OPERATION EXCHANGE TOOLS INDUSTRIAL CHEMISTRY CLASS XII SEMESTER























- Slides: 35

OPERATION EXCHANGE TOOLS INDUSTRIAL CHEMISTRY CLASS : XII SEMESTER : 6

The Principle of Ion Exchange v. The most common water softening method called "ion exchange, " is a reversible chemical process of exchanging hard water ions for soft water ions. v. Calcium and magnesium are the hardness ions, sodium can be considered the "softness" ions and they are exchanged to create soft water.

The Principle of Ion Exchange v. Ion exchange takes place in a "resin bed" made up of tiny bead-like material often made of styrene and divynil benzene. v. The beads, having a negative charge, attract and hold positively charged ions such as sodium, but will exchange them whenever the beads encounter another positively charged ion, such as calcium or magnesium minerals.

v. This ion exchange happens very easily since the sodium ions have a positive charge of only one, while magnesium and calcium have a more powerful positive charge of two Teknologi dan Rekayasa

Ion Exchange Reactions v. Ion exchange is a reversible chemical reaction wherein an ion (an atom or molecule that has lost or gained an electron and thus acquired an electrical charge) from solution is exchanged for a similarly charged ion attached to an immobile solid particle.

v. These solid ion exchange particles are either naturally occurring inorganic zeolites or synthetically produced organic resins. v. The synthetic organic resins are the predominant type used today because their characteristics can be tailored to specific applications. Teknologi dan Rekayasa

An organic ion exchange resin v. An organic ion exchange resin is composed of high-molecular-weight polyelectrolytes that can exchange their mobile ions for ions of similar charge from the surrounding medium. v. Each resin has a distinct number of mobile ion sites that set the maximum quantity of exchanges per unit of resin.

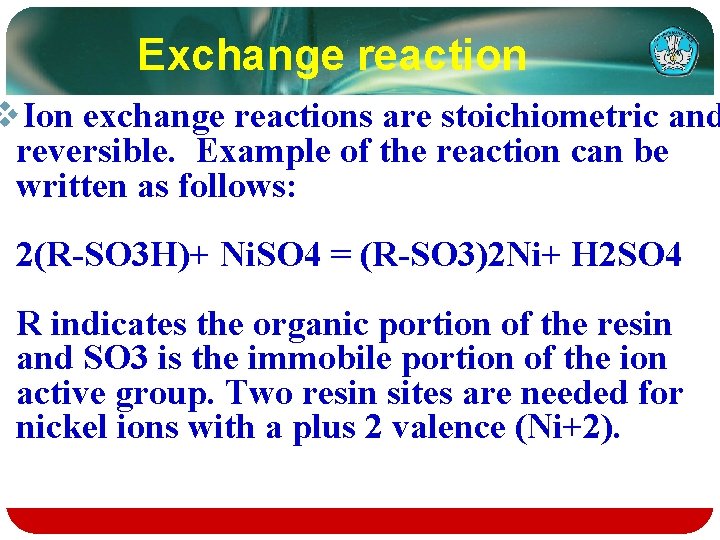
Exchange reaction v. Ion exchange reactions are stoichiometric and reversible. Example of the reaction can be written as follows: 2(R-SO 3 H)+ Ni. SO 4 = (R-SO 3)2 Ni+ H 2 SO 4 R indicates the organic portion of the resin and SO 3 is the immobile portion of the ion active group. Two resin sites are needed for nickel ions with a plus 2 valence (Ni+2).

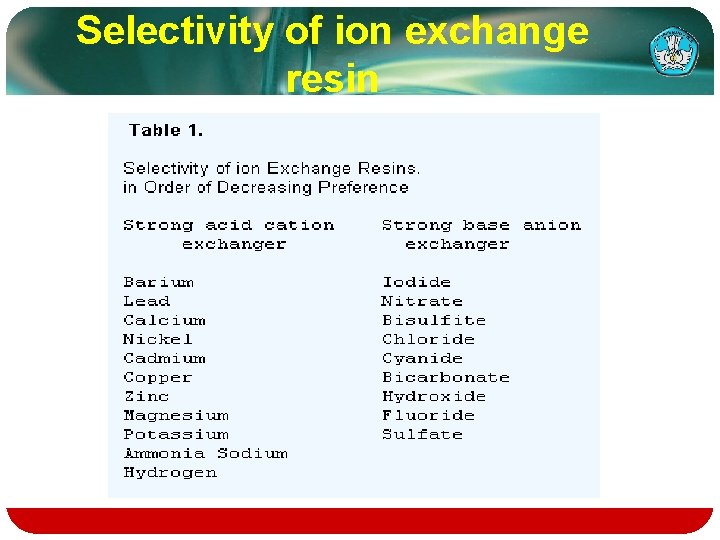
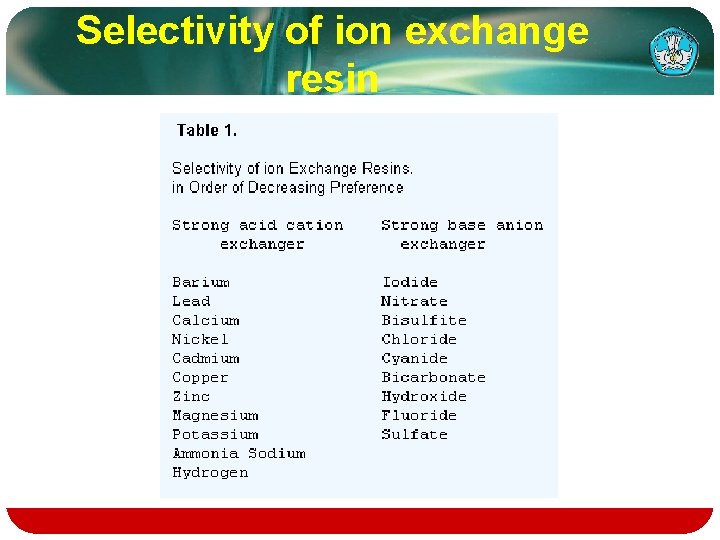
v. The ion exchange reaction is reversible. The selectivity of a resin for a given ion is measured by the selectivity coefficient. K. which in its simplest form for the reaction R-A++B+=R--B++A+ is expressed as: K = (concentration of B+ in resin/concentration of A+ in resin) X (concentration of A+ in solution/concentration of B+ in solution). Teknologi dan Rekayasa

Selectivity of ion exchange resin

Typical examples of ions that can bind to ion exchangers: v. H+ (proton) and OH− (hydroxide) v. Single charged mono atomic ions like Na+, K+, or Cl− v. Double charged mono atomic ions like Ca 2+ or Mg 2+ v. Polyatomic inorganic ions like SO 42− or PO 43−

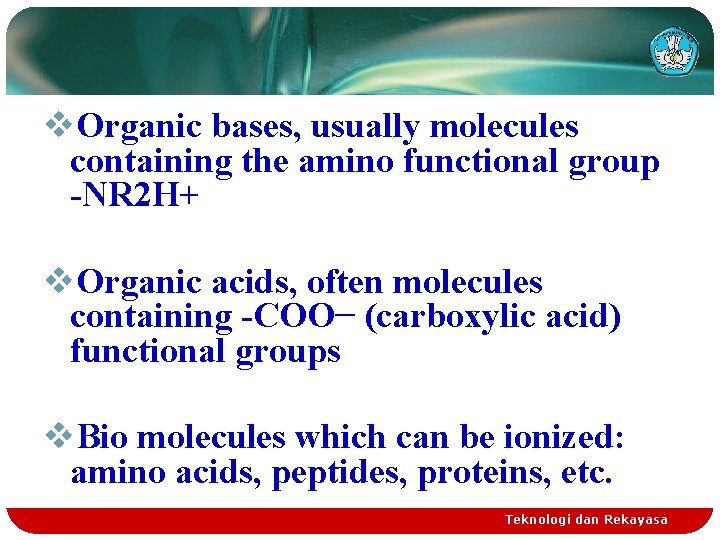
v. Organic bases, usually molecules containing the amino functional group -NR 2 H+ v. Organic acids, often molecules containing -COO− (carboxylic acid) functional groups v. Bio molecules which can be ionized: amino acids, peptides, proteins, etc. Teknologi dan Rekayasa

Selectivity of ion exchange resin

Ion-exchange resin v. An ion-exchange resin or ionexchange polymer is an insoluble matrix (or support structure) normally in the form of small (1 -2 mm diameter) beads, usually white or yellowish, fabricated from an organic polymer substrate.

v. The material has highly developed structure of pores on the surface of which are sites with easily trapped and released ions. v. The trapping of ions takes place only with simultaneous releasing of other ions; thus the process is called ionexchange. Teknologi dan Rekayasa

Resin Types There are four main types differing in their functional groups: vstrongly acidic (typically, sulfonic acid groups, eg. sodium polystyrene sulfonate or poly. AMPS) vstrongly basic, (quaternary amino groups, for example, trimethylammonium groups, eg. poly. APTAC) vweakly acidic (mostly, carboxylic acid groups) vweakly basic (primary, secondary, and/or ternary amino groups, eg. polyethylene amine)

There also specialised types: vchelating resins (iminodiacetic acid, thiourea, and many others) Teknologi dan Rekayasa

Ion exchange resin beads

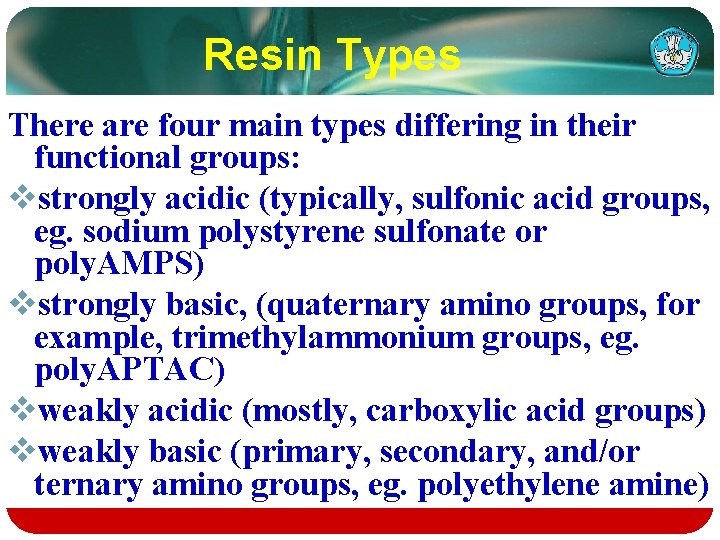
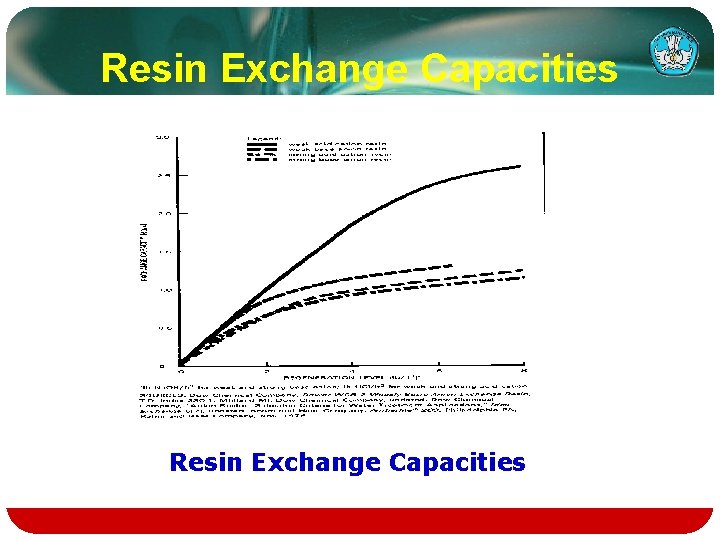
Exchange capacity Exchange Capacity of Weak Acid Cation and Weak Base Anion Resins as a Function of solution p. H

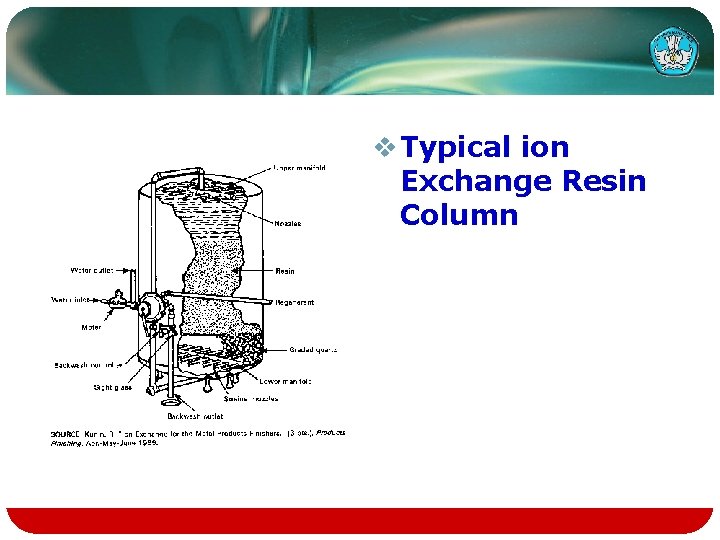
Batch and Column Exchange Systems v. Ion exchange processing can be accomplished by either a batch method or a column method. v. In the first method, the resin and solution are mixed in a batch tank, the exchange is allowed to come to equilibrium, then the resin is separated from solution.

v. Passing a solution through a column containing a bed of exchange resin is analogous to treating the solution in an infinite series of batch tanks. Teknologi dan Rekayasa

Column Exchange Systems

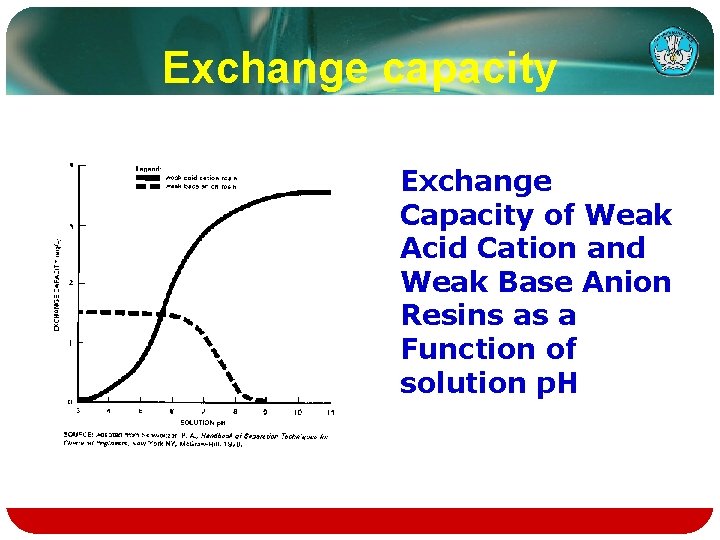
Ion Exchange Process Equipment and Operation v. Most industrial applications of ion exchange use fixed-bed column systems, the basic component of which is the resin column

v Typical ion Exchange Resin Column

Exchange Batch Tanks v Concentration Profile in a Series of ion Exchange Batch Tanks

Resin Exchange Capacities

Water Softening Plants v. Softeners are based on ion exchange process. v. Steel pressure vessels containing cation exchange resin in sodium form.

v. These resin eliminate dissolved ions like calcium and magnesium from water to give high quality soft water. v. Necessary salt preparation tank with injection systems is also offered with the system. Teknologi dan Rekayasa

Water softener

Water softening units

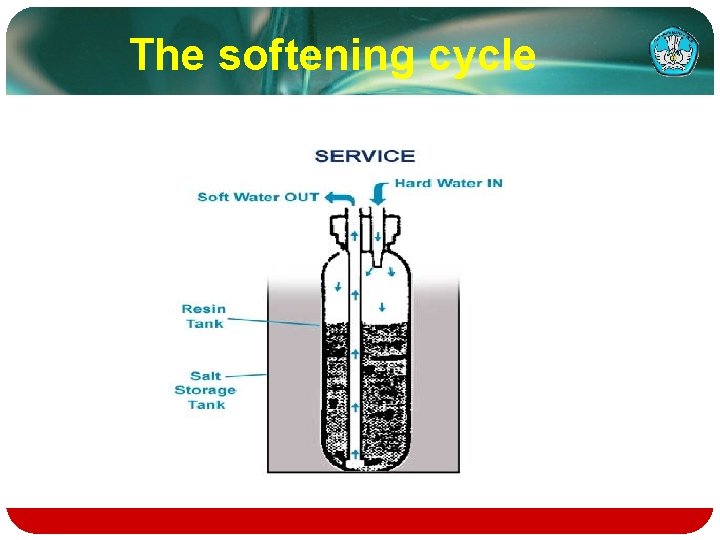
The softening cycle

Regeneration consists of 5 cycles: 1. Brine Fill 2. Brining 3. Brine Rinse 4. Backwash 5. Fast Rinse

v. Salt, dissolved in water, is called brine and is needed to clean the minerals from the resin beads. v. To make brine, water flows into the salt storage area during the fill stage as shown. v. The softener continues to produce soft water during this initial stage

Usage v. Processes of purification v. Separation v. Decontamination of aqueous and other ion-containing solutions

Application v. Water softening v. Water purification v. Catalysis v. Pharmaceuticals v. Chemical & petrochemical, v. Sugar & sweeteners, v. Protein purification. v. Food & beverage, v. Hydrometallurgical, v. Metals finishing, v. Nuclear, v. Semiconductor, v. Power.