Optical Methods of Analysis Chem 321 Prof Dr


- Slides: 28

Optical Methods of Analysis Chem 321 Prof. Dr. Faten A. Nour El-Dien 1/31/2022 Prof. Faten A. Nour El-Dien

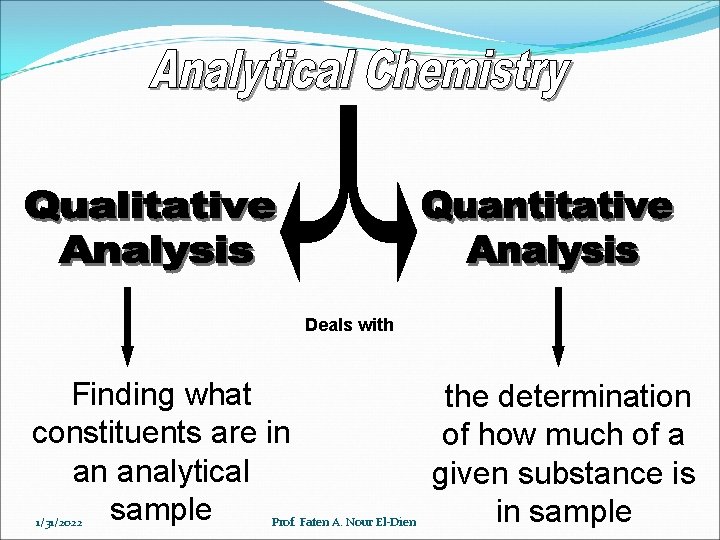
Deals with Finding what constituents are in an analytical sample 1/31/2022 Prof. Faten A. Nour El-Dien the determination of how much of a given substance is in sample

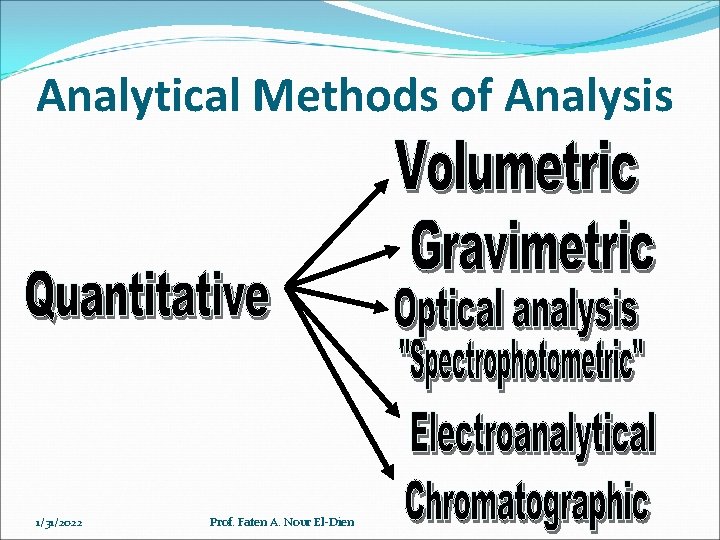
Analytical Methods of Analysis 1/31/2022 Prof. Faten A. Nour El-Dien

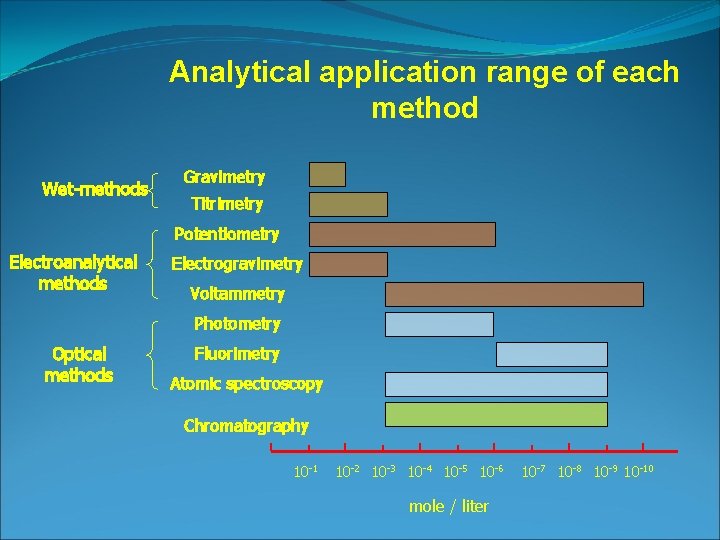
Analytical application range of each method Wet-methods Gravimetry Titrimetry Potentiometry Electroanalytical methods Electrogravimetry Voltammetry Photometry Optical methods Fluorimetry Atomic spectroscopy Chromatography 10 -1 10 -2 10 -3 10 -4 10 -5 10 -6 mole / liter 10 -7 10 -8 10 -9 10 -10

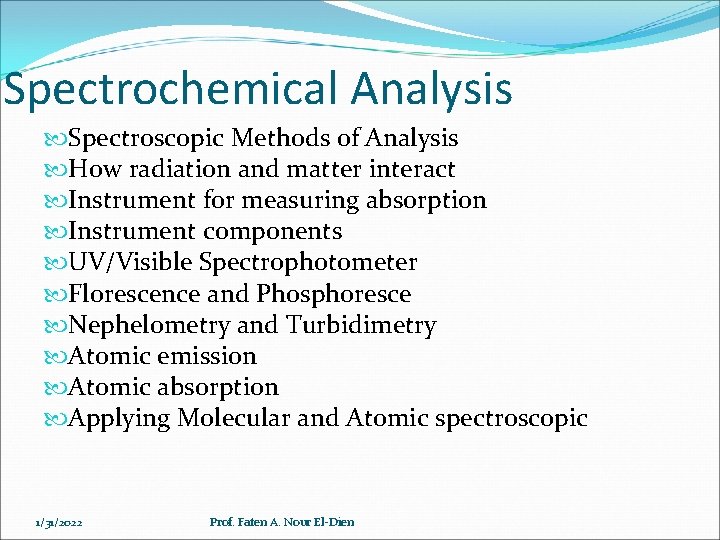
Spectrochemical Analysis Spectroscopic Methods of Analysis How radiation and matter interact Instrument for measuring absorption Instrument components UV/Visible Spectrophotometer Florescence and Phosphoresce Nephelometry and Turbidimetry Atomic emission Atomic absorption Applying Molecular and Atomic spectroscopic 1/31/2022 Prof. Faten A. Nour El-Dien

Spectroscopic Methods of Analysis 1/31/2022 Prof. Faten A. Nour El-Dien

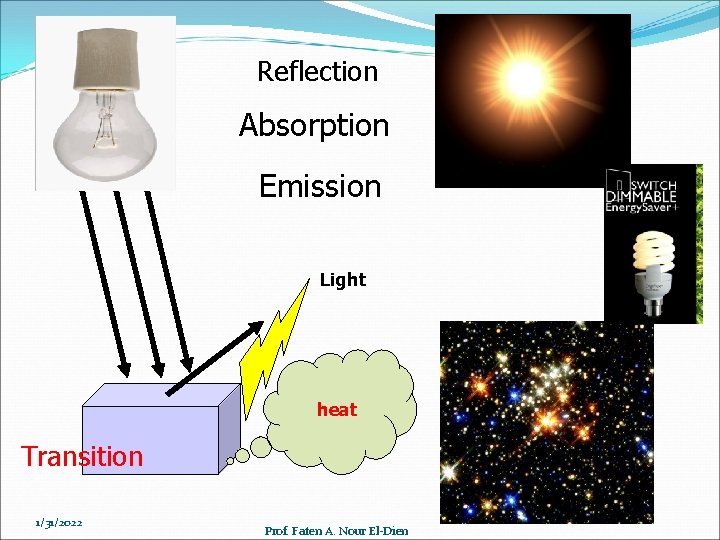
Reflection Absorption Emission Light heat Transition 1/31/2022 Prof. Faten A. Nour El-Dien

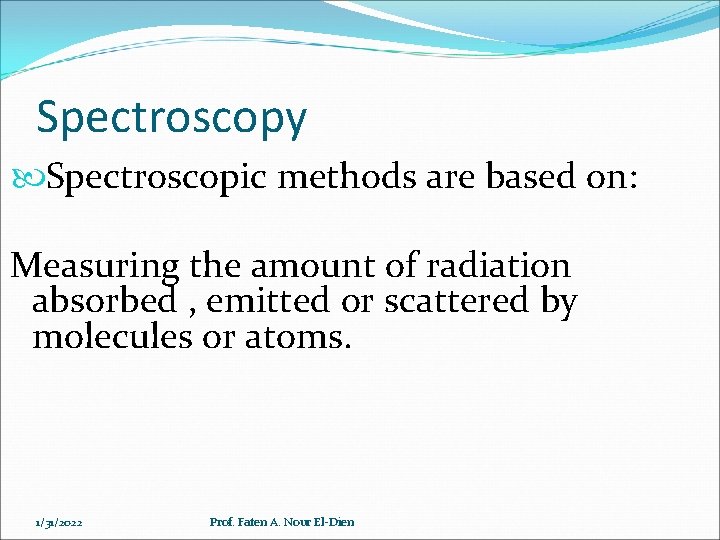
Spectroscopy Spectroscopic methods are based on: Measuring the amount of radiation absorbed , emitted or scattered by molecules or atoms. 1/31/2022 Prof. Faten A. Nour El-Dien

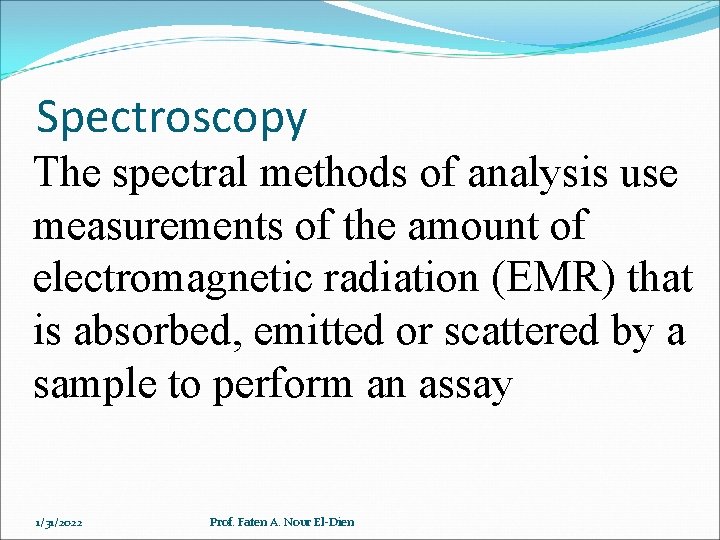
Spectroscopy The spectral methods of analysis use measurements of the amount of electromagnetic radiation (EMR) that is absorbed, emitted or scattered by a sample to perform an assay 1/31/2022 Prof. Faten A. Nour El-Dien

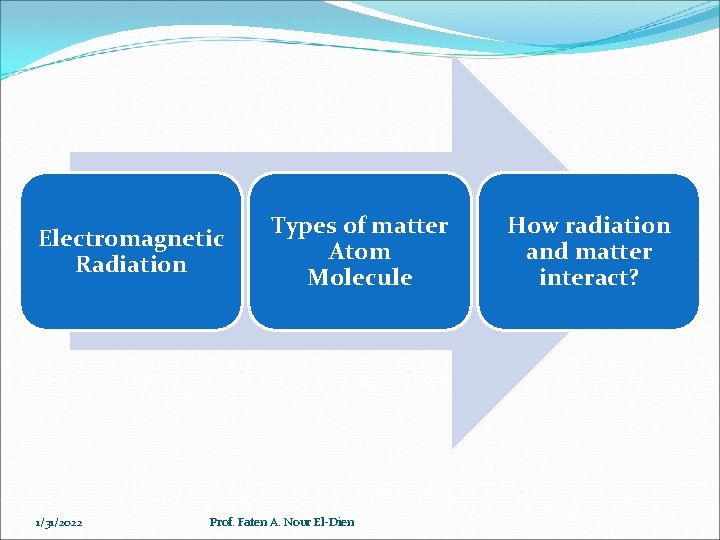
Electromagnetic Radiation 1/31/2022 Types of matter Atom Molecule Prof. Faten A. Nour El-Dien How radiation and matter interact?

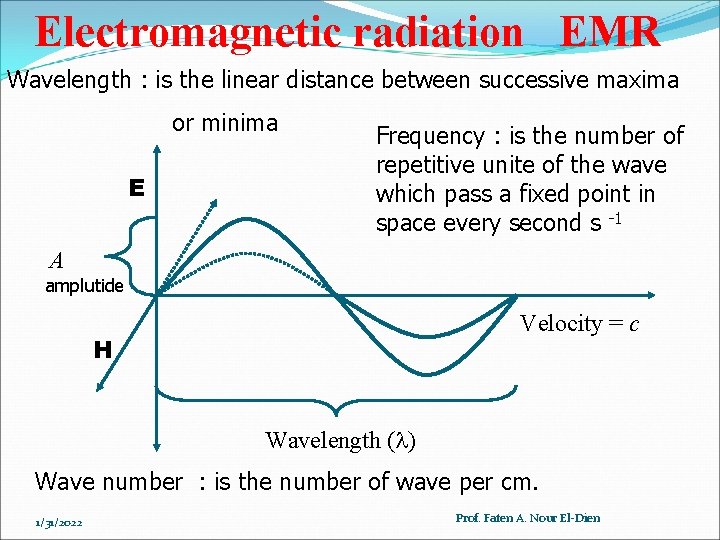
Electromagnetic radiation EMR Wavelength : is the linear distance between successive maxima or minima E Frequency : is the number of repetitive unite of the wave which pass a fixed point in space every second s -1 A amplutide Velocity = c H Wavelength ( ) Wave number : is the number of wave per cm. 1/31/2022 Prof. Faten A. Nour El-Dien

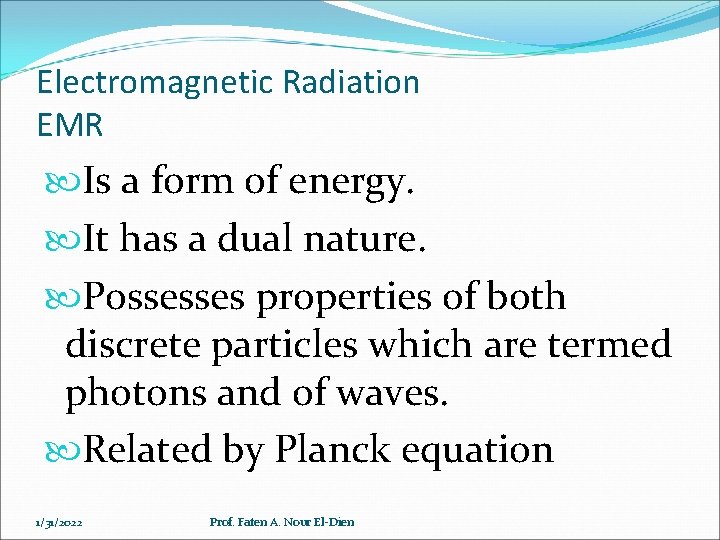
Electromagnetic Radiation EMR Is a form of energy. It has a dual nature. Possesses properties of both discrete particles which are termed photons and of waves. Related by Planck equation 1/31/2022 Prof. Faten A. Nour El-Dien

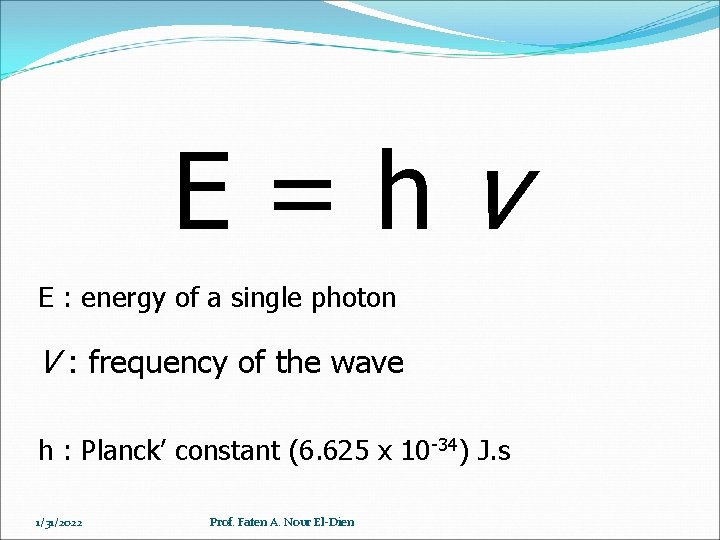
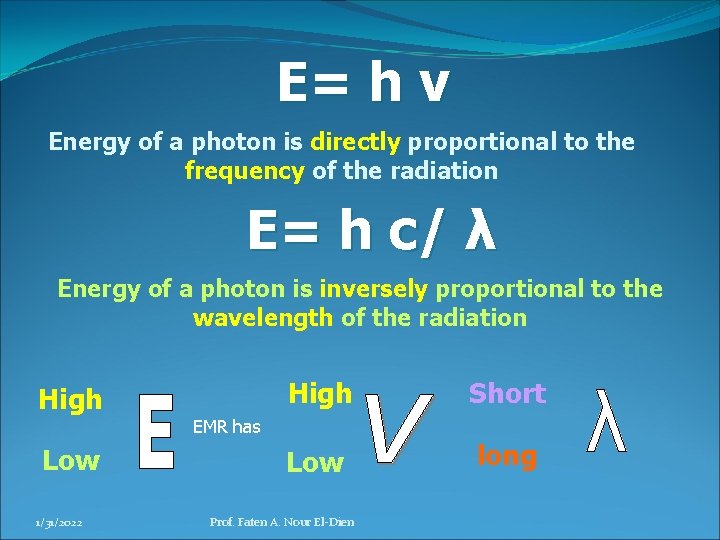
E=hv E : energy of a single photon V : frequency of the wave h : Planck’ constant (6. 625 x 10 -34) J. s 1/31/2022 Prof. Faten A. Nour El-Dien

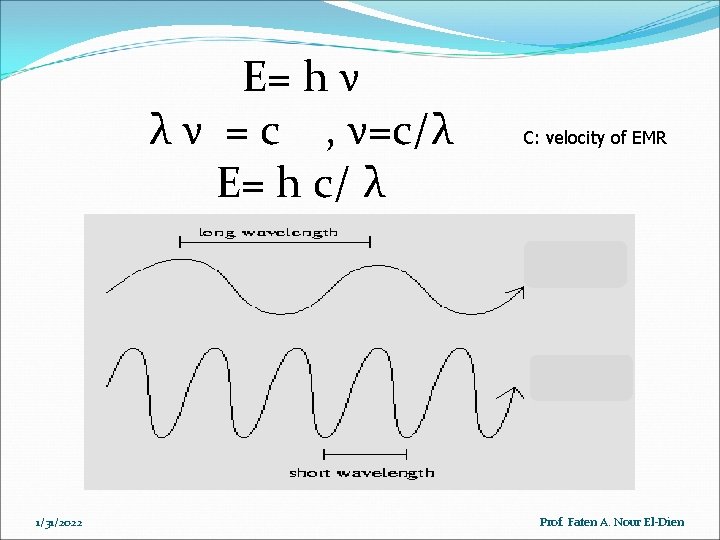
E= h ν λ ν = c , ν=c/λ E= h c/ λ 1/31/2022 C: velocity of EMR Prof. Faten A. Nour El-Dien

E= h ν Energy of a photon is directly proportional to the frequency of the radiation E= h c/ λ Energy of a photon is inversely proportional to the wavelength of the radiation High Short Low long EMR has Low 1/31/2022 Prof. Faten A. Nour El-Dien

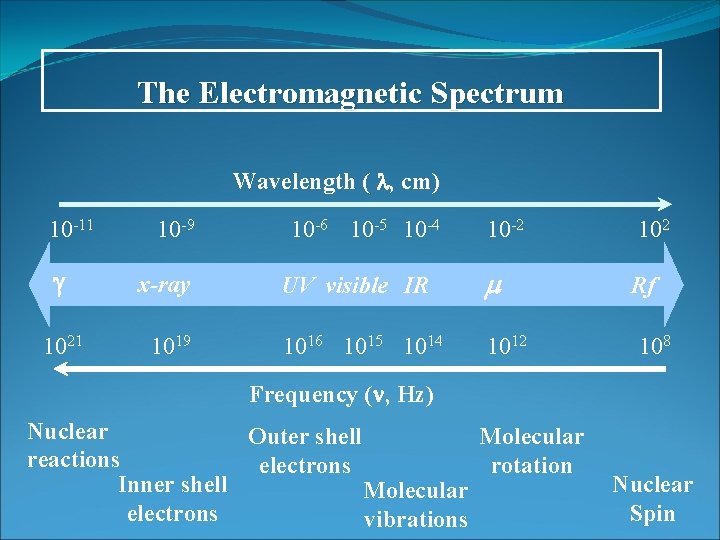
The Electromagnetic Spectrum Wavelength ( , cm) 10 -11 10 -9 x-ray 1021 1019 10 -6 10 -5 10 -4 10 -2 102 UV visible IR Rf 1016 1015 1014 1012 108 Frequency ( , Hz) Nuclear Outer shell Molecular reactions electrons rotation Inner shell Molecular electrons vibrations Nuclear Spin

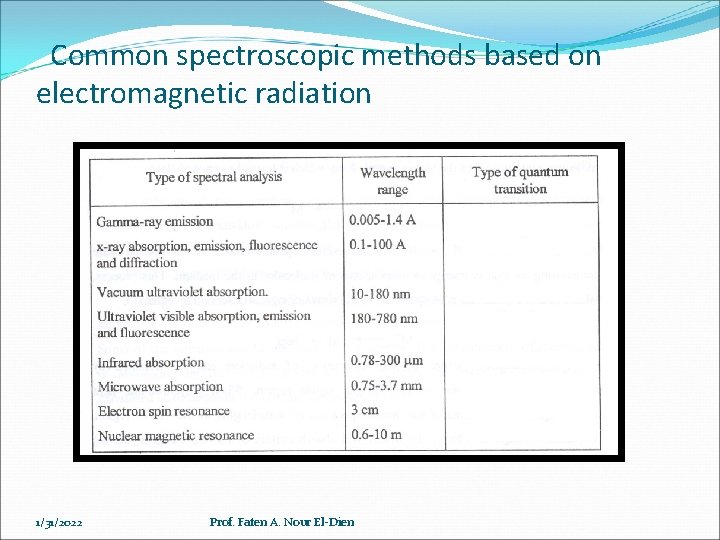
Common spectroscopic methods based on electromagnetic radiation 1/31/2022 Prof. Faten A. Nour El-Dien

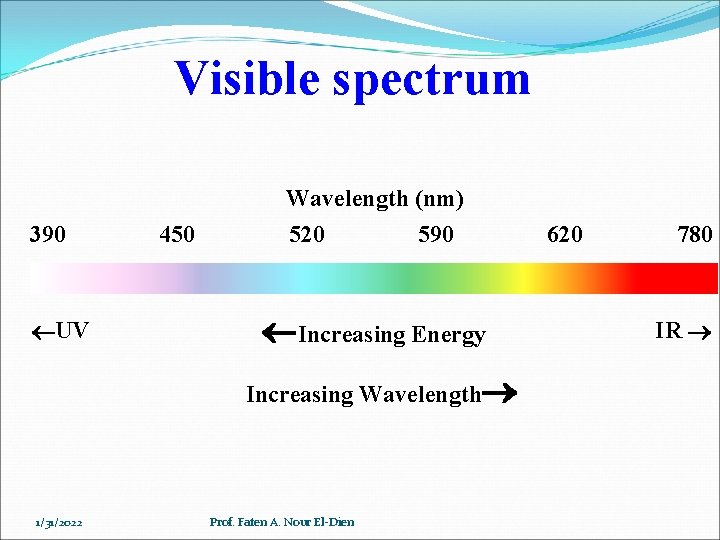
Visible spectrum 390 UV 450 Wavelength (nm) 520 590 Increasing Energy Increasing Wavelength 1/31/2022 Prof. Faten A. Nour El-Dien 620 780 IR

1/31/2022 Prof. Faten A. Nour El. Dien

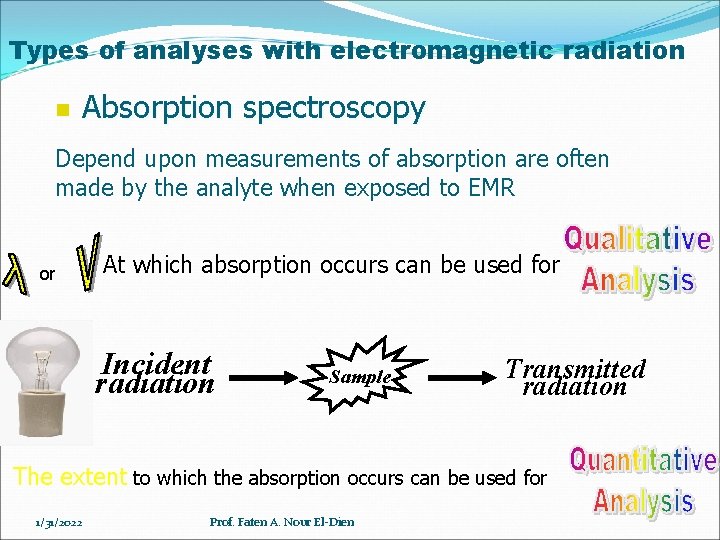
Types of analyses with electromagnetic radiation n Absorption spectroscopy Depend upon measurements of absorption are often made by the analyte when exposed to EMR or At which absorption occurs can be used for Incident radiation Sample Transmitted radiation The extent to which the absorption occurs can be used for 1/31/2022 Prof. Faten A. Nour El-Dien

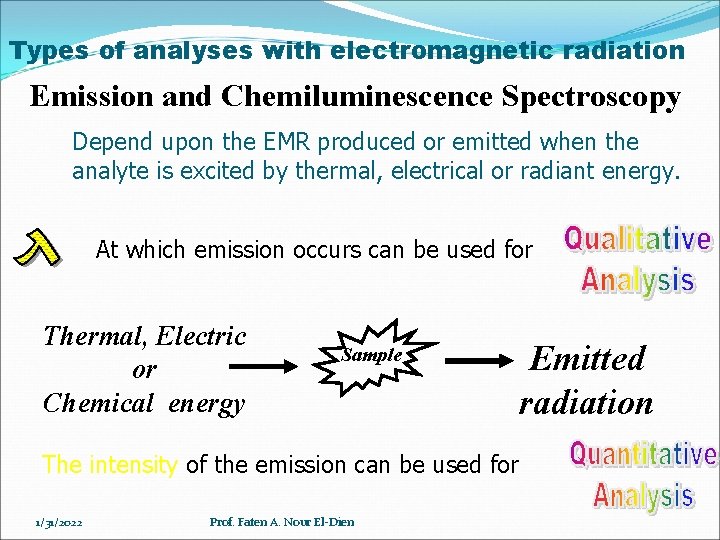
Types of analyses with electromagnetic radiation Emission and Chemiluminescence Spectroscopy Depend upon the EMR produced or emitted when the analyte is excited by thermal, electrical or radiant energy. At which emission occurs can be used for Thermal, Electric or Chemical energy Sample Emitted radiation The intensity of the emission can be used for 1/31/2022 Prof. Faten A. Nour El-Dien

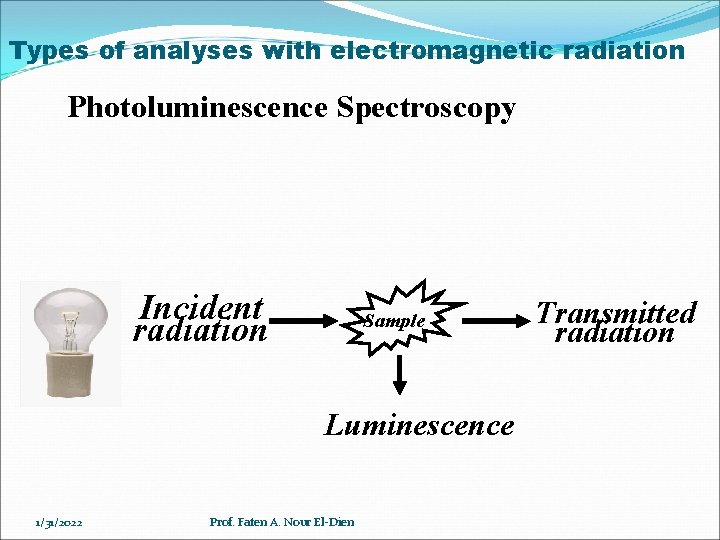
Types of analyses with electromagnetic radiation Photoluminescence Spectroscopy Incident radiation Sample Luminescence 1/31/2022 Prof. Faten A. Nour El-Dien Transmitted radiation

1/31/2022 Prof. Faten A. Nour El. Dien

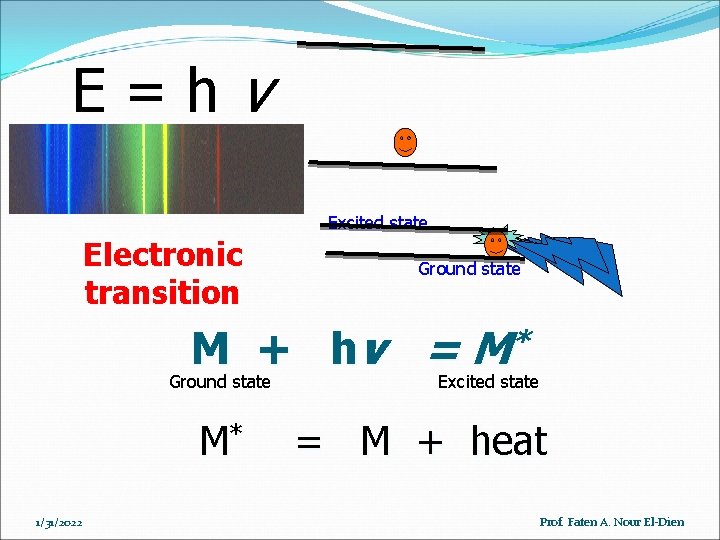
E=hv Excited state Electronic transition Ground state M + hv = M* Ground state M* 1/31/2022 Excited state = M + heat Prof. Faten A. Nour El-Dien

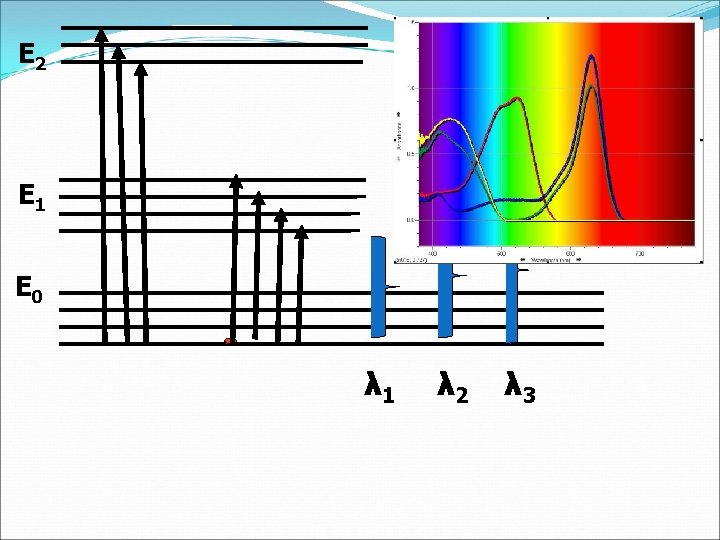
E 2 E 1 E 0 λ 1 λ 2 λ 3

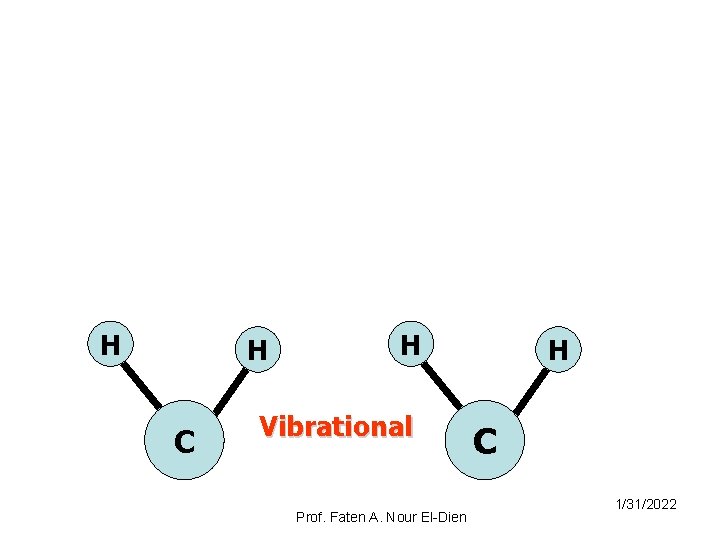
Rotational H H C H Vibrational Prof. Faten A. Nour El-Dien H C 1/31/2022

Rotational transition: where the molecule absorb energy to rotate a round various axes. Vibrational transition: where atoms or group of atoms vibrate relative to each other. Electronic transition: where electrons of a molecule, ion or atom may be raised to higher energy levels. 1/31/2022 Prof. Faten A. Nour El-Dien

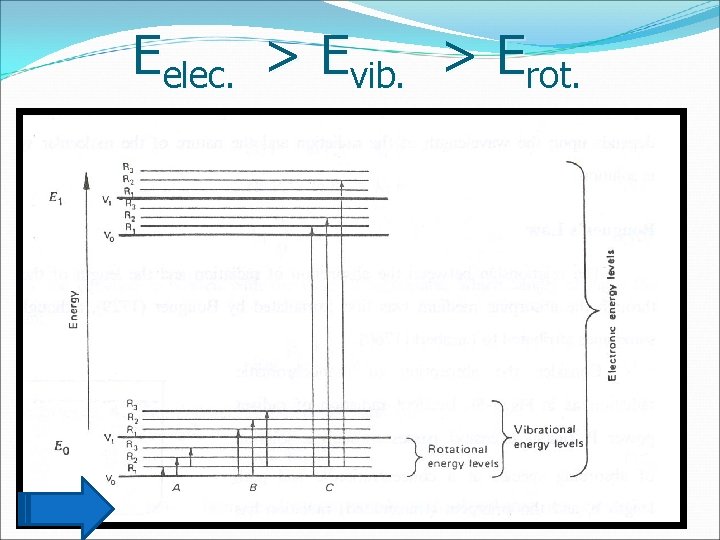
Eelec. > Evib. > Erot. 1/31/2022 Prof. Faten A. Nour El-Dien