Oral Sustained Controlled Drug Delivery System Introduction v


















- Slides: 34

Oral Sustained & Controlled Drug Delivery System

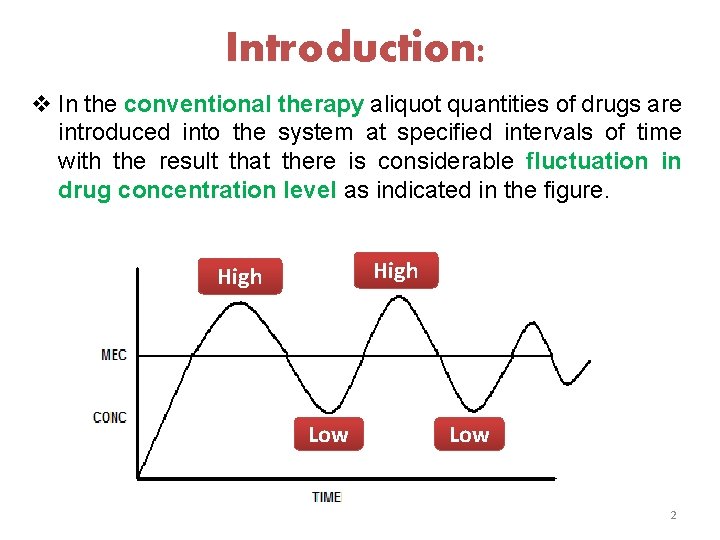
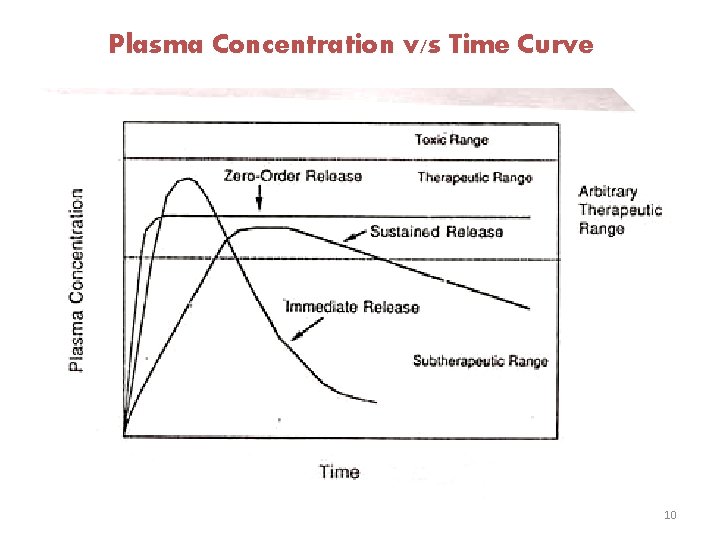
Introduction: v In the conventional therapy aliquot quantities of drugs are introduced into the system at specified intervals of time with the result that there is considerable fluctuation in drug concentration level as indicated in the figure. High Low 2

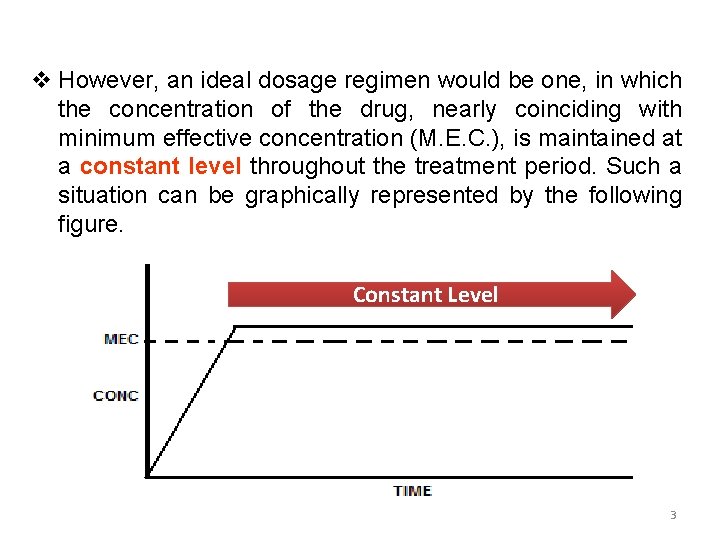
v However, an ideal dosage regimen would be one, in which the concentration of the drug, nearly coinciding with minimum effective concentration (M. E. C. ), is maintained at a constant level throughout the treatment period. Such a situation can be graphically represented by the following figure. Constant Level 3

Sustained Release Dosage Form: v “Drug Delivery system that are designed to achieve prolonged therapeutic effect by continuously releasing medication over an extended period of time after administration of single dose. ” v The basic goal of therapy is to achieve steady state blood level that is therapeutically effective and non toxic for an extended period of time. v The design of proper dosage regimen is an important element in accomplishing this goal. 4

Advantages: 1. Improved patient compliance 2. Improved efficiency of treatment 3. Better drug utilization 4. Bio-availability of certain drugs can be increased 5. Increased safety margin of high potency drugs and decreased local and systemic side effects 6. Reduces nursing and hospitalization time 5

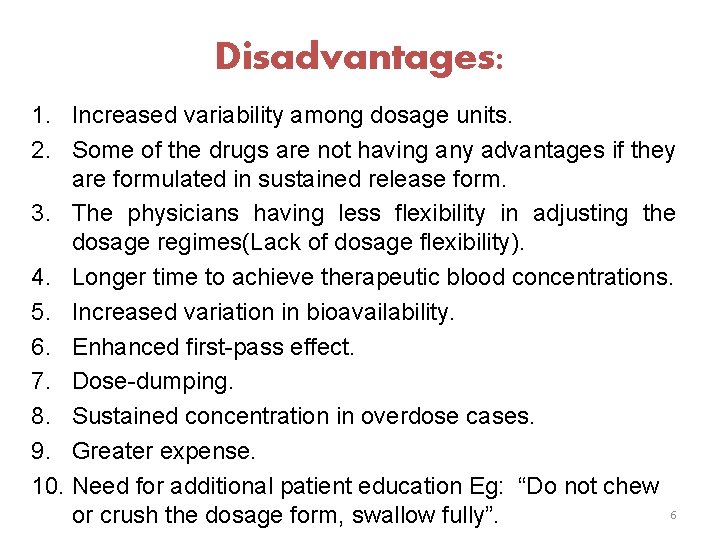
Disadvantages: 1. Increased variability among dosage units. 2. Some of the drugs are not having any advantages if they are formulated in sustained release form. 3. The physicians having less flexibility in adjusting the dosage regimes(Lack of dosage flexibility). 4. Longer time to achieve therapeutic blood concentrations. 5. Increased variation in bioavailability. 6. Enhanced first-pass effect. 7. Dose-dumping. 8. Sustained concentration in overdose cases. 9. Greater expense. 10. Need for additional patient education Eg: “Do not chew 6 or crush the dosage form, swallow fully”.

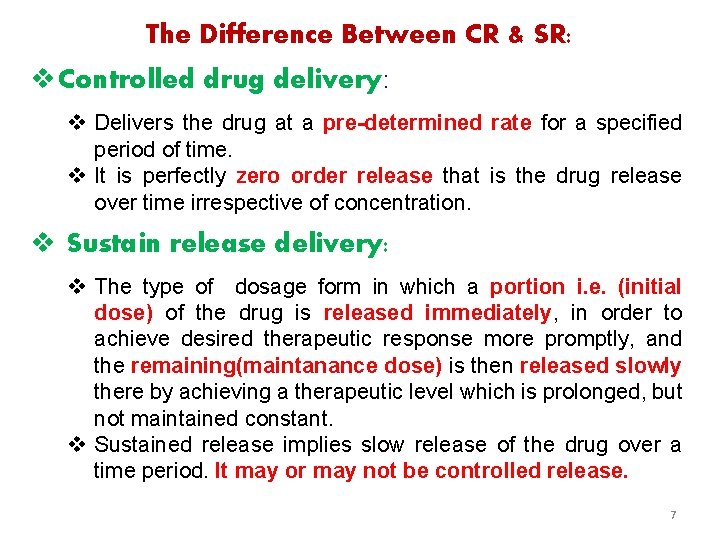
The Difference Between CR & SR: v Controlled drug delivery: v Delivers the drug at a pre-determined rate for a specified period of time. v It is perfectly zero order release that is the drug release over time irrespective of concentration. v Sustain release delivery: v The type of dosage form in which a portion i. e. (initial dose) of the drug is released immediately, in order to achieve desired therapeutic response more promptly, and the remaining(maintanance dose) is then released slowly there by achieving a therapeutic level which is prolonged, but not maintained constant. v Sustained release implies slow release of the drug over a time period. It may or may not be controlled release. 7

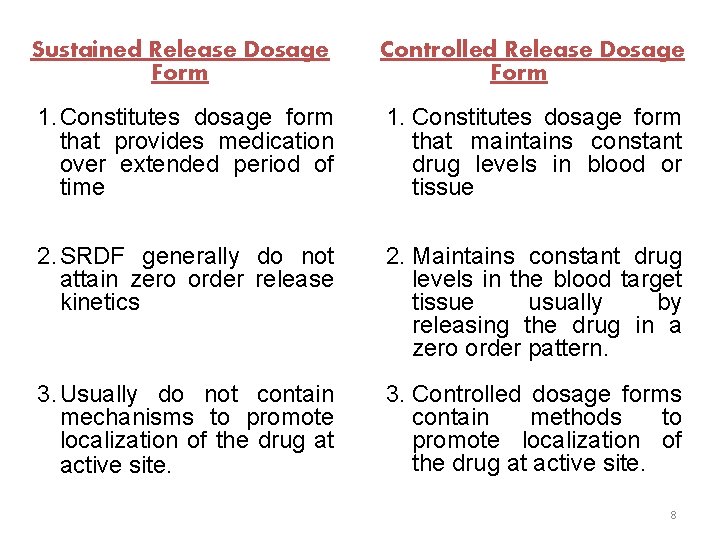
Sustained Release Dosage Form Controlled Release Dosage Form 1. Constitutes dosage form that provides medication over extended period of time 1. Constitutes dosage form that maintains constant drug levels in blood or tissue 2. SRDF generally do not attain zero order release kinetics 2. Maintains constant drug levels in the blood target tissue usually by releasing the drug in a zero order pattern. 3. Usually do not contain mechanisms to promote localization of the drug at active site. 3. Controlled dosage forms contain methods to promote localization of the drug at active site. 8

Rationality Behind S. R. Dosage Form: . v The basic objective in dosage form design is to optimize the delivery of medication to achieve the control of therapeutic effect in the face of uncertain fluctuation in the vivo environment in which drug release take place. v This is usually concerned with maximum drug availability by attempting to attain a maximum rate and extent of drug absorption however, control of drug action through formulation also implies controlling bioavailability to reduce drug absorption rates. v To improve patient compliance by reducing dosing frequency. v To improve efficiency of treatment, by maintaining safety margin of high potency drugs and decreased local and systemic side effects. v To reduce nursing and hospitalization time. 9

Plasma Concentration v/s Time Curve 10

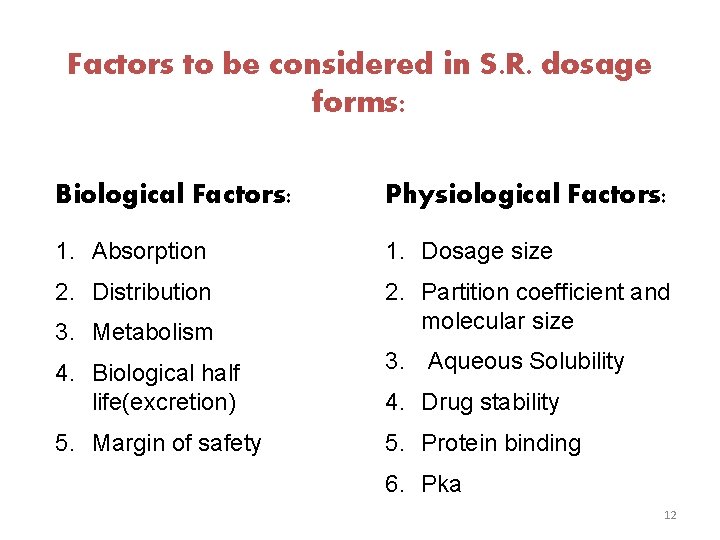
Drug Properties Relevant to SR Formulation: § The design of sustained release delivery system is subjected to several variables and each of variables are inter-related. § For the purpose of discussion it is convenient to describe the properties of the drugs as being either physicochemical or biological , these may be divided in two types: 1. Physicochemical properties 2. Biological properties 11

Factors to be considered in S. R. dosage forms: Biological Factors: Physiological Factors: 1. Absorption 1. Dosage size 2. Distribution 2. Partition coefficient and molecular size 3. Metabolism 4. Biological half life(excretion) 3. Aqueous Solubility 5. Margin of safety 5. Protein binding 4. Drug stability 6. Pka 12

Biological Factors: CONTD… Biological Factors: Absorption: § Absorption of drug need dissolution in fluid before it reaches to systemic circulation. § The rate, extent and uniformity in absorption of drug are important factor when considering its formulation in to controlled release system. Absorption and Dissolution § The characteristics of absorption of a drug can be greatly effects its suitability of sustained release product. 13

Biological Factors: CONTD… § The maximum half-life for absorption should be approximately 3 -4 hr otherwise, the device will pass out of potential absorptive region before drug release is complete. § Compounds that demonstrate true lower absorption rate constants will probably be poor candidates for sustaining systems. § The rate, extent and uniformity of absorption of a drug are important factors considered while formulation of sustained release formulation. § As the rate limiting step in drug delivery from a sustainedrelease system is its release from a dosage form, rather than absorption. 14

Biological Factors: CONTD… § The transit time of drug must be in the absorptive areas of the GI tract is about 8 -12 hrs. § If the rate of absorption is below 0. 17/hr and above the 0. 23/hr then it is difficult to prepare sustained release formulation. § As the rate limiting step in drug delivery from a sustainedrelease system is its release from a dosage form, rather than absorption. § Rapid rate of absorption of drug, relative to its release is essential if the system is to be successful. 15

Biological Factors: CONTD… Distribution: § The distribution of drugs into tissues can be important factor in the overall drug elimination kinetics. § Since it not only lowers the concentration of drug but it also can be rate limiting in its equilibrium with blood and extra vascular tissue, consequently apparent volume of distribution assumes different values depending on time course of drug disposition. § For design of sustained/ controlled release products, one must have information of disposition of drug. § The apparent volume of distribution Vd is nearly a proportional constant that release drug concentration in the blood or plasma to the amount of drug in the body. 16

Biological Factors: CONTD… Metabolism: § There are two areas of concern relative to metabolism that significantly restrict sustained release formulation. 1. If drug upon chronic administration is capable of either inducing or inhibition enzyme synthesis it will be poor candidate for sustained release formulation because of difficulty of maintaining uniform blood levels of drugs. 2. If there is a variable blood level of drug through a first-pass effect, this also will make preparation of sustained release product difficult. § Drug that are significantly metabolized before absorption, either in lumen of intestine, can show decreased bio-availability from slower-releasing dosage 17 forms.

Biological Factors: CONTD… Biological Half Life: § The usual goal of sustained release product is to maintain therapeutic blood level over an extended period, to this drug must enter the circulation at approximately the same rate at which it is eliminated. The elimination rate is quantitatively described by the half-life (t 1/2). § Therapeutic compounds with short half life are excellent candidates for sustained release preparation since these can reduce dosing frequency. § Drugs with half-life shorter than 2 hours. Such as e. g. : Furosemide, levodopa are poor for sustained release formulation because it requires large rates and large dose compounds with long half-life. § More than 8 hours are also generally not used in sustaining forms, since their effect is already sustained. E. g. ; Digoxin, 18 Warfarin, Phenytoin etc.

Biological Factors: CONTD… Margin of Safety: § In general the larger the volume of therapeutic index safer the drug. § Drug with very small values of therapeutic index usually are poor candidates for SRDF due to pharmacological limitation of control over release rate. e. g. - induced digtoxin, Phenobarbital, phenotoin. = TD 50/ED 50 § Larger the TI ratio the safer is drug. § It is imperative that the drug release pattern is precise so that the plasma drug concentration achieved in under therapeutic range. 19

Physiological Factors: 1. Dosage size. 2. Partition coefficient and molecular size. 3. Aqueous Solubility. 4. Drug stability. 5. Protein binding. 6. PKa 20

Physiological Factors: CONTD… Dosage Size: § In general a single dose of 0. 5 - 1. 0 gm is considered for a conventional dosage form this also holds for sustained release dosage forms. § If an oral product has a dose size greater that 500 mg it is a poor candidate for sustained release system, since addition of sustaining dose and possibly the sustaining mechanism will, in most cases generates a substantial volume product that unacceptably large. 21

Physiological Factors: CONTD… Partition Coefficient & Molecular Size: § When the drug is administered to the GIT, it must cross a variety of biological membranes to produce therapeutic effects in another area of the body. § It is common to consider that these membranes are lipidic, therefore the Partition coefficient of oil soluble drugs becomes important in determining the effectiveness of membranes barrier penetration. § Partition coefficient is the fraction of drug in an oil phase to that of an adjacent aqueous phase. § High partition coefficient compound are predominantly lipid soluble and have very low aqueous solubility and thus these compound persist in the body for long periods. 22

Physiological Factors: CONTD… § Partition coefficient and molecular size influence not only the penetration of drug across the membrane but also diffusion across the rate limiting membrane. § The ability of drug to diffuse through membranes its so called diffusivity & diffusion coefficient is function of molecular size (or molecular weight). § Thus high molecular weight drugs or polymeric drugs should be expected to display very slow release kinetics in sustained release device using diffusion through polymer membrane. 23

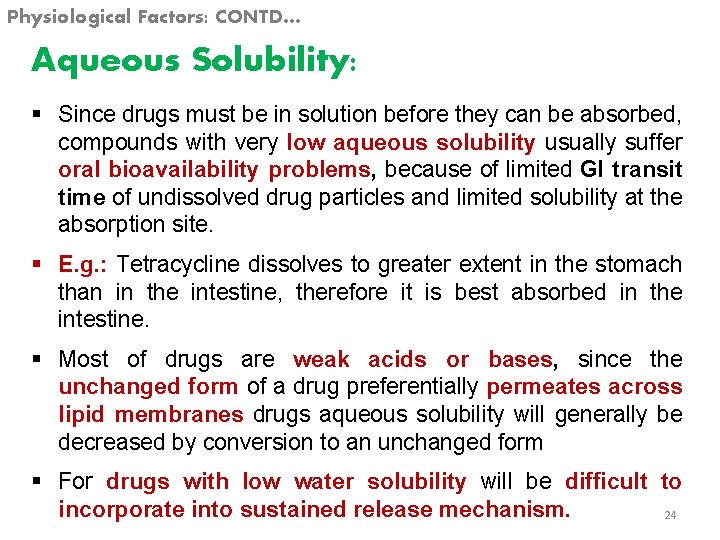
Physiological Factors: CONTD… Aqueous Solubility: § Since drugs must be in solution before they can be absorbed, compounds with very low aqueous solubility usually suffer oral bioavailability problems, because of limited GI transit time of undissolved drug particles and limited solubility at the absorption site. § E. g. : Tetracycline dissolves to greater extent in the stomach than in the intestine, therefore it is best absorbed in the intestine. § Most of drugs are weak acids or bases, since the unchanged form of a drug preferentially permeates across lipid membranes drugs aqueous solubility will generally be decreased by conversion to an unchanged form § For drugs with low water solubility will be difficult to incorporate into sustained release mechanism. 24

Physiological Factors: CONTD… Aqueous solubility and p. Ka § These are the most important to influence its absorptive behavior and its aqueous solubility (if it’s a weak acid or base) and its p. Ka. § The aqueous solubility of the drug influences its dissolution rate. 25

Physiological Factors: CONTD… Drug Stability: § The stability of drug in environment to which it is exposed, is another physico-chemical factor to be considered in design at sustained/ controlled release systems, drugs that are unstable in stomach can be placed in slowly soluble forms or have their release delayed until they reach the small intestine. § Orally administered drugs can be subject to both acid, base hydrolysis and enzymatic degradation. § Degradation will proceed at the reduced rate for drugs in the solid state. § For drugs that are unstable in stomach, systems that prolong delivery ever the entire course of transit in GI tract 26 are beneficial.

Physiological Factors: CONTD… § Compounds that are unstable in the small intestine may demonstrate decreased bioavailability when administered form a sustaining dosage from. § This is because more drug is delivered in small intestine and hence subject to degradation. § However for some drugs which are unstable in small intestine are undergo extensive gut–wall metabolism have decreased the bioavailability. § When these drugs are administered from a sustained dosage form to achieve better bioavailability, different routes of the drugs administered should be chosen. E. g. Nitroglycerine. 27

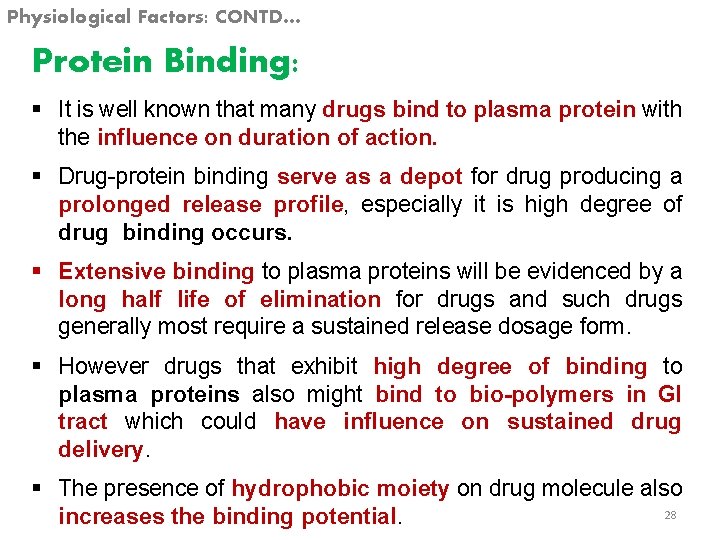
Physiological Factors: CONTD… Protein Binding: § It is well known that many drugs bind to plasma protein with the influence on duration of action. § Drug-protein binding serve as a depot for drug producing a prolonged release profile, especially it is high degree of drug binding occurs. § Extensive binding to plasma proteins will be evidenced by a long half life of elimination for drugs and such drugs generally most require a sustained release dosage form. § However drugs that exhibit high degree of binding to plasma proteins also might bind to bio-polymers in GI tract which could have influence on sustained drug delivery. § The presence of hydrophobic moiety on drug molecule also 28 increases the binding potential.

Physiological Factors: CONTD… § The binding of the drugs to plasma proteins (e. g. Albumin) results in retention of the drug into the vascular space the drug protein complex can serves as reservoir in the vascular space for sustained drug release to extra vascular tissue but only for those drugs that exhibited a high degree of binding. § The main force of attraction are Wander-Vals forces, hydrogen binding, electrostatic binding. § In general charged compound have a greater tendency to bind a protein then uncharged compound, due to electrostatic effect. § E. g. Amitryptline, Cumarin, Dicaumarol, Novobiocin. Diazepam, Digoxide, 29

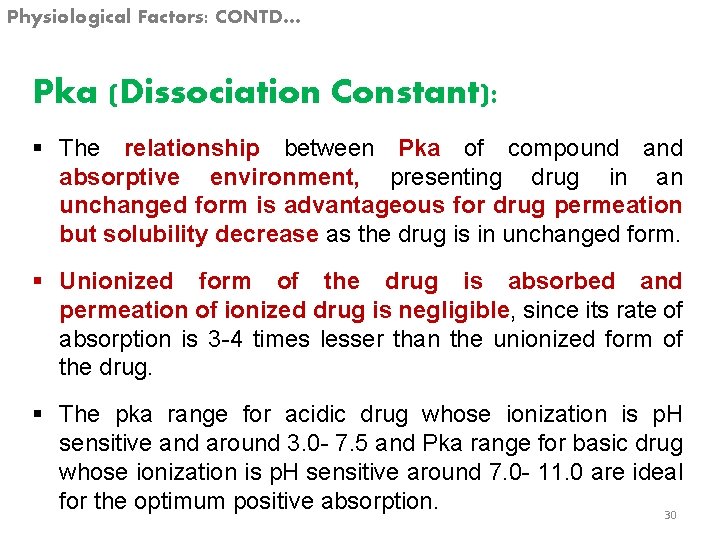
Physiological Factors: CONTD… Pka (Dissociation Constant): § The relationship between Pka of compound absorptive environment, presenting drug in an unchanged form is advantageous for drug permeation but solubility decrease as the drug is in unchanged form. § Unionized form of the drug is absorbed and permeation of ionized drug is negligible, since its rate of absorption is 3 -4 times lesser than the unionized form of the drug. § The pka range for acidic drug whose ionization is p. H sensitive and around 3. 0 - 7. 5 and Pka range for basic drug whose ionization is p. H sensitive around 7. 0 - 11. 0 are ideal for the optimum positive absorption. 30

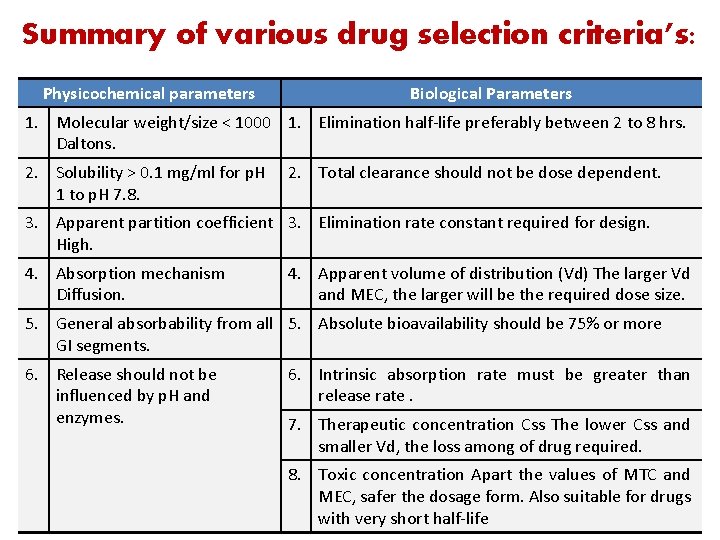
Summary of various drug selection criteria’s: Physicochemical parameters Biological Parameters 1. Molecular weight/size < 1000 1. Elimination half-life preferably between 2 to 8 hrs. Daltons. 2. Solubility > 0. 1 mg/ml for p. H 1 to p. H 7. 8. 2. Total clearance should not be dose dependent. 3. Apparent partition coefficient 3. Elimination rate constant required for design. High. 4. Absorption mechanism Diffusion. 4. Apparent volume of distribution (Vd) The larger Vd and MEC, the larger will be the required dose size. 5. General absorbability from all 5. Absolute bioavailability should be 75% or more GI segments. 6. Release should not be influenced by p. H and enzymes. 6. Intrinsic absorption rate must be greater than release rate. 7. Therapeutic concentration Css The lower Css and smaller Vd, the loss among of drug required. 8. Toxic concentration Apart the values of MTC and MEC, safer the dosage form. Also suitable for drugs 31 with very short half-life

2 Marks 1. 2. 3. 4. 5. 6. 7. 8. 9. Question Bank: Give various advantages of sustained drug delivery system. ** Define sustained and controlled drug delivery system. State different synonyms for sustained drug delivery system. What are disadvantages of sustained drug delivery system? Differentiate between controlled release and sustained release formulations. ** Define for maintenance & loading dose and give there formula. * What is loading dose? Give formula for calculation of loading dose. State various evaluation parameters used for evaluation of sustained or controlled-release tablets. Define sustained release dosage form. State its 32

5 Marks 1. Give classification of oral controlled & sustained release systems. ** Add a note on altered density systems. 2. Give the model drug selection criteria based on biopharmaceutical characteristics for sustained drug delivery system. 3. Give the model drug selection criteria based on PK-PD characteristics for sustained drug delivery system. 4. Explain the evaluation of sustained release tablets. **** 5. Write in brief osmotic & hydrodynamic controlled system. 6. How sustained release dosage forms differ from controlled release dosage form? Add a note on osmotic pressure controlled release system. 33

10 Marks 1. Discuss in detail about model drug selection criteria for sustained drug delivery system. *** 2. Give objective of sustained drug delivery system. State and explain drug selection criteria for sustained drug delivery system. Mention different evaluation parameters for sustained release tablets. 3. Give detail classification of oral controlled systems. Explain them in very brief. * 34