Oral anticoagulant therapy a look to the future














- Slides: 35

Oral anticoagulant therapy : a look to the future Alexander G. G. Turpie Department of Medicine HHS General Hospital Hamilton, Canada

Antithrombotics That Have Changed Clinical Practice Anticoagulants § Low molecular weight heparin Antiplatelet Drugs § Thienopyridines § Glycoprotein IIb/IIIa Inhibitors

But…………. . for oral anticoagulation, Vitamin K antagonists (warfarin) remain the only available option

The ‘ideal’ oral anticoagulant § Oral, preferably once daily § Rapid onset and offset of action § Predictable PK and PD § Low propensity for food and drug interactions § Fixed doses § Wide therapeutic window § Easy to use with no need for monitoring

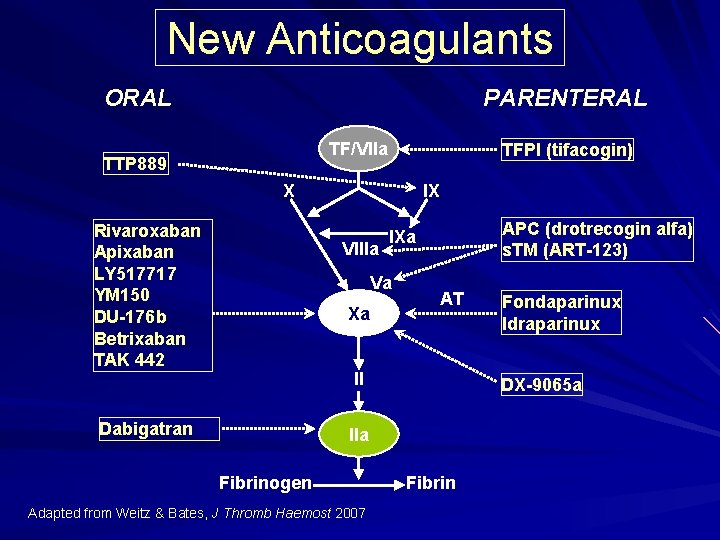
New Anticoagulants ORAL PARENTERAL TF/VIIa TTP 889 TFPI (tifacogin) X Rivaroxaban Apixaban LY 517717 YM 150 DU-176 b Betrixaban TAK 442 IX VIIIa Va Xa APC (drotrecogin alfa) s. TM (ART-123) IXa AT II Dabigatran DX-9065 a IIa Fibrinogen Adapted from Weitz & Bates, J Thromb Haemost 2007 Fondaparinux Idraparinux Fibrin

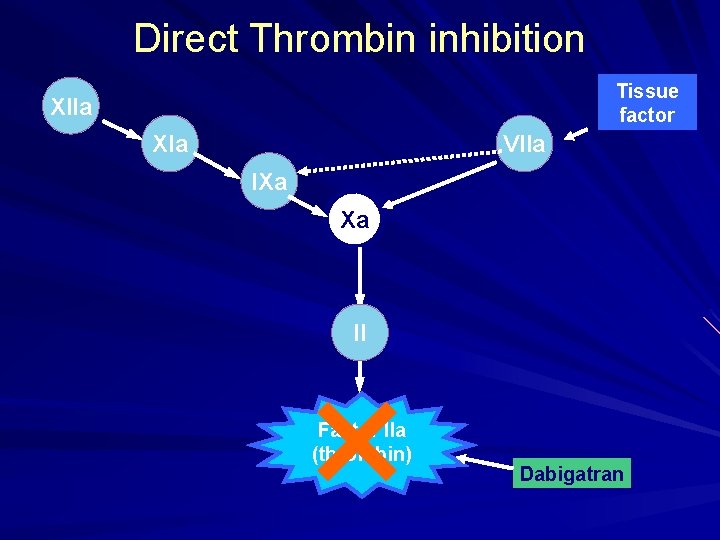
Direct Thrombin inhibition Tissue factor XIIa XIa VIIa IXa Xa II × Factor IIa (thrombin) Dabigatran

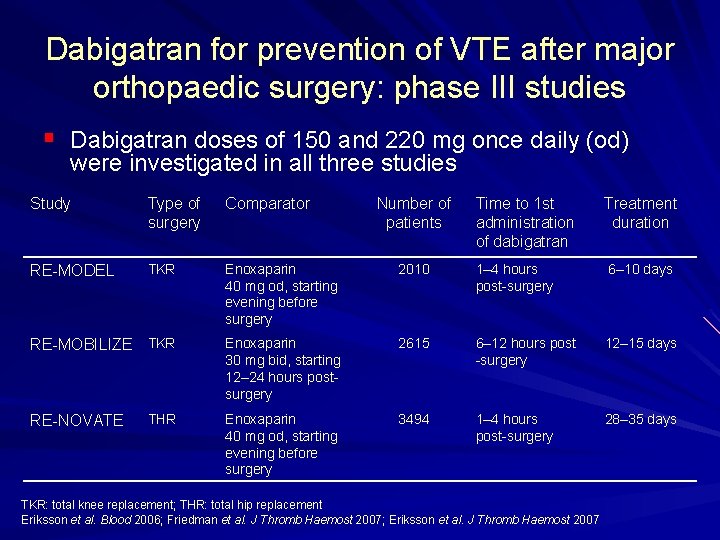
Dabigatran for prevention of VTE after major orthopaedic surgery: phase III studies § Dabigatran doses of 150 and 220 mg once daily (od) were investigated in all three studies Study Type of surgery Comparator RE MODEL TKR Enoxaparin 40 mg od, starting evening before surgery RE MOBILIZE TKR THR RE NOVATE Number of patients Time to 1 st administration of dabigatran Treatment duration 2010 1– 4 hours post surgery 6– 10 days Enoxaparin 30 mg bid, starting 12– 24 hours post surgery 2615 6– 12 hours post surgery 12– 15 days Enoxaparin 40 mg od, starting evening before surgery 3494 1– 4 hours post surgery 28– 35 days TKR: total knee replacement; THR: total hip replacement Eriksson et al. Blood 2006; Friedman et al. J Thromb Haemost 2007; Eriksson et al. J Thromb Haemost 2007

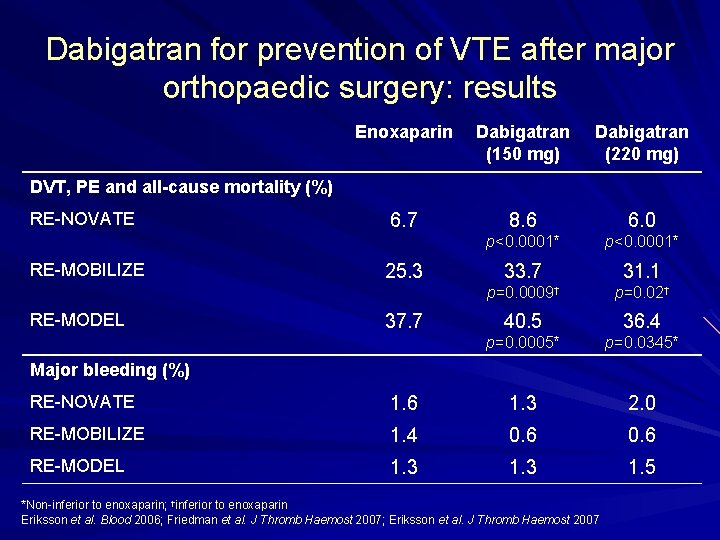
Dabigatran for prevention of VTE after major orthopaedic surgery: results Enoxaparin Dabigatran (150 mg) Dabigatran (220 mg) 6. 7 8. 6 6. 0 p<0. 0001* 33. 7 31. 1 p=0. 0009† p=0. 02† 40. 5 36. 4 p=0. 0005* p=0. 0345* DVT, PE and all-cause mortality (%) RE NOVATE RE MOBILIZE RE MODEL 25. 3 37. 7 Major bleeding (%) RE NOVATE 1. 6 1. 3 2. 0 RE MOBILIZE 1. 4 0. 6 RE MODEL 1. 3 1. 5 *Non inferior to enoxaparin; †inferior to enoxaparin Eriksson et al. Blood 2006; Friedman et al. J Thromb Haemost 2007; Eriksson et al. J Thromb Haemost 2007

Dabigatran: phase III studies § RE LY (stroke prevention in patients with AF) – Planned enrolment 15, 000 patients – Dabigatran 110 and 150 mg bid compared with warfarin – Treatment duration up to 3 years § RE SOLVE, RE COVER and RE MEDY – Ongoing studies in treatment and secondary prevention of VTE

New Anticoagulants ORAL PARENTERAL TF/VIIa TTP 889 TFPI (tifacogin) X Rivaroxaban Apixaban LY 517717 YM 150 DU-176 b Betrixaban TAK 442 IX VIIIa Va Xa APC (drotrecogin alfa) s. TM (ART-123) IXa AT II Dabigatran DX-9065 a IIa Fibrinogen Adapted from Weitz & Bates, J Thromb Haemost 2007 Fondaparinux Idraparinux Fibrin

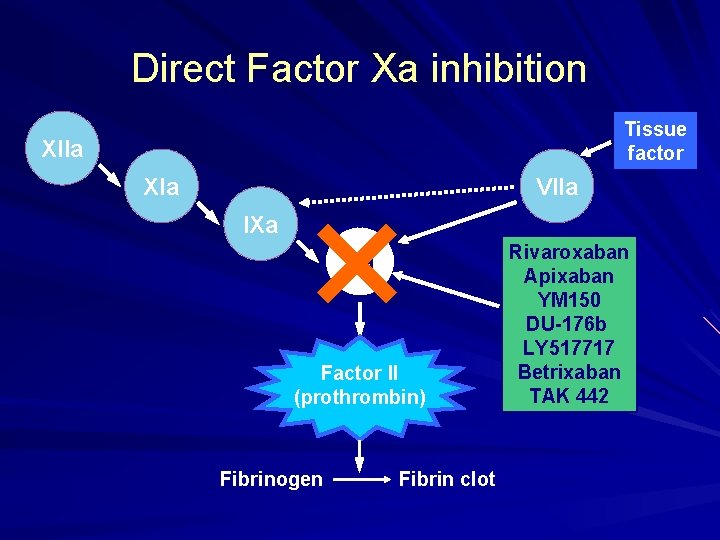
Direct Factor Xa inhibition XIIa XIa IXa × Xa Factor II (prothrombin) Fibrinogen Fibrin clot Tissue factor VIIa Rivaroxaban Apixaban YM 150 DU-176 b LY 517717 Betrixaban TAK 442

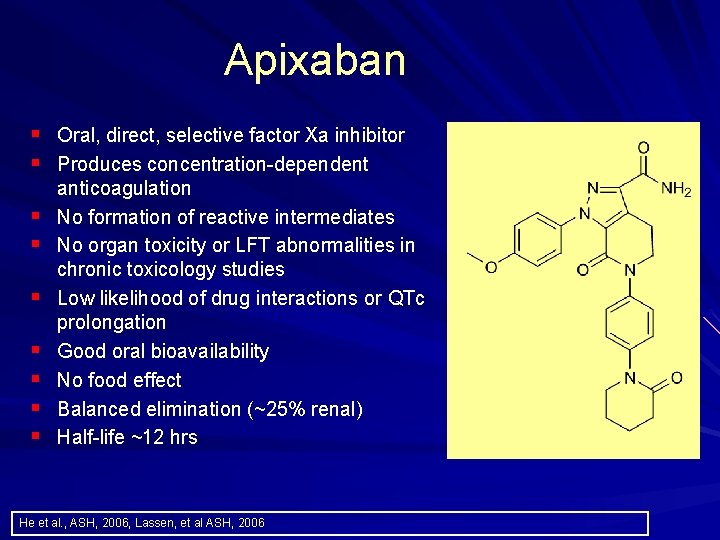
Apixaban § Oral, direct, selective factor Xa inhibitor § Produces concentration dependent § § § § anticoagulation No formation of reactive intermediates No organ toxicity or LFT abnormalities in chronic toxicology studies Low likelihood of drug interactions or QTc prolongation Good oral bioavailability No food effect Balanced elimination (~25% renal) Half life ~12 hrs He et al. , ASH, 2006, Lassen, et al ASH, 2006

Apixaban : Phase II Apropos – orthopaedic surgery Botticelli – treatment Adapt – advanced cancer Appraise 1 – ACS

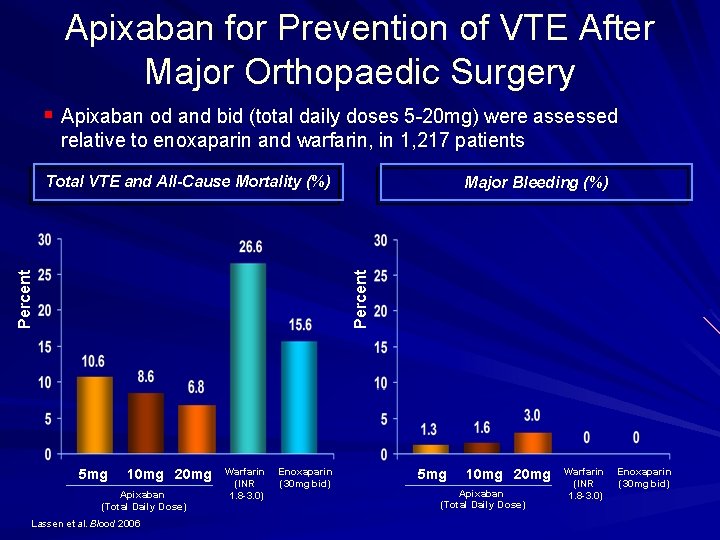
Apixaban for Prevention of VTE After Major Orthopaedic Surgery § Apixaban od and bid (total daily doses 5 20 mg) were assessed relative to enoxaparin and warfarin, in 1, 217 patients Total VTE and All-Cause Mortality (%) Percent Major Bleeding (%) 5 mg 10 mg 20 mg Apixaban (Total Daily Dose) Lassen et al. Blood 2006 Warfarin (INR 1. 8 -3. 0) Enoxaparin (30 mg bid) 5 mg 10 mg 20 mg Apixaban (Total Daily Dose) Warfarin (INR 1. 8 -3. 0) Enoxaparin (30 mg bid)

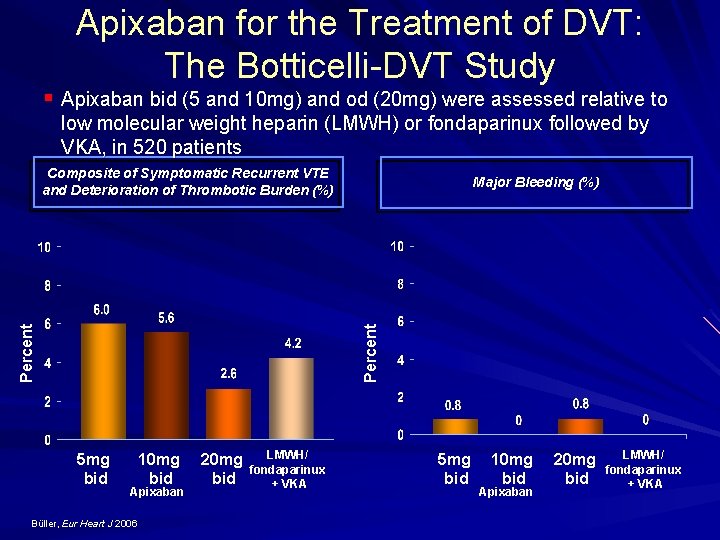
Apixaban for the Treatment of DVT: The Botticelli DVT Study § Apixaban bid (5 and 10 mg) and od (20 mg) were assessed relative to low molecular weight heparin (LMWH) or fondaparinux followed by VKA, in 520 patients Composite of Symptomatic Recurrent VTE and Deterioration of Thrombotic Burden (%) Percent Major Bleeding (%) 5 mg bid 10 mg bid Apixaban Büller, Eur Heart J 2006 20 mg bid LMWH/ fondaparinux + VKA 5 mg bid 10 mg bid Apixaban 20 mg bid LMWH/ fondaparinux + VKA

Apixaban : Phase III Advance 1, 2, 3 – orthopaedic surgery Adopt – medically ill Aristotle atrial fibrillation Appraise 2 ACS

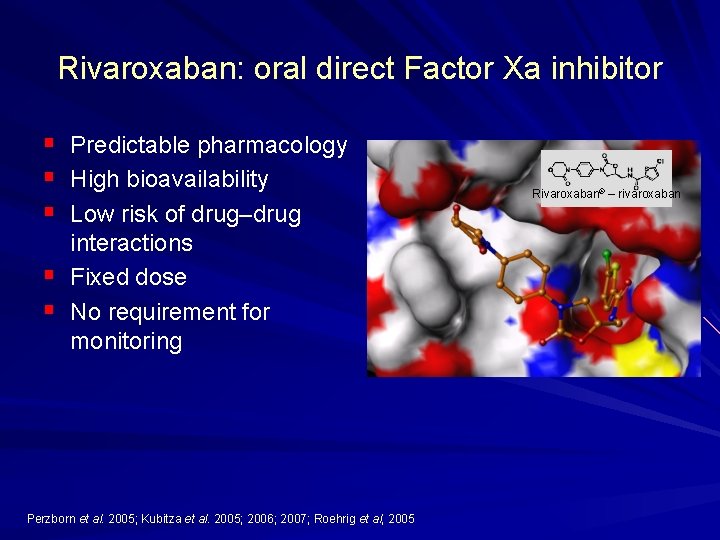
Rivaroxaban: oral direct Factor Xa inhibitor § § § Predictable pharmacology High bioavailability Low risk of drug–drug interactions Fixed dose No requirement for monitoring Perzborn et al. 2005; Kubitza et al. 2005; 2006; 2007; Roehrig et al, 2005 Rivaroxaban® – rivaroxaban

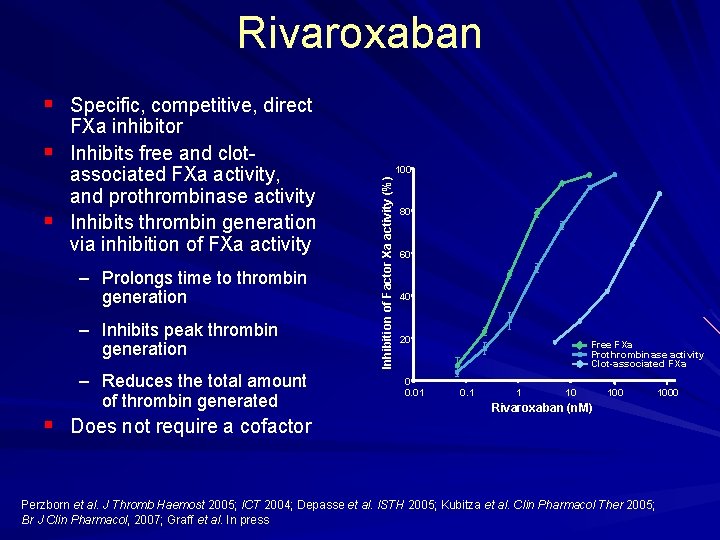
Rivaroxaban § Specific, competitive, direct § – Prolongs time to thrombin generation – Inhibits peak thrombin generation – Reduces the total amount of thrombin generated § Does not require a cofactor 100 Inhibition of Factor Xa activity (%) § FXa inhibitor Inhibits free and clot associated FXa activity, and prothrombinase activity Inhibits thrombin generation via inhibition of FXa activity 80 60 40 20 0 0. 01 Free FXa Prothrombinase activity Clot-associated FXa 0. 1 1 10 100 Rivaroxaban (n. M) Perzborn et al. J Thromb Haemost 2005; ICT 2004; Depasse et al. ISTH 2005; Kubitza et al. Clin Pharmacol Ther 2005; Br J Clin Pharmacol, 2007; Graff et al. In press 1000

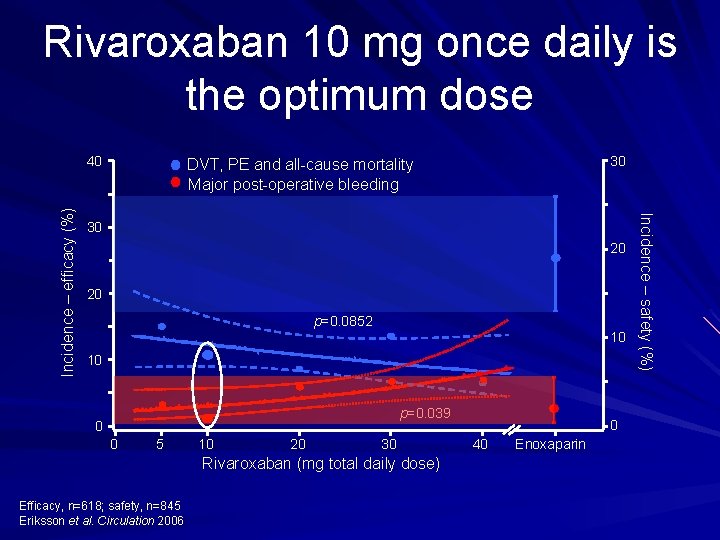
Rivaroxaban 10 mg once daily is the optimum dose 30 DVT, PE and all cause mortality Major post operative bleeding 30 20 20 p=0. 0852 10 10 p=0. 039 0 0 5 10 20 30 Rivaroxaban (mg total daily dose) Efficacy, n=618; safety, n=845 Eriksson et al. Circulation 2006 0 40 Enoxaparin Incidence – safety (%) Incidence – efficacy (%) 40

Rivaroxaban: VTE Treatment Trials Deep Vein Thrombosis Pulmonary Embolism r T m t a e t n e

Rivaroxaban for the treatment and secondary prevention of VTE § Two large, phase II studies of rivaroxaban for 3 months for the treatment and long term secondary prevention of VTE: – ODIXa DVT : Rivaroxaban 10– 30 mg bid and 40 mg od – EINSTEIN DVT : Rivaroxaban 20– 40 mg od – LMWH followed by a VKA comparator in both studies Agnelli et al. Circulation 2007; Büller. Eur Heart J 2006

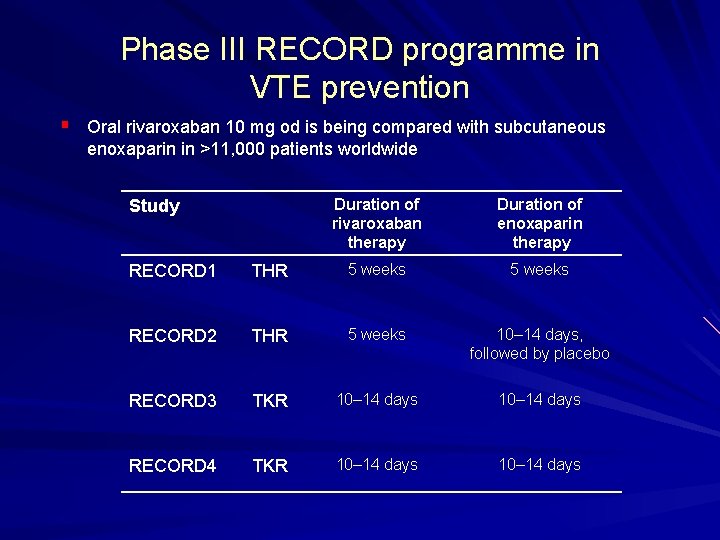
Phase III RECORD programme in VTE prevention § Oral rivaroxaban 10 mg od is being compared with subcutaneous enoxaparin in >11, 000 patients worldwide Study Duration of rivaroxaban therapy Duration of enoxaparin therapy RECORD 1 THR 5 weeks RECORD 2 THR 5 weeks 10– 14 days, followed by placebo RECORD 3 TKR 10– 14 days RECORD 4 TKR 10– 14 days

Efficacy endpoints Primary § Total venous thromboembolism (VTE): any deep vein thrombosis (DVT), non fatal pulmonary embolism (PE), and all cause mortality Secondary § Major VTE: proximal DVT, non fatal PE, and VTE related death § DVT: any, proximal, distal § Symptomatic VTE All endpoints were adjudicated centrally by independent, blinded committees

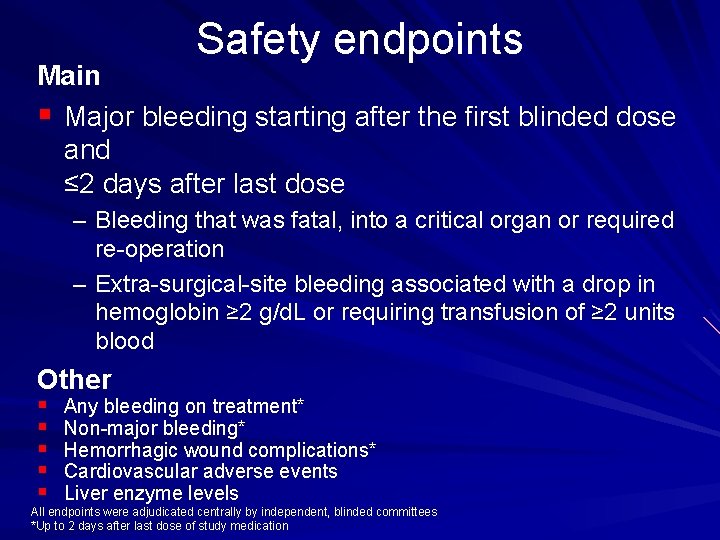
Safety endpoints Main § Major bleeding starting after the first blinded dose and ≤ 2 days after last dose – Bleeding that was fatal, into a critical organ or required re operation – Extra surgical site bleeding associated with a drop in hemoglobin ≥ 2 g/d. L or requiring transfusion of ≥ 2 units blood Other § § § Any bleeding on treatment* Non major bleeding* Hemorrhagic wound complications* Cardiovascular adverse events Liver enzyme levels All endpoints were adjudicated centrally by independent, blinded committees *Up to 2 days after last dose of study medication

Rivaroxaban – an oral, direct Factor Xa inhibitor – for the prevention of venous thromboembolism in total knee arthroplasty surgery

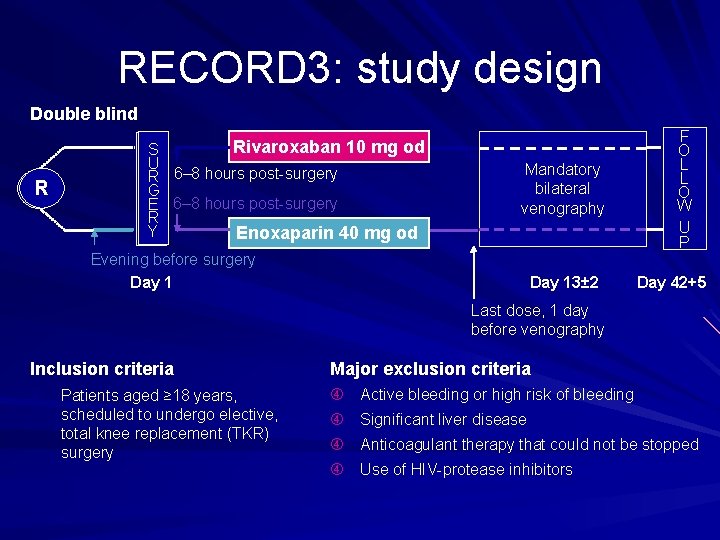
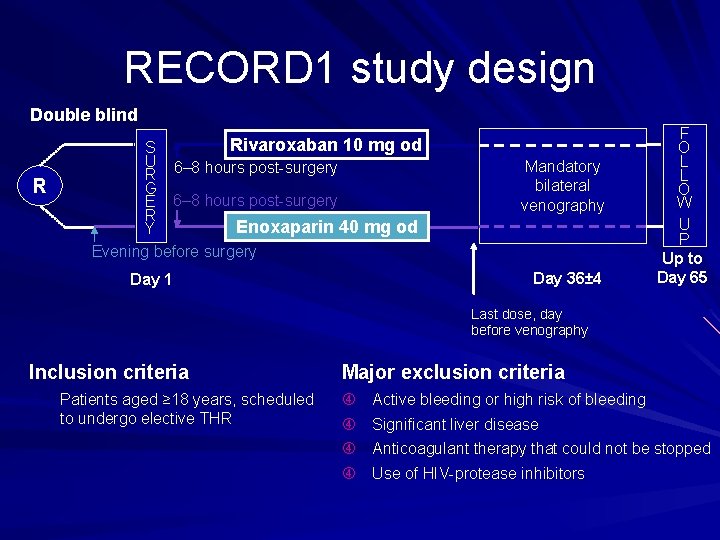
RECORD 3: study design Double blind R R Rivaroxaban 10 mg od S S U U R 6– 8 hours post surgery R G G E 6– 8 hours post surgery E R R Y Y Enoxaparin 40 mg od Mandatory bilateral venography Evening before surgery Day 13± 2 F O L L O W U P Day 42+5 Last dose, 1 day before venography Inclusion criteria Major exclusion criteria Patients aged ≥ 18 years, scheduled to undergo elective, total knee replacement (TKR) surgery Active bleeding or high risk of bleeding Significant liver disease Anticoagulant therapy that could not be stopped Use of HIV protease inhibitors

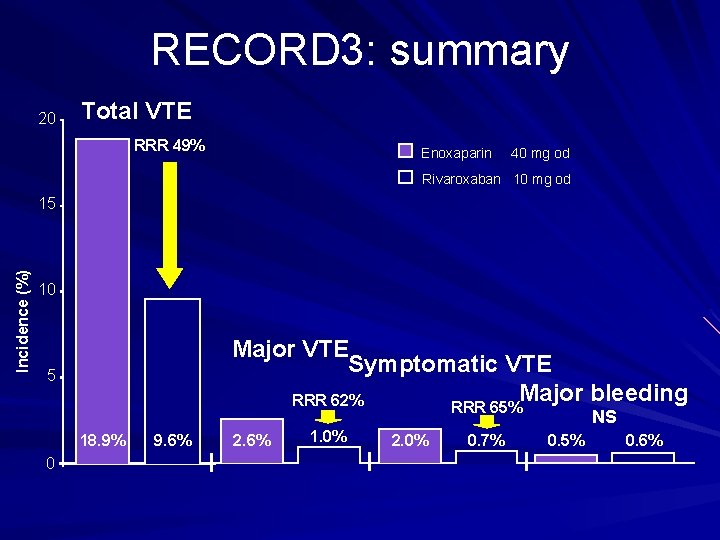
RECORD 3: summary 20 Total VTE RRR 49% Enoxaparin 40 mg od Rivaroxaban 10 mg od Incidence (%) 15 10 Major VTE Symptomatic VTE Major bleeding RRR 62% RRR 65% 5 NS 18. 9% 0 9. 6% 2. 6% 1. 0% 2. 0% 0. 7% 0. 5% 0. 6%

Study background § ACCP guidelines: grade 1 A recommendation for up to 35 days’ prophylaxis after elective hip replacement surgery Geerts et al. , 2004

Oral rivaroxaban compared with subcutaneous enoxaparin for extended thromboprophylaxis after total hip arthroplasty

RECORD 1 study design Double blind Rivaroxaban 10 mg od R S U 6– 8 hours post surgery R G E 6– 8 hours post surgery R Enoxaparin 40 mg od Y Evening before surgery Day 1 Mandatory bilateral venography Day 36± 4 F O L L O W U P Up to Day 65 Last dose, day before venography Inclusion criteria Major exclusion criteria Patients aged ≥ 18 years, scheduled to undergo elective THR Active bleeding or high risk of bleeding Significant liver disease Anticoagulant therapy that could not be stopped Use of HIV protease inhibitors

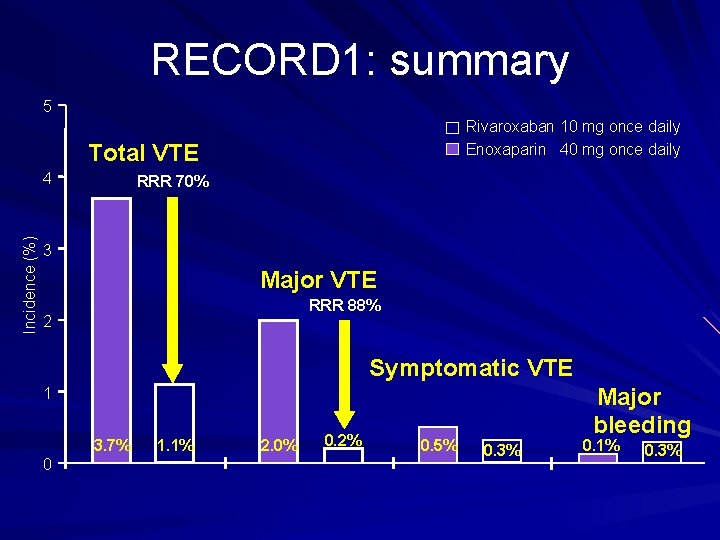
RECORD 1: summary 5 Rivaroxaban 10 mg once daily Enoxaparin 40 mg once daily Total VTE Incidence (%) 4 RRR 70% 3 Major VTE RRR 88% 2 Symptomatic VTE Major bleeding 1 3. 7% 0 1. 1% 2. 0% 0. 2% 0. 5% 0. 3% 0. 1% 0. 3%

Extended thromboprophylaxis with rivaroxaban compared with short term thromboprophylaxis with low molecular weight heparin after total hip arthroplasty

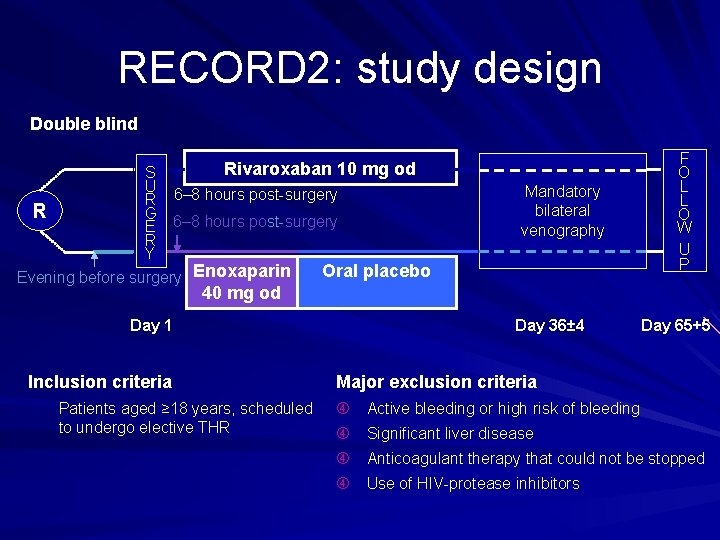
RECORD 2: study design Double blind R Rivaroxaban 10 mg od S U R 6– 8 hours post surgery G E 6– 8 hours post surgery R Y Evening before surgery Enoxaparin Mandatory bilateral venography Oral placebo F O L L O W U P 40 mg od Day 1 Day 36± 4 Inclusion criteria Major exclusion criteria Patients aged ≥ 18 years, scheduled to undergo elective THR Active bleeding or high risk of bleeding Day 65+5 Significant liver disease Anticoagulant therapy that could not be stopped Use of HIV protease inhibitors

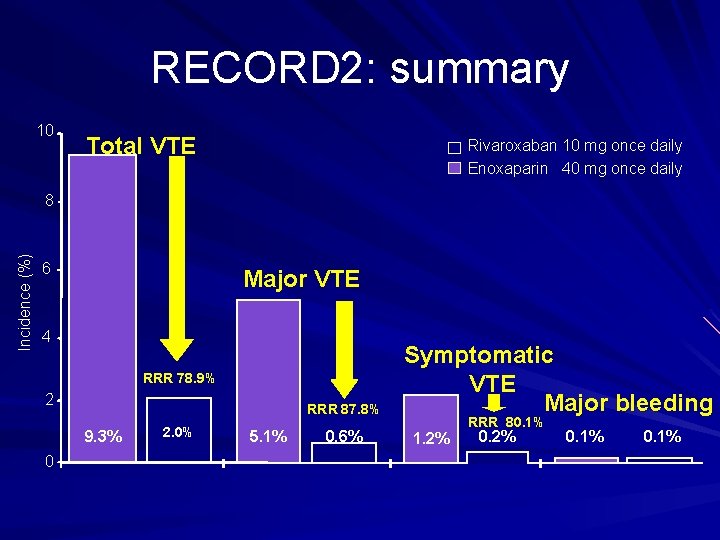
RECORD 2: summary 10 Total VTE Rivaroxaban 10 mg once daily Enoxaparin 40 mg once daily Incidence (%) 8 6 Major VTE 4 RRR 78. 9% 2 RRR 87. 8% 9. 3% 0 2. 0% 5. 1% 0. 6% Symptomatic VTE Major bleeding 1. 2% RRR 80. 1% 0. 2% 0. 1%

Clinical programme overview: 50, 000 patients to be enrolled Phase II VTE prevention after major orthopaedic surgery § ODIXa HIP 1 § ODIXa HIP 2 § ODIXa KNEE § ODIXa OD HIP Phase III § RECORD 1 § RECORD 2 § RECORD 3 § RECORD 4 VTE prevention in hospitalized medically ill patients VTE treatment § ODIXa DVT § EINSTEIN DVT Stroke prevention in atrial fibrillation § EINSTEIN DVT § EINSTEIN PE § EINSTEIN EXT Japanese Phase III study Secondary prevention of acute coronary syndromes ~8, 000 >42, 000
 Look left right
Look left right Anticoagulant
Anticoagulant Jean marie connors
Jean marie connors An anticoagulant is a substance that prevents
An anticoagulant is a substance that prevents Anticoagulant
Anticoagulant Thrombolytic vs anticoagulant
Thrombolytic vs anticoagulant Anticoagulant drugs
Anticoagulant drugs Anticoagulant examples
Anticoagulant examples Warfarin strengths and colors
Warfarin strengths and colors Heparin anticoagulant
Heparin anticoagulant Future perfect vs future continuous
Future perfect vs future continuous Future perfect simple and future continuous exercises
Future perfect simple and future continuous exercises Oral placement therapy
Oral placement therapy Psychoanalytic vs humanistic
Psychoanalytic vs humanistic Humanistic therapy aims to
Humanistic therapy aims to Bioness integrated therapy system price
Bioness integrated therapy system price Look at each picture
Look at each picture Look at activity 1 and answer
Look at activity 1 and answer Picture analysis activity 1
Picture analysis activity 1 Activity 1 look out
Activity 1 look out 45ü
45ü Summary tenses
Summary tenses Nulti i prvi kondicional
Nulti i prvi kondicional Future nurse programme
Future nurse programme Future plans and finished future actions
Future plans and finished future actions Future perfect interrogative
Future perfect interrogative Present progressive repetition with always
Present progressive repetition with always Future continuous vs future perfect
Future continuous vs future perfect Future continuous tense vs future simple
Future continuous tense vs future simple Present continuous for future activities
Present continuous for future activities See future continuous
See future continuous Thể thơ truyền thống
Thể thơ truyền thống Phép trừ bù
Phép trừ bù Hát lên người ơi
Hát lên người ơi Sự nuôi và dạy con của hươu
Sự nuôi và dạy con của hươu Diễn thế sinh thái là
Diễn thế sinh thái là






















































