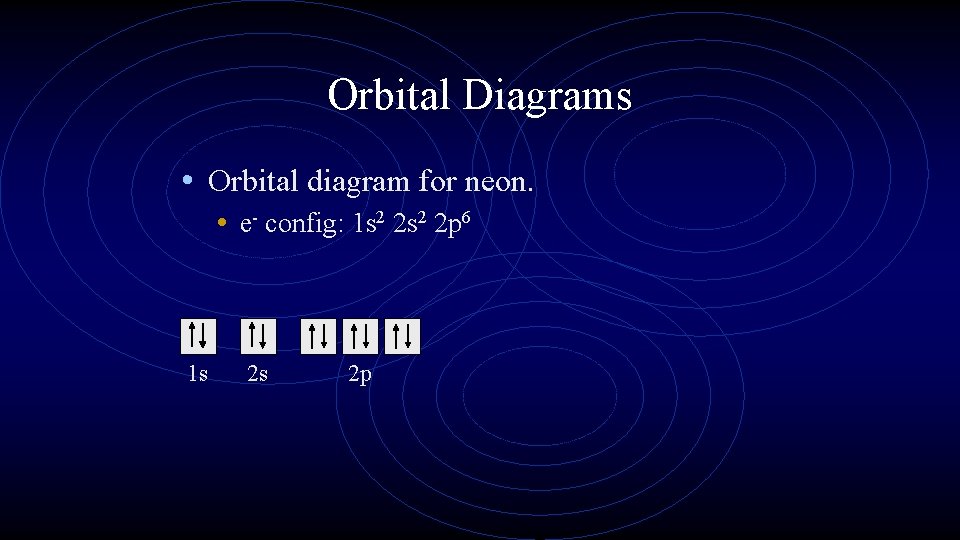
Orbital Diagrams • Orbital diagram for neon. • e- config: 1 s 2 2 p 6 1 s 2 s 2 p
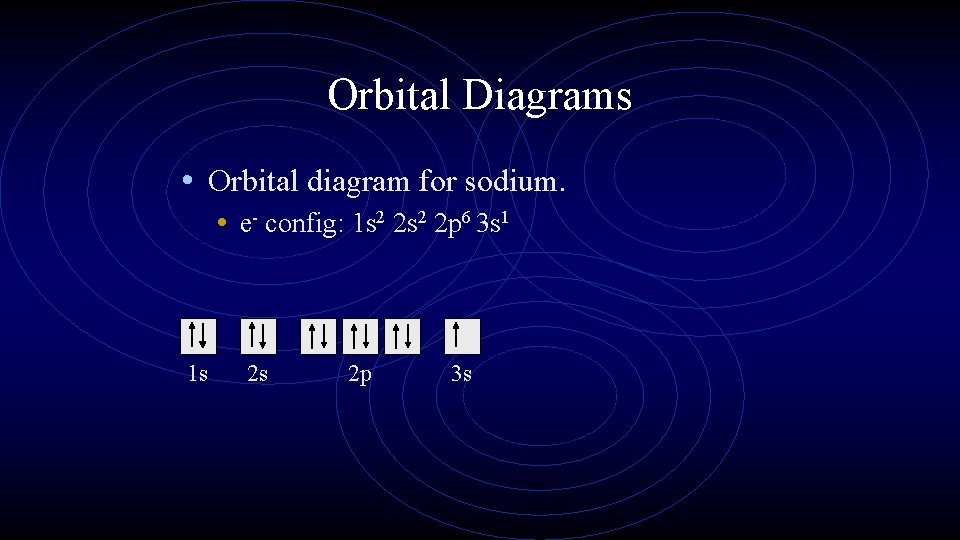
Orbital Diagrams • Orbital diagram for sodium. • e- config: 1 s 2 2 p 6 3 s 1 1 s 2 s 2 p 3 s
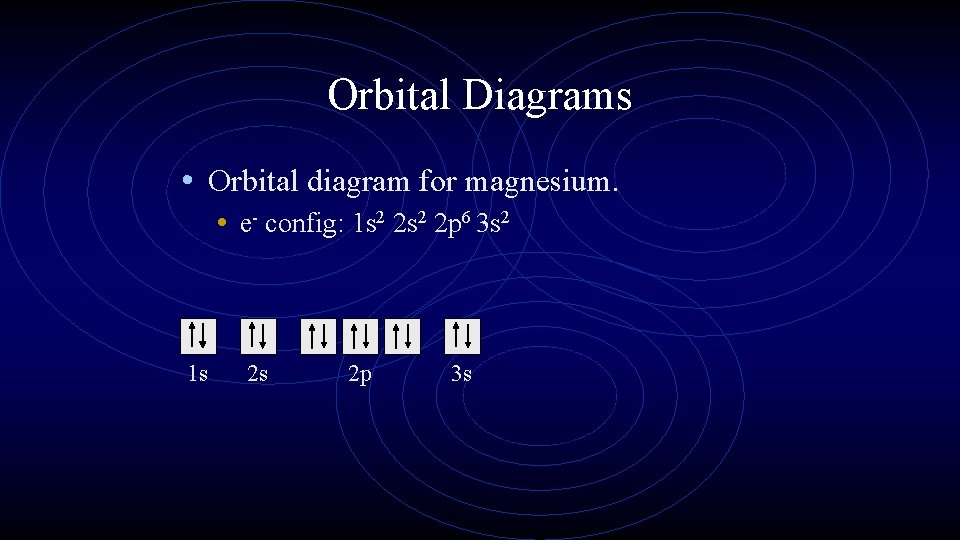
Orbital Diagrams • Orbital diagram for magnesium. • e- config: 1 s 2 2 p 6 3 s 2 1 s 2 s 2 p 3 s
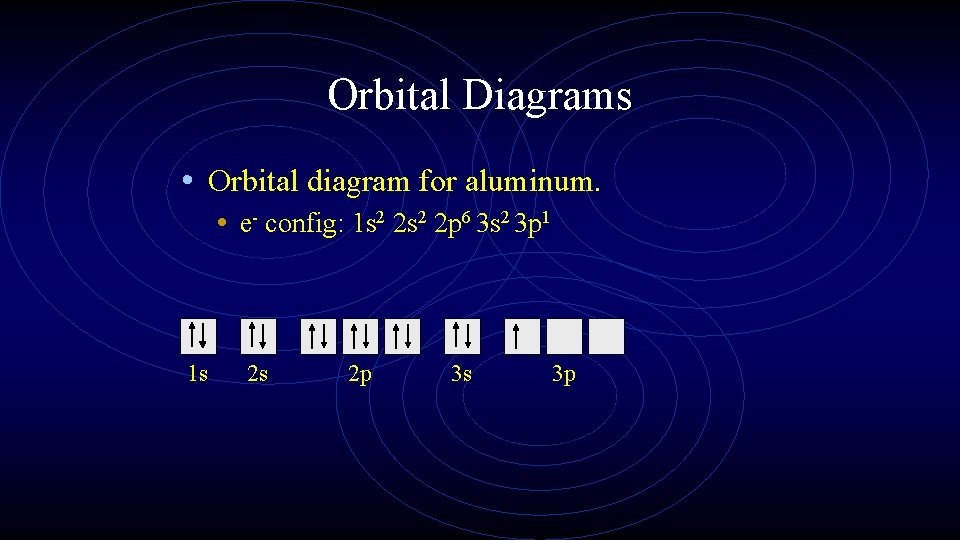
Orbital Diagrams • Orbital diagram for aluminum. • e- config: 1 s 2 2 p 6 3 s 2 3 p 1 1 s 2 s 2 p 3 s 3 p

Orbital Diagrams Practice Board

Orbital Diagrams Practice Sheet

Opening video. Journal, how do you explain? Diamagnetic levitation
Do magnetic example from Ms. Wilson, it is in the storage room

Paramagnetic Diamagnetic Molecules
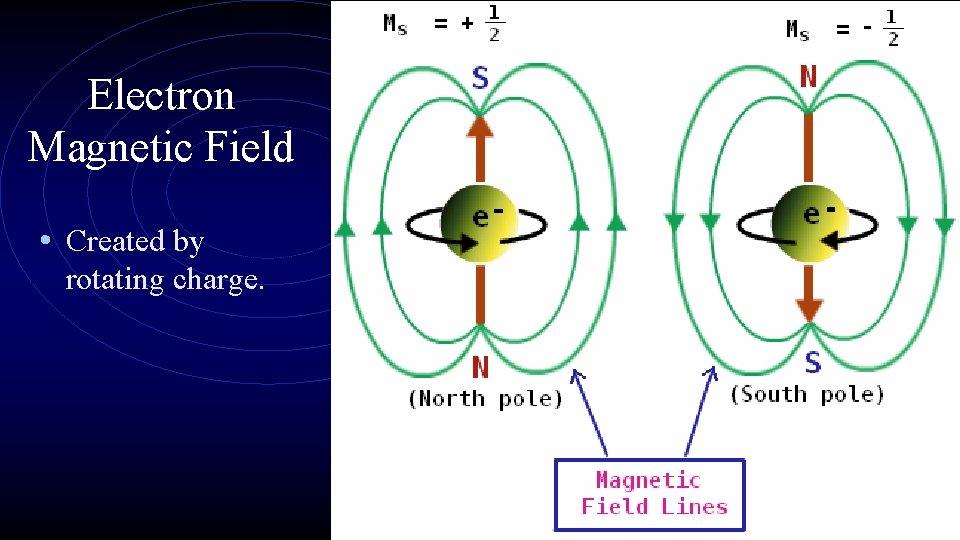
Electron Magnetic Field • Created by rotating charge.
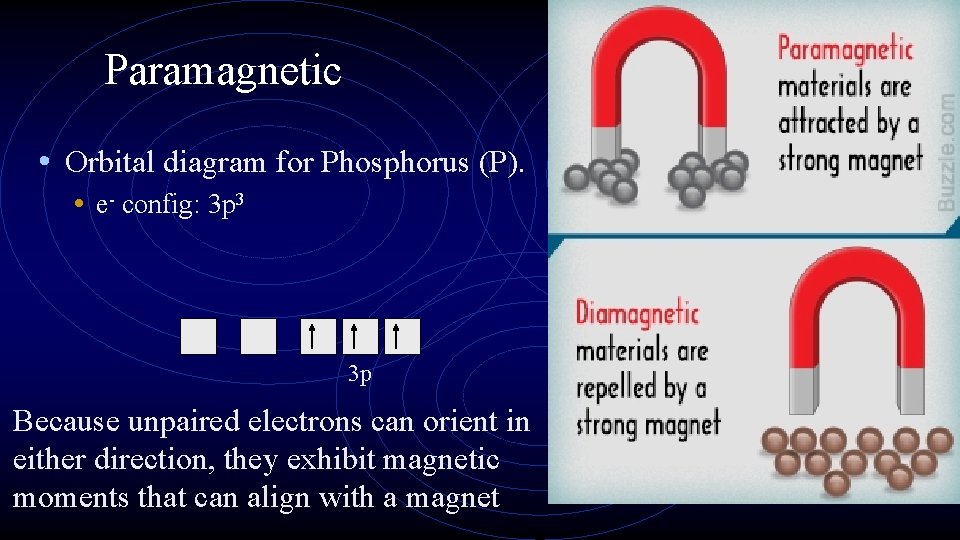
Paramagnetic • Orbital diagram for Phosphorus (P). • e- config: 3 p 3 3 p Because unpaired electrons can orient in either direction, they exhibit magnetic moments that can align with a magnet
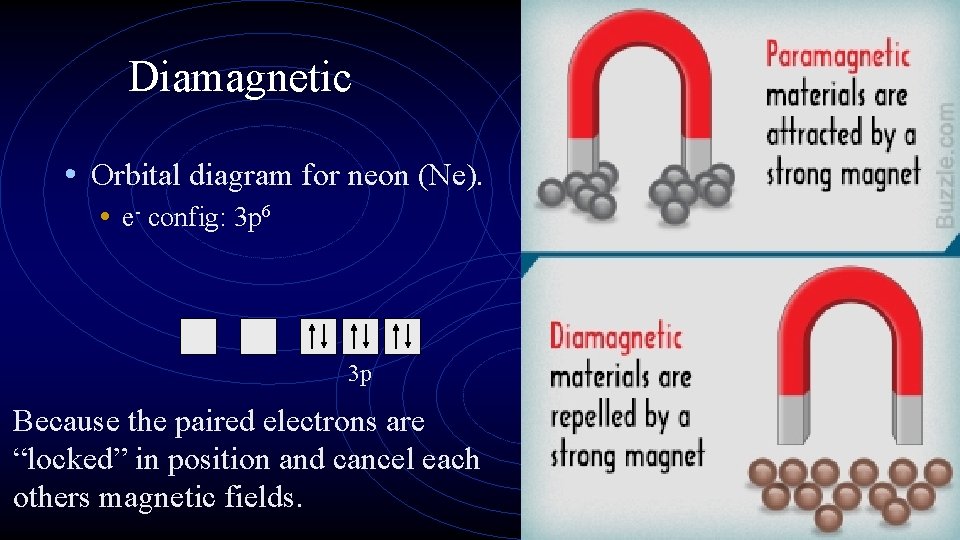
Diamagnetic • Orbital diagram for neon (Ne). • e- config: 3 p 6 3 p Because the paired electrons are “locked” in position and cancel each others magnetic fields.

Diamagnetic Levitation • Video – a frog in a strong magnetic field • Graphite on magnets • MRI application video

Diamagnetism of Water • How many electrons in Oxygen? • How many electrons in Hydrogen? • Write orbital diagram for each. • Write combined orbital diagram for molecular water.
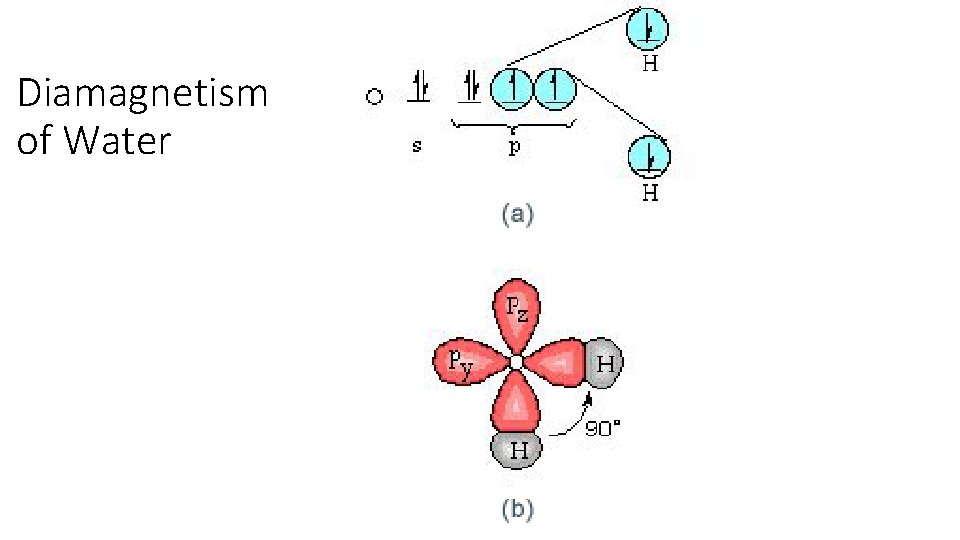
Diamagnetism of Water
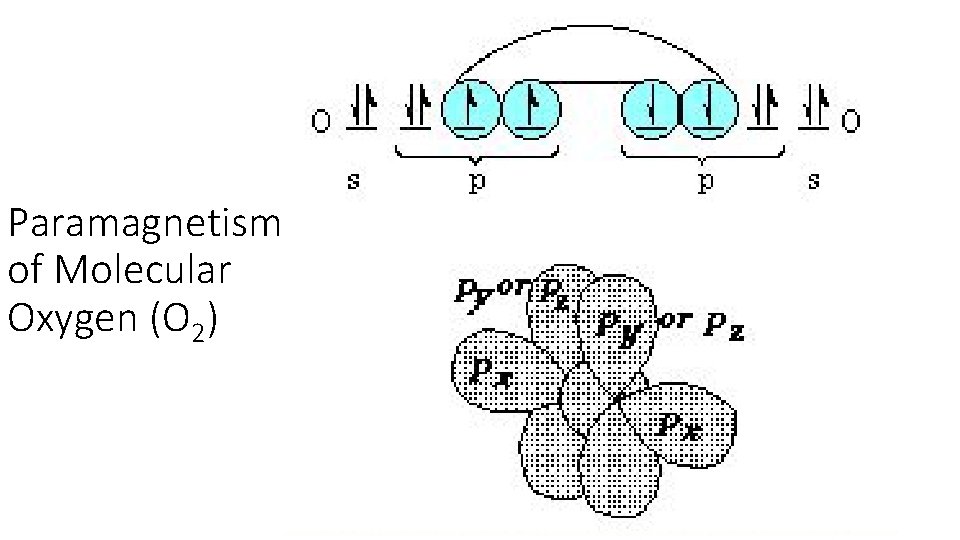
Paramagnetism of Molecular Oxygen (O 2)
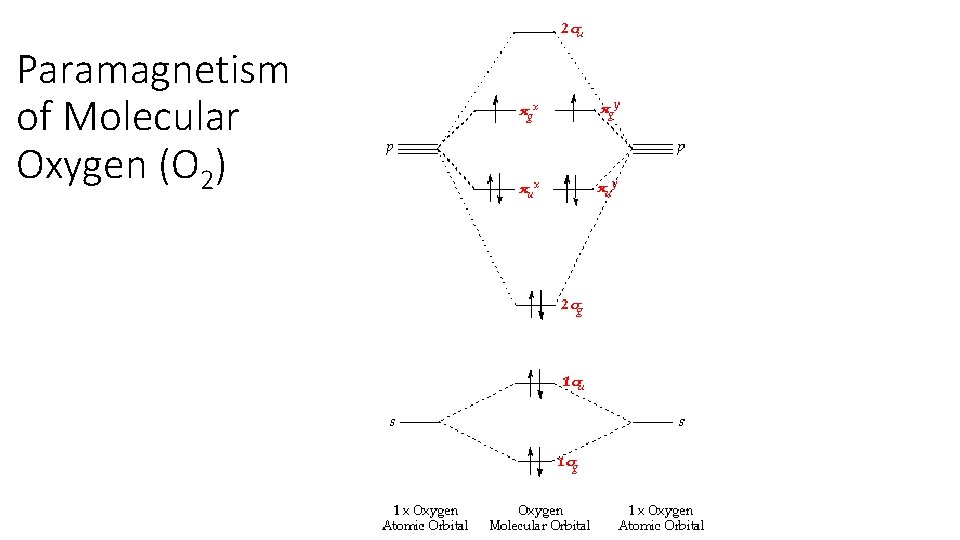
Paramagnetism of Molecular Oxygen (O 2)
Video • Video – liquid nitrogen vs. liquid Oxygen: magnetism
- Slides: 32