Oops That Wasnt Supposed to Happen NoncomplianceProtocol Deviations




























- Slides: 34

Oops, That Wasn’t Supposed to Happen (Noncompliance/Protocol Deviations) JERI R. BARNEY, JD, MS HRPP COMPLIANCE MANAGER YALE UNIVERSITY HUMAN RESEARCH PROTECTION PROGRAM WWW. YALE. EDU/HRPP MAY 2013

Today’s Discussion HRPP Compliance What is Noncompliance? Minor Noncompliance Serious Noncompliance Continuing Noncompliance Protocol deviations Reporting: How to report to the IRBs and when Corrective and preventive action (CAPA) plans IRB Actions Lapses in IRB Approval

HRPP Compliance Unit Mission: To facilitate compliance with the federal regulations & University policies, and protection of research participants IRB What do they say they will do? Compliance Are they doing what they said they would do?

Regulatory Oversight § Office for Human Research Protections (OHRP) § 45 CFR Part 46 § U. S. Food & Drug Administration (FDA) § 21 CFR Parts 50, 56, 312 and 812 § Office for Civil Rights (OCR) § 45 CFR Parts 160, 162 and 164

Noncompliance OHRP Guidance FDA Guidance http: //www. fda. gov/downloads/Drugs/Guidanc e. Compliance. Regulatory. Information/Guidances/ UCM 187772. pdf Association for the Accreditation of Human Research Protection Programs (AAHRPP) Standards

Definition of Noncompliance Defined as “any action or activity associated with the conduct or oversight of research involving human participants that fails to comply with either the research plan as approved by a designated IRB, or federal regulations or institutional policies governing human subject research. ” HRPP Policy 700 (http: //www. yale. edu/hrpp/policies/index. html)

What is Noncompliance? Noncompliance may range from minor to serious, be unintentional or willful, and may occur once or several times. Noncompliance includes failure to have protocols reviewed by the IRB as required, protocol deviations from IRB-approved protocols, including deviations made in the interest of a single participant such as changing a participant’s scheduled study visits.

What is Noncompliance? Noncompliance may result from the action of the investigator, research personnel, or a participant, and may or may not impact the rights and welfare of research participants or others or the integrity of the study. Complaints or reports of noncompliance from someone other than the Principal Investigator or study team personnel are handled as allegations of noncompliance until such time that the report is validated or found to be invalidated or dismissed.

Minor Noncompliance Defined as any behavior, action or omission in the conduct or oversight of research involving human participants that deviates from the approved research plan, federal regulations or institutional policies but, because of its nature, the research project, or subject population, does or did not: harm or pose an increased risk of substantive harm to a research participant; result in a detrimental change to a participant’s clinical or emotional condition or status; have a substantive effect on the value of the data collected; and result from willful or knowing misconduct on the part of the investigator(s) or study staff.

Examples of Minor Noncompliance Examples of minor noncompliance may include, but are not limited to, the following: Changing study personnel (excluding PI) without notifying the IRB; Shortening the duration between planned study visits; Implementing minor wording changes in study questionnaires without first obtaining IRB approval; Routine lab missed at scheduled visit and re-drawn.

Serious Noncompliance Defined as any behavior, action or omission in the conduct or oversight of human research that, in the judgment of a convened IRB, has been determined to: adversely affect the rights and welfare of participants; result in a detrimental change to a participant’s clinical or emotional condition or status; compromise the integrity or validity of the research; result from willful or knowing misconduct on the part of the investigator(s) or study staff; or harm or pose an increased risk of substantive harm to a research participant.

Examples of Serious Noncompliance Conducting research that requires direct interaction or interventions with human participants without first obtaining IRB approval; Enrolling participants who fail to meet the inclusion or exclusion criteria in a protocol that in the opinion of the IRB Chair, designee, or convened IRB, places the participant(s) at greater risk; Failing to submit a continuing review application to the IRB before study expiration for an ongoing study (resulting in a lapse of IRB approval) and continuing with engaged activities; Failing to obtain and/or document a participant’s informed consent provided the IRB has not granted a waiver of consent;

More Examples Failing to retain copies of informed consent forms (signed or unsigned); Performing a study procedure not approved by the IRB; or failing to perform a required study visit or procedure that, in either case, may affect subject safety or data integrity; Failing to follow the safety monitoring plan; Enrolling study subjects after the IRB-approval of a study has expired; or Failing to report serious adverse events and/or unanticipated problems to the IRB in accordance with IRB Policy 710, Reporting Adverse Events and Unanticipated Problems.

Continuing Noncompliance Defined as a pattern of noncompliance that indicates a lack of understanding or disregard for the regulations or institutional requirements that protect the rights and welfare of participants and others, suggests a likelihood that noncompliance will continue without intervention, or involves frequent instances of minor noncompliance. Continuing noncompliance may also include failure to respond to a request from the IRB to resolve an episode of noncompliance or a pattern of minor noncompliance.

Examples of Continuing Noncompliance Multiple lapses in IRB approval Continuing to conduct research after IRB orders a stop due to an issue of noncompliance 10% of consents missing on 5 protocols “PI doesn’t get it”

What is a Protocol Deviation? Defined as “any alteration/modification to an IRB- approved protocol made without prior IRB approval. ” A protocol deviation constitutes noncompliance.

Level of Noncompliance Whether a protocol deviation qualifies as minor or serious noncompliance depends heavily on the specific facts of the situation. The key depends upon whether, under the specific circumstances, it may adversely affect the rights and welfare of participants, harm or pose an increased risk of substantive harm to a research participant, have a substantive effect on the value of the data collected, or result from willful or knowing misconduct on the part of the investigator(s) or study staff. A pattern of protocol deviations may constitute continuing noncompliance, which may have serious ramifications for the PI.

Reporting Requirement Principal Investigators, co/sub-investigators, research personnel, or other individuals who believe that an instance of serious or continuing noncompliance has occurred must report it to the IRB within five (5) working days of becoming aware of the noncompliance. All instances of minor noncompliance should be summarized for the IRB at the time of continuing review.

Additional Reporting Requirements Principal Investigators are also required to report results of audits or inspections conducted by sponsors, other external entities such as the FDA or internal oversight committees. Investigators are not required to report instances of noncompliance that occur at other sites unless a Yale investigator serves as the lead PI or managing investigator acting as the lead coordinating center for a multi-center study.

Reporting Form for Noncompliance Form can be found at http: //www. yale. edu/hrpp/forms-templates/biomedical. html

What corrective actions are necessary? Issue may warrant consideration of substantive changes in the research protocol or informed consent process/document or other corrective actions in order to protect the safety, welfare or rights of the subjects or others. The PI must include a proposed corrective and preventive action (CAPA) plan with the report of noncompliance.

Corrective and Preventive Action Plan In crafting an effective plan, the investigator needs to really think about why the event occurred. Is it a system problem? Problem with procedure or something in the protocol? A training issue? Once the “why” is determined, the plan must address ways to prevent it from happening again Additional education/training is virtually always appropriate The IRB will make the final determination regarding the sufficiency of the CAPA

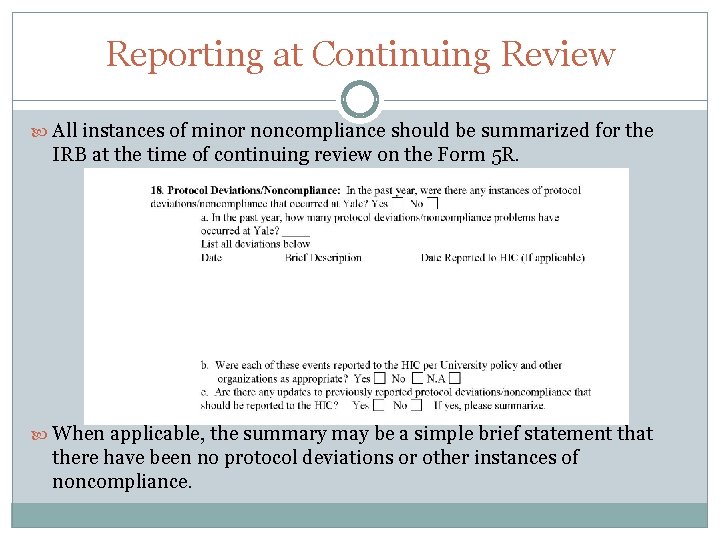
Reporting at Continuing Review All instances of minor noncompliance should be summarized for the IRB at the time of continuing review on the Form 5 R. When applicable, the summary may be a simple brief statement that there have been no protocol deviations or other instances of noncompliance.

HRPP Review & Inquiry The HRPP Compliance Manager, HRPP Director, an IRB Chair or Manager, or other qualified designee will initially assess a report/allegation of noncompliance and make a preliminary determination as to the seriousness or continuing nature of the noncompliance. Next, a fact-finding inquiry will be conducted which may include: examination of study records; and discussions with the research team, other personnel, research participants, witnesses, the complainant (if applicable and not anonymous), and others, as appropriate. If the inquiry suggests that the incident may constitute serious or continuing noncompliance, then the matter will be referred to a fully-convened IRB.

Actions By IRB The IRB has the authority to take whatever action it deems appropriate, up to and including suspending or terminating approval of research. Such actions may include, but are not limited to: Remediation or educational measures required of PI and research team. Monitoring of research activities by appropriate person(s). Notification of past or current research participants. Re-consenting of participants.

Additional Actions By IRB Additional actions may include, but are not limited to: More frequent continuing review (renewal of approval) schedule. Periodic audits by the HRPP Compliance Manager. Restrictions to the PI’s research practice (e. g. , limiting the privilege to minimal risk or supervised projects). PI may put the study on a voluntary partial or full hold. Suspension or termination of approval for one or more of the PI’s studies.

Consequences for Serious and/or Continuing Noncompliance Could result in Reporting to federal agencies (OHRP and FDA), including funding agencies (e. g. , NIH) Mandatory re-education/independent certification of the PI and study team Removal of PI from study or all studies Tarnished reputation Loss of funding or sponsorship Debarment from research

Regulatory Requirement for Continuing Review DHHS regulations 45 CFR 46. 109(e)

Regulatory Requirement for Continuing Review FDA regulations 21 CFR 56. 109(f)

Lapse in IRB Approval: Preventive Measures Lapse in IRB approval constitutes noncompliance with the federal regulations Approval & reapproval letters include reminders regarding regulatory obligations Reminder letters are sent out of Coeus at 60, 45 and 30 days (HIC) and 60 & 30 days (HSC) prior to expiration of the approval period

Reminder Notices Current template language: This letter serves as a reminder that the above-cited protocol is due for reapproval by the HIC. It is the primary responsibility of the Principal Investigator to ensure continued reapproval status for protocols. All protocols must be reviewed and approved annually by the HIC unless shorter intervals have been specified. It is a violation of Yale policy and federal regulations to continue human research activities after the (IRB) approval period has expired. If the HIC does not receive renewal materials within reasonable time to review and reapprove this research by its current expiration date, all enrollment, research activities and intervention on previously enrolled subjects must stop. If the approval of this protocol lapses, and if the research activities (including the analysis of identifiable private information) are supported by a sponsored award(s), no further expenses related to the research activities may be incurred and charged to the sponsored award(s) until the protocol has been reapproved. Should the research activities no longer involve human participants and you are only conducting data analysis of de-identified data, IRB approval is no longer required but the IRB does require notification of this change in order to close the study. Note also that some research sponsors may require documentation that IRB approval is no longer required.

Need Assistance or Have Questions Call HRPP Compliance Manager at 785 -6471 or send email to jeri. barney@yale. edu Call HRPP office directly at 785 -4688

References http: //www. yale. edu/hrpp/policies/index. html http: //www. yale. edu/hrpp/responsibility/complian ce. html http: //www. fda. gov/downloads/Drugs/Guidance. Co mpliance. Regulatory. Information/Guidances/UCM 18 7772. pdf http: //answers. hhs. gov/ohrp/search/results? q=non compliance

Questions