OPUS Help with math Recall column summary Organization

















- Slides: 38

OPUS! Help with math. Recall column summary Organization of Physics Undergraduate Students. Tutoring Schedule: Posted on the door of the tutoring lab. Where the tunnel from Armes meets the tunnel going to University Centre. Face the bank machine, look left Allen Or follow me after class Building. Go into Allen and go right to the tutoring office. Or go down left hand corridor to find their office and directions to the tutoring office.

Phys 1830: Lecture 7 summary Recall column Discussion Group: time so far: Mon 3: 30 pm during office/tutorial hour. CAN BE CHANGED Tutorial/Office hour 3 pm practice math and prepare for tests 2 tutorials away! • Change of password • iclicker report on wall. • check the number on the clicker. • attach it to your name. • if your student number isn’t on the roster then email me with your name, iclicker number, and student number AND COURSE.

Special Club! University of Manitoba Astronomy Club Contact: umastroclub@gmail. com Wonder about the origins of the Universe? Curious about black holes? Come out and help design the club activities! No need to be a geek First Meeting Monday Sept 23 at 7 pm in Allen 330

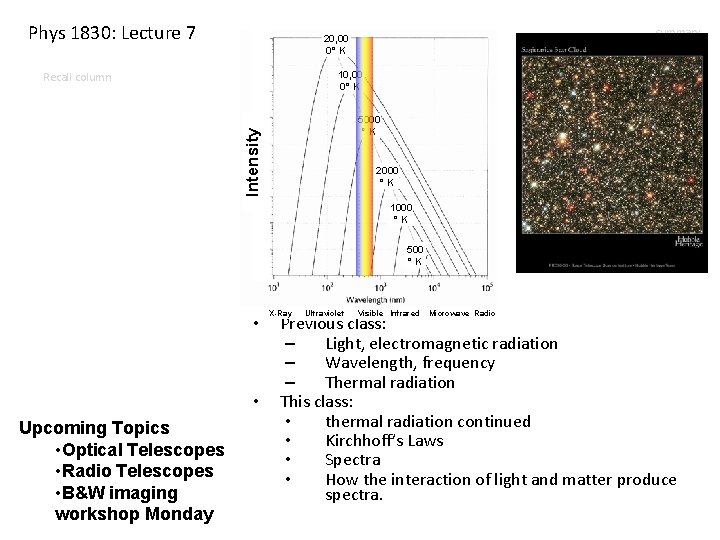
Phys 1830: Lecture 7 summary 20, 00 0° K 10, 00 0° K Recall column Intensity 5000 °K 2000 °K 1000 °K 500 °K • • Upcoming Topics • Optical Telescopes • Radio Telescopes • B&W imaging workshop Monday X-Ray Ultraviolet Visible Infrared Microwave Radio Previous class: – Light, electromagnetic radiation – Wavelength, frequency – Thermal radiation This class: • thermal radiation continued • Kirchhoff’s Laws • Spectra • How the interaction of light and matter produce spectra.

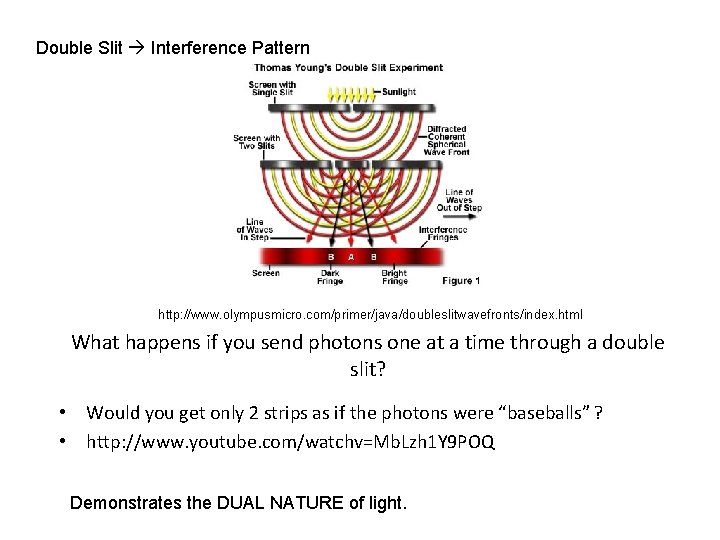
Double Slit Interference Pattern http: //www. olympusmicro. com/primer/java/doubleslitwavefronts/index. html What happens if you send photons one at a time through a double slit? • Would you get only 2 strips as if the photons were “baseballs” ? • http: //www. youtube. com/watchv=Mb. Lzh 1 Y 9 POQ Demonstrates the DUAL NATURE of light.

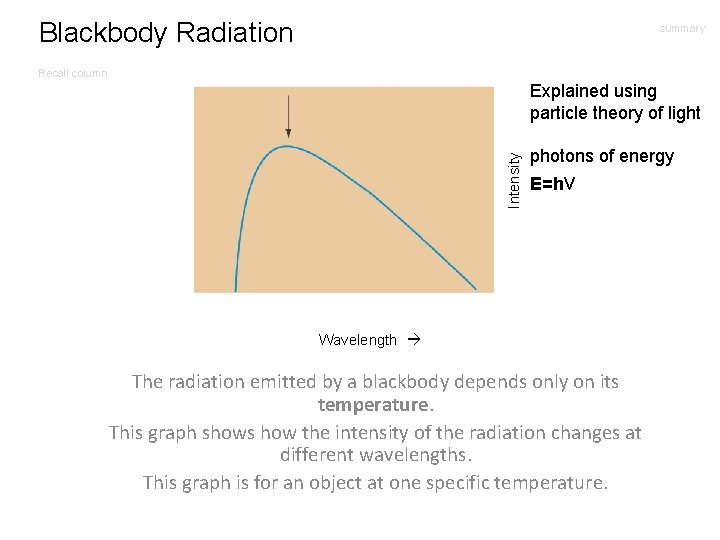
Blackbody Radiation summary Recall column Intensity Explained using particle theory of light photons of energy E=hν Wavelength The radiation emitted by a blackbody depends only on its temperature. This graph shows how the intensity of the radiation changes at different wavelengths. This graph is for an object at one specific temperature.

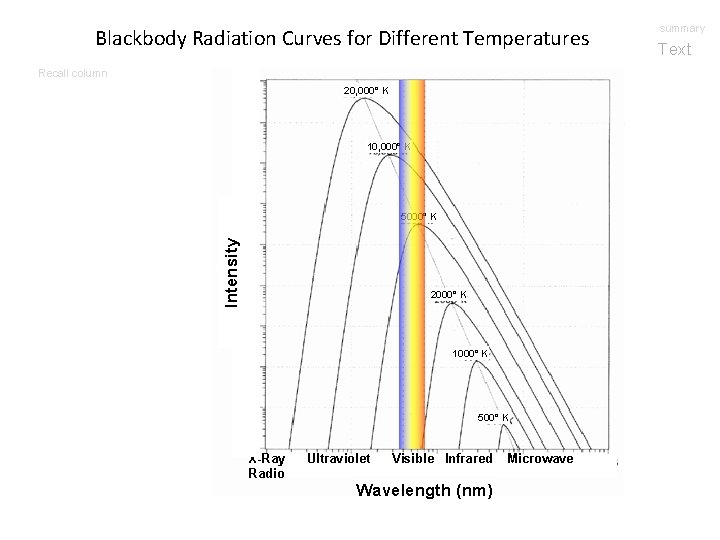
Blackbody Radiation Curves for Different Temperatures Recall column 20, 000° K 10, 000° K Intensity 5000° K 2000° K 1000° K 500° K X-Ray Radio Ultraviolet Visible Infrared Wavelength (nm) Microwave summary Text

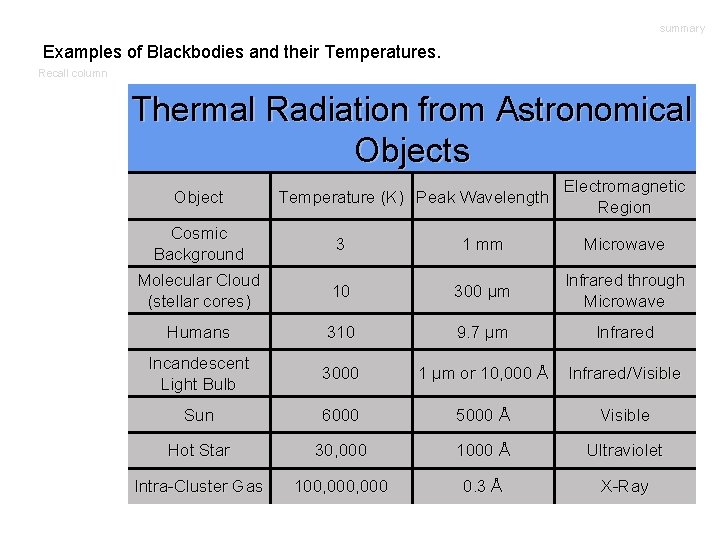
summary Examples of Blackbodies and their Temperatures. Recall column Thermal Radiation from Astronomical Objects Object Temperature (K) Peak Wavelength Electromagnetic Region Cosmic Background 3 1 mm Microwave Molecular Cloud (stellar cores) 10 300 μm Infrared through Microwave Humans 310 9. 7 μm Infrared Incandescent Light Bulb 3000 1 μm or 10, 000 Å Infrared/Visible Sun 6000 5000 Å Visible Hot Star 30, 000 1000 Å Ultraviolet Intra-Cluster Gas 100, 000 0. 3 Å X-Ray

Objects and Peak of Emission Dense, spherical clouds: radio and Far-IR. • Exercise for at home: Match these objects to peak temperatures in the previous table. Globule of dust: IR. Sun: visible White dwarf star/planetary nebula: UV

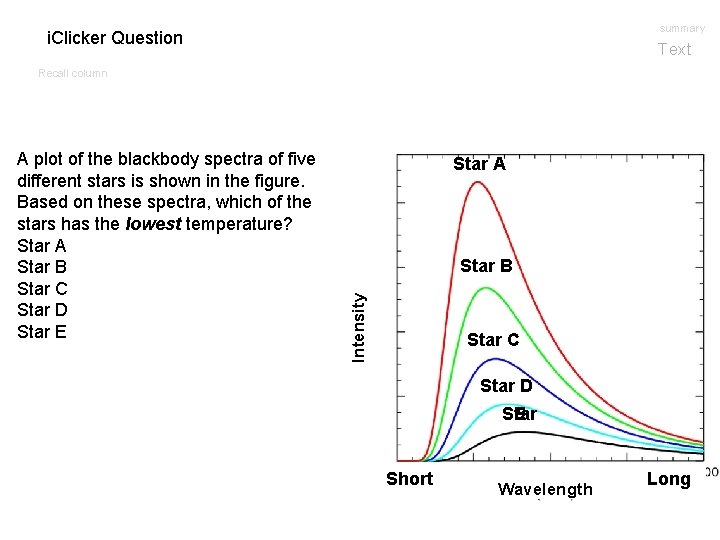
summary i. Clicker Question Text Recall column Star A Star B Intensity A plot of the blackbody spectra of five different stars is shown in the figure. Based on these spectra, which of the stars has the lowest temperature? Star A Star B Star C Star D Star E Short Wavelength Long

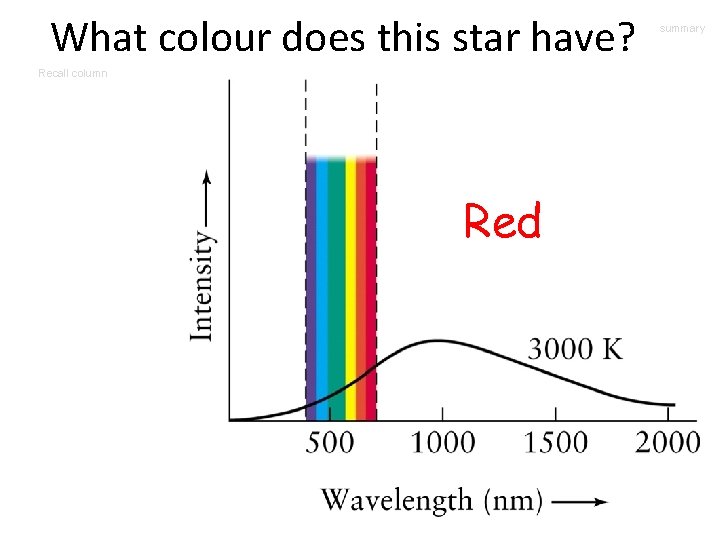
What colour does this star have? Recall column Red summary

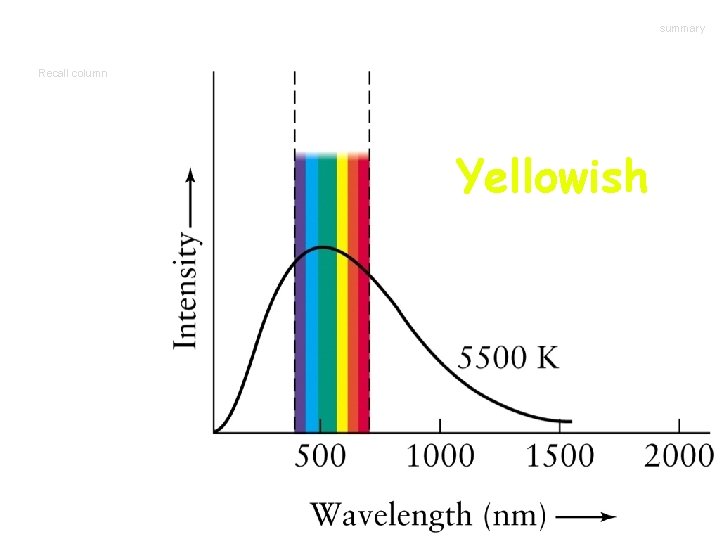
summary Recall column Yellowish

summary Recall column Blue

what is hot & what is not? Recall column The hottest stars in this image appear: a)Blueish b)Reddish summary

Contrast with everyday experience! Recall column summary

The Interaction of light and matter. summary Recall column • Photons and matter interact creating spectra. • spectra can be used to assess • temperature (blackbody curve type spectrum) • processes that produce light or absorb it (i. e. what is going on) (Animation)

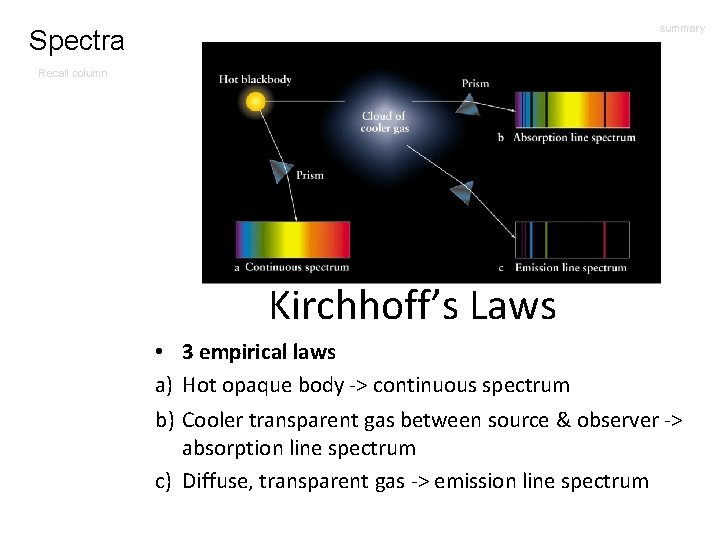
summary Spectra Recall column Kirchhoff’s Laws • 3 empirical laws a) Hot opaque body -> continuous spectrum b) Cooler transparent gas between source & observer -> absorption line spectrum c) Diffuse, transparent gas -> emission line spectrum

Spectra summary Recall column • This kind of spectrum (continuum) is caused by a) Hot, low density gas b) Hot, dense blackbody c) Cooler transparent gas

Spectra summary Recall column • Our sun and other stars have an atmosphere. Imagine that you are in a spaceship far above the Earth’s atmosphere. Which of the following spectra would you observe when analyzing sunlight? a) Continuum rainbow-like spectrum b) Dark line absorption spectrum c) Bright line emission spectrum

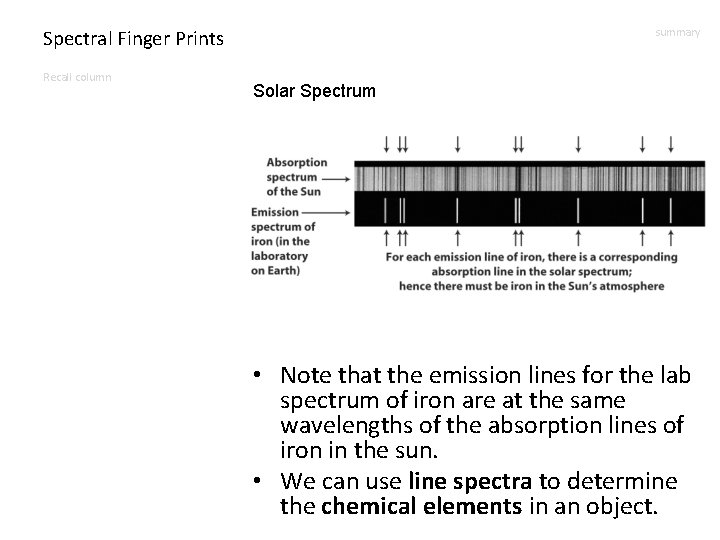
Spectral Finger Prints Recall column summary Solar Spectrum • Note that the emission lines for the lab spectrum of iron are at the same wavelengths of the absorption lines of iron in the sun. • We can use line spectra to determine the chemical elements in an object.

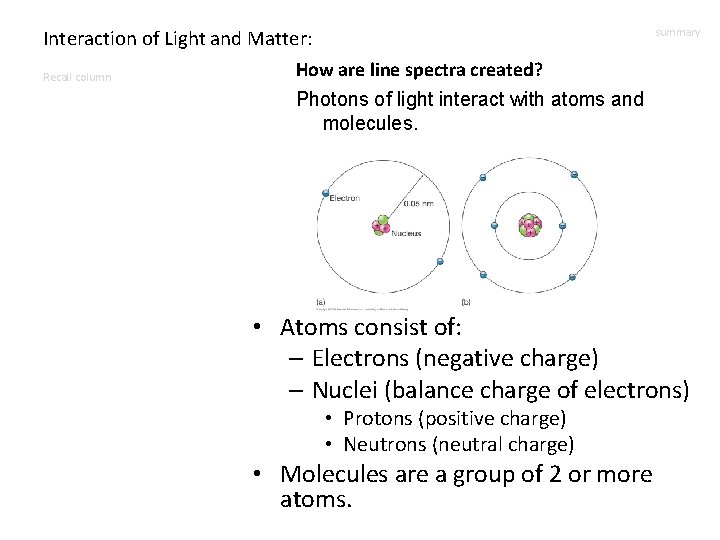
Interaction of Light and Matter: How are line spectra created? Recall column Photons of light interact with atoms and molecules. summary • Atoms consist of: – Electrons (negative charge) – Nuclei (balance charge of electrons) • Protons (positive charge) • Neutrons (neutral charge) • Molecules are a group of 2 or more atoms.

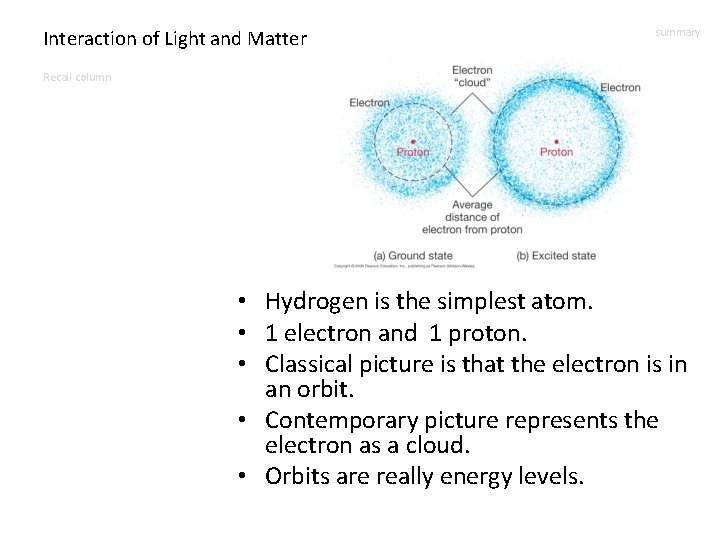
Interaction of Light and Matter summary Recall column • Hydrogen is the simplest atom. • 1 electron and 1 proton. • Classical picture is that the electron is in an orbit. • Contemporary picture represents the electron as a cloud. • Orbits are really energy levels.

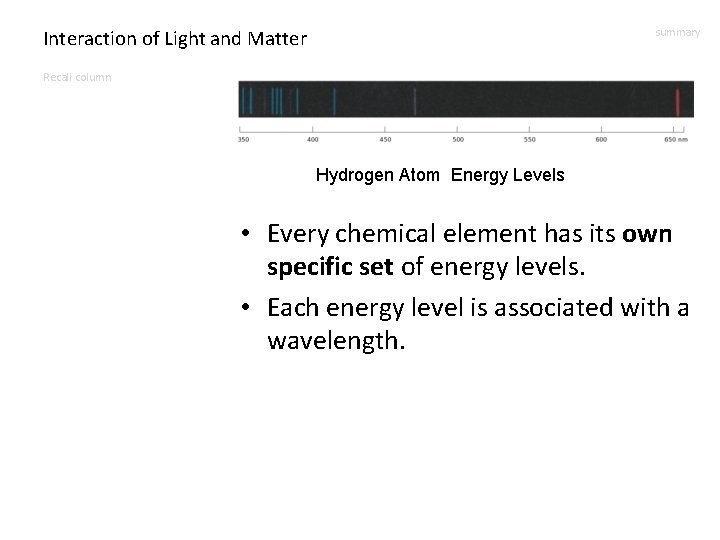
Interaction of Light and Matter summary Recall column Hydrogen Atom Energy Levels • Every chemical element has its own specific set of energy levels. • Each energy level is associated with a wavelength.

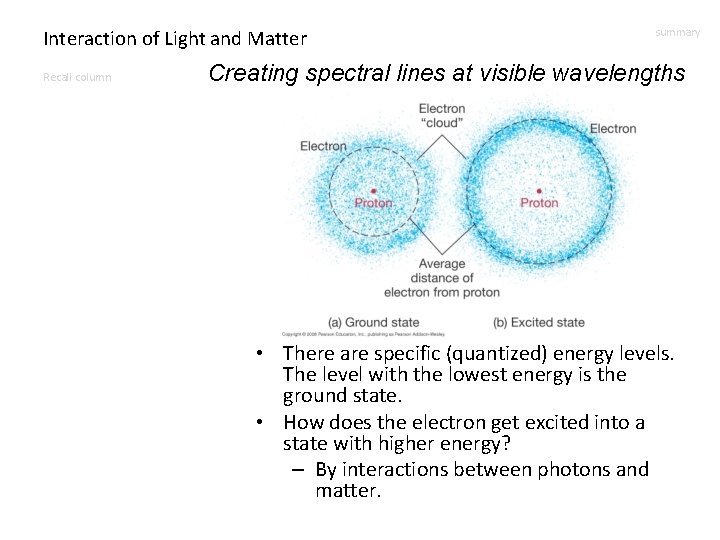
Interaction of Light and Matter Recall column summary Creating spectral lines at visible wavelengths • There are specific (quantized) energy levels. The level with the lowest energy is the ground state. • How does the electron get excited into a state with higher energy? – By interactions between photons and matter.

Interaction of Light and Matter: Recall column summary Creating spectral lines at visible wavelengths • The electron can shift between energy levels by absorption and emission of photons.

Interaction of Light and Matter Recall column summary Creating spectral lines at visible wavelengths Absorption: 1. If the photon’s energy is not matched to any energy level then the photon passes by the atom. The atom is unchanged.

Interaction of Light and Matter Recall column summary Creating spectral lines at visible wavelengths Absorption: 2. If the photon’s energy matches the energy needed to cause an electron to jump to a larger energy level, then the atom absorbs the photon (i. e. absorbs energy) and the electron jumps to that energy level. The atom is now in an excited state.

Interaction of Light and Matter Recall column summary Creating spectral lines at visible wavelengths Absorption: 3. If the photon’s energy is larger than any jump within the atom, then the atom absorbs energy, the photon disappears, and an electron (or more) are kicked out of the atom creating an ion. (In an ion the charge is not balanced. ) We say that the atom is ionized.

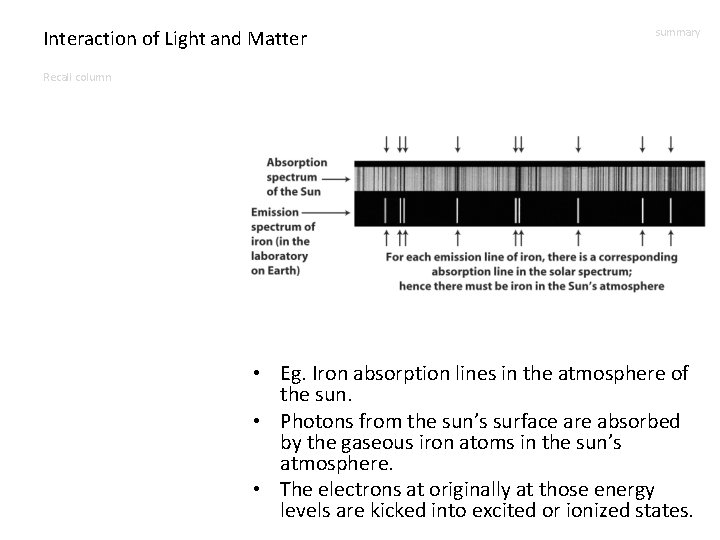
Interaction of Light and Matter summary Recall column • Eg. Iron absorption lines in the atmosphere of the sun. • Photons from the sun’s surface are absorbed by the gaseous iron atoms in the sun’s atmosphere. • The electrons at originally at those energy levels are kicked into excited or ionized states.

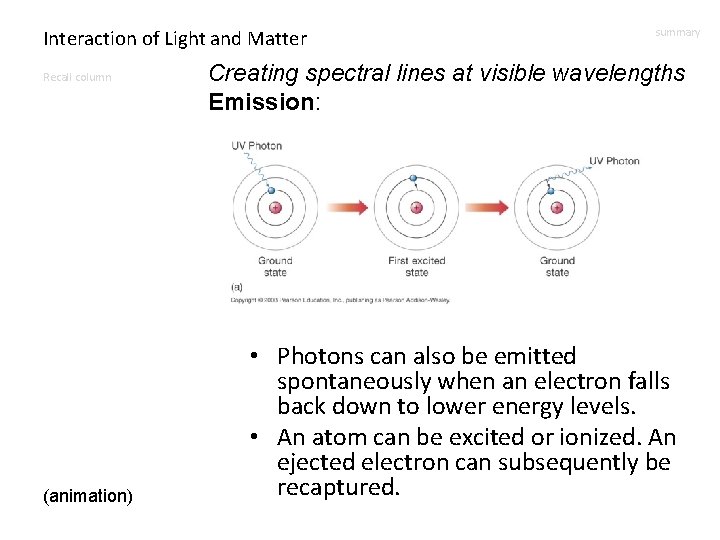
Interaction of Light and Matter Recall column (animation) summary Creating spectral lines at visible wavelengths Emission: • Photons can also be emitted spontaneously when an electron falls back down to lower energy levels. • An atom can be excited or ionized. An ejected electron can subsequently be recaptured.

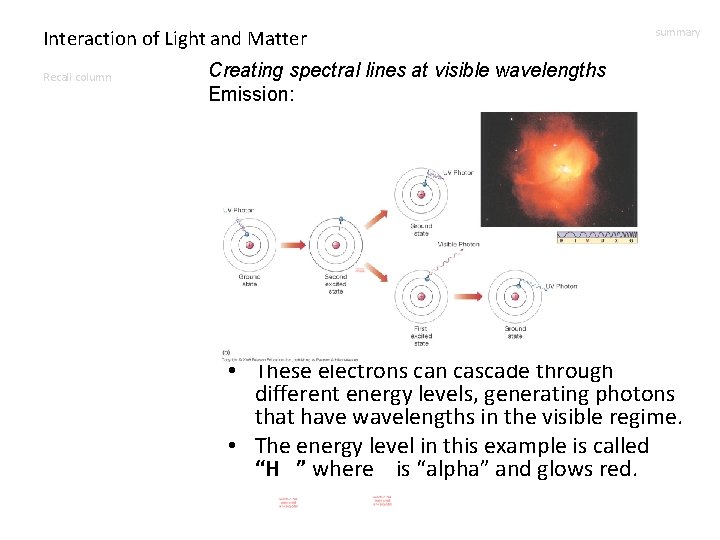
Interaction of Light and Matter Recall column summary Creating spectral lines at visible wavelengths Emission: • These electrons can cascade through different energy levels, generating photons that have wavelengths in the visible regime. • The energy level in this example is called “H ” where is “alpha” and glows red.

Interaction of Light and Matter Creating spectral lines at visible wavelengths Recall column Emission: summary The Orion Nebula David Malin • Clouds of gas that glow due to this process have a few names: – Emission nebulae – H II regions – H regions • If they are very bright, they are pinker. • The ionizing photons come from hot stars.

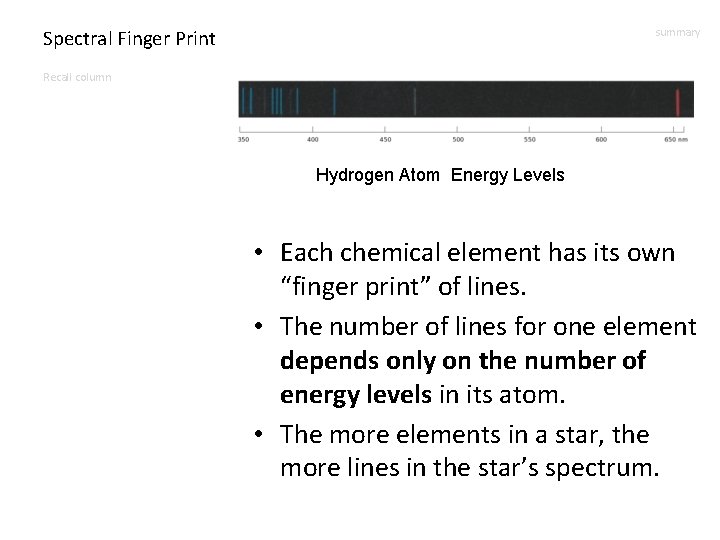
Spectral Finger Print summary Recall column Hydrogen Atom Energy Levels • Each chemical element has its own “finger print” of lines. • The number of lines for one element depends only on the number of energy levels in its atom. • The more elements in a star, the more lines in the star’s spectrum.

Spectral Finger Print summary Recall column • The strength of the absorption lines gives the number of atoms of that element in the gas. • Comparison of strengths of absorption lines of different elements in the gas gives – Density – Temperature • Can get these characteristics for the outer layers of a star from its absorption line spectrum.

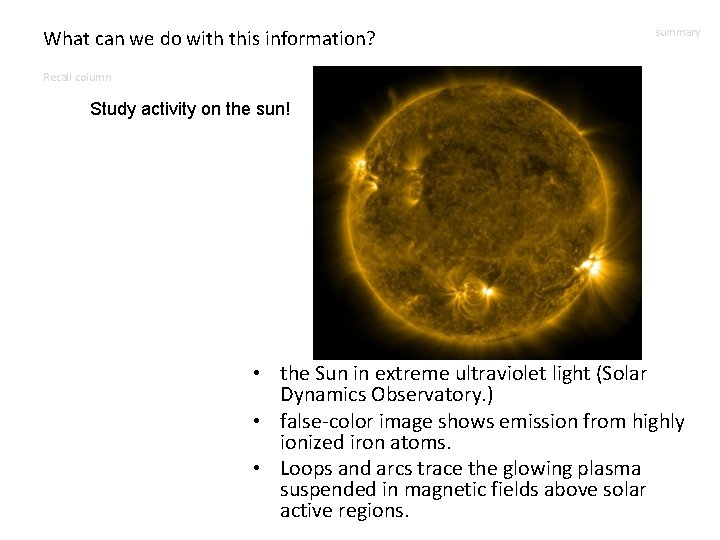
What can we do with this information? summary Recall column Study activity on the sun! • the Sun in extreme ultraviolet light (Solar Dynamics Observatory. ) • false-color image shows emission from highly ionized iron atoms. • Loops and arcs trace the glowing plasma suspended in magnetic fields above solar active regions.

What can we do with this information? Recall column • (note asteroid trail in upper right corner) • Consider stars. . . summary

Spectral Finger Print summary Recall column • What can we do with this spectral information? • If 2 stars have the same elements, same density, and same temperature then they have the same intrinsic luminosity. • If they have the same intrinsic luminosity we can use their apparent brightnesses to derive their relative distances using the Inverse Square Brightness Law!

Spectral Finger Print summary Recall column • The uniqueness of the spectral line pattern of any element is caused by a) The density of the gas in the stellar atmosphere. b) The temperature of the gas in the stellar atmosphere. c) The energy level structure of the atom.