Online Entrance Preparatio n WITH NARAYAN SAPKOTA The





























- Slides: 41

Online Entrance Preparatio n WITH NARAYAN SAPKOTA

The force of attraction between unit point masses separated by unit distance is called A. Gravitational potential B. Acceleration due to gravity C. Gravitational field D. Universal Gravitational constant An Intellectual Property of Narayan Prasad Sapkota

Law of gravitation gives the gravitational force between A. The earth and a point mass only B. The earth and sun only C. any two bodies having some mass D. two charged body only An Intellectual Property of Narayan Prasad Sapkota

force exerted by earth on apple and F 2 is magnitude of force applied by apple on earth then A. F 1 is very much greater than F 2 B. F 2 is very much greater than F 1 C. F 2 is only little greater than F 1 D. F 1=F 1 An Intellectual Property of Narayan Prasad Sapkota

The gravitational force between objects is F. if masses of both objects are halved with changing distance between them then gravitational force become A. F/4 B. F/2 C. F D. 2 F An Intellectual Property of Narayan Prasad Sapkota

two objects is X. Keeping the masses of the objects same , if distance is halved then gravitational force will become A. X/4 B. X/2 C. 2 X D. 4 X An Intellectual Property of Narayan Prasad Sapkota

The value of g on the surface of the moon A. is same to that of earth B. is less than that of earth C. is more than that of earth D. keeps changing Day by day An Intellectual Property of Narayan Prasad Sapkota

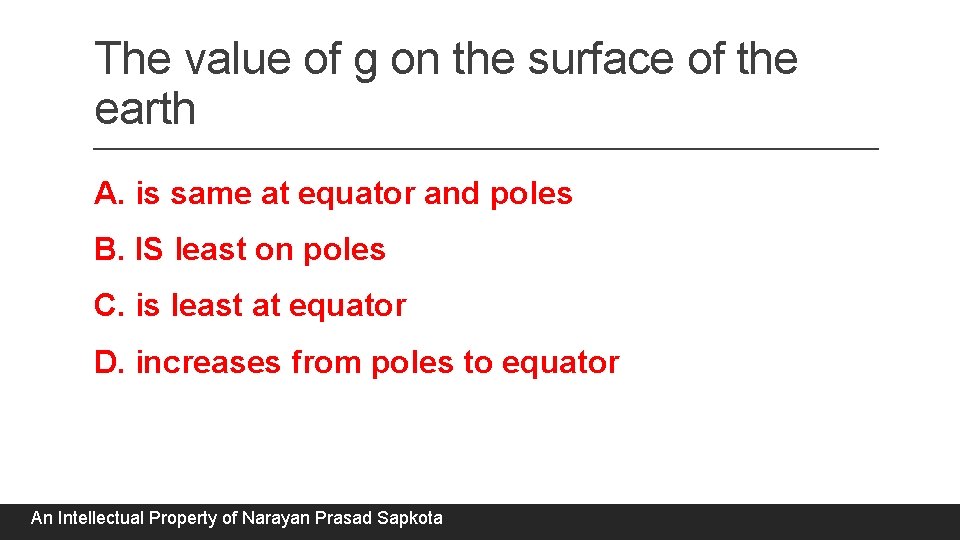
The value of g on the surface of the earth A. is same at equator and poles B. IS least on poles C. is least at equator D. increases from poles to equator An Intellectual Property of Narayan Prasad Sapkota

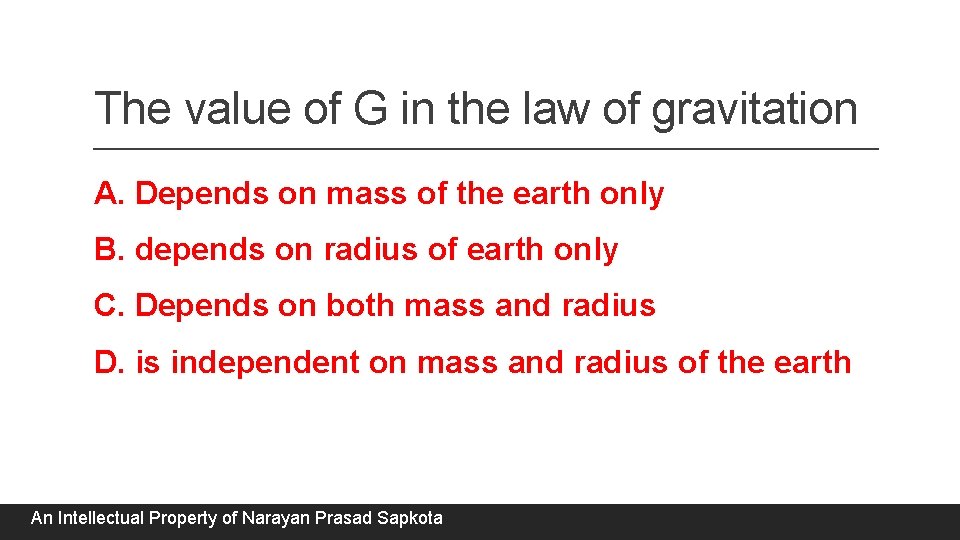
The value of G in the law of gravitation A. Depends on mass of the earth only B. depends on radius of earth only C. Depends on both mass and radius D. is independent on mass and radius of the earth An Intellectual Property of Narayan Prasad Sapkota

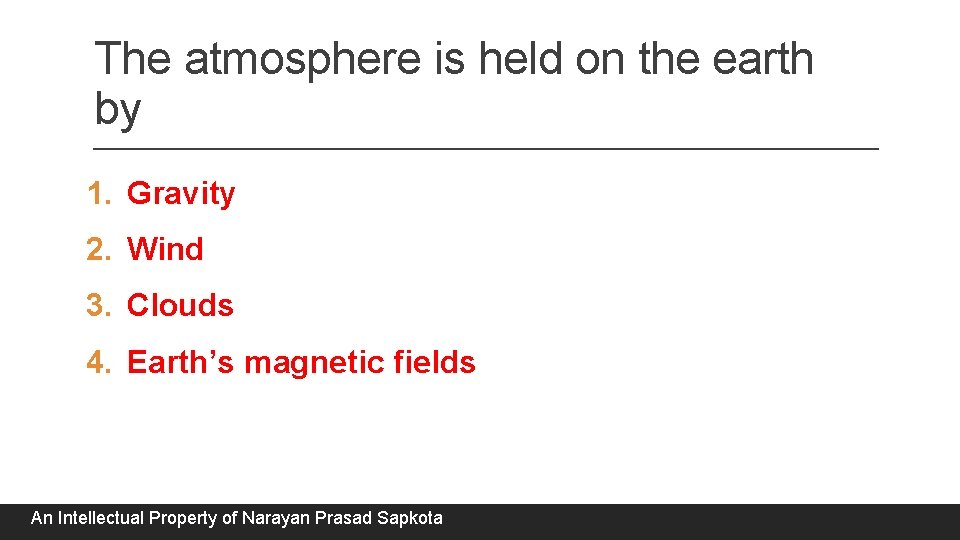
The atmosphere is held on the earth by 1. Gravity 2. Wind 3. Clouds 4. Earth’s magnetic fields An Intellectual Property of Narayan Prasad Sapkota

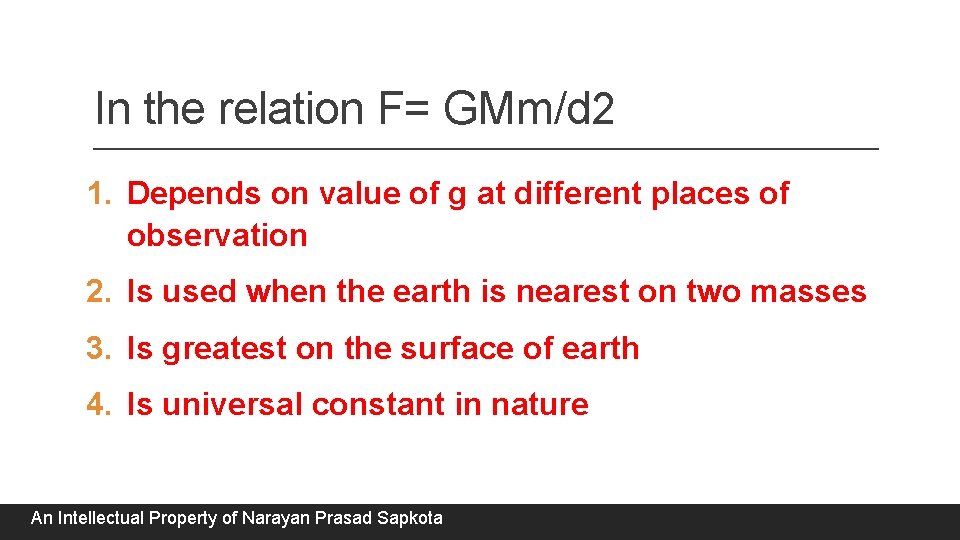
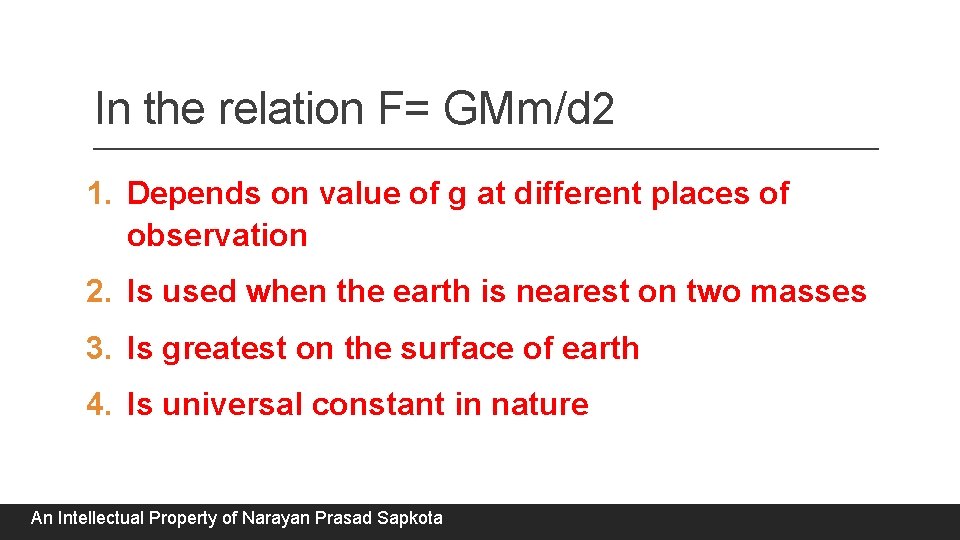
In the relation F= GMm/d 2 1. Depends on value of g at different places of observation 2. Is used when the earth is nearest on two masses 3. Is greatest on the surface of earth 4. Is universal constant in nature An Intellectual Property of Narayan Prasad Sapkota

In the relation F= GMm/d 2 1. Depends on value of g at different places of observation 2. Is used when the earth is nearest on two masses 3. Is greatest on the surface of earth 4. Is universal constant in nature An Intellectual Property of Narayan Prasad Sapkota

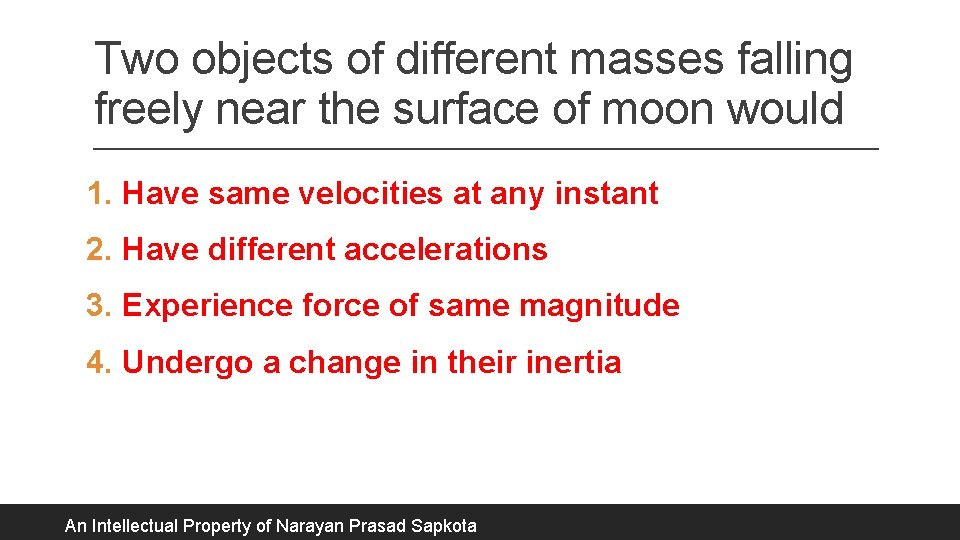
Two objects of different masses falling freely near the surface of moon would 1. Have same velocities at any instant 2. Have different accelerations 3. Experience force of same magnitude 4. Undergo a change in their inertia An Intellectual Property of Narayan Prasad Sapkota

A boy is whirling a stone tied with a string in a horizontal circular path. If string breaks the stone 1. Will continue to move in circular path 2. Will move along a straight line towards the centre in circular path 3. Will move along a straight line tangential to the circular path 4. Will move along a straight line perpendicular to the circular path away from the boy An Intellectual Property of Narayan Prasad Sapkota

Two particles are placed at some diastance. If mass of each of the two particles is doubled. Keeping the distance same, the value of gravitional force will be 1. ¼ times 2. 4 times 3. ½ times 4. Unchanged An Intellectual Property of Narayan Prasad Sapkota

The weight of the object at the centre of earth with radius R will be 1. O 2. Infinite 3. Times the more as of surface of earth 4. 1/R 2 times the surface of earth An Intellectual Property of Narayan Prasad Sapkota

According to Mendeleev’s Periodic law, elements in periodic table arranged 1. Increasing atomic weight 2. Decreasing atomic number 3. Increasing atomic number 4. Decreasing atomic weight An Intellectual Property of Narayan Prasad Sapkota

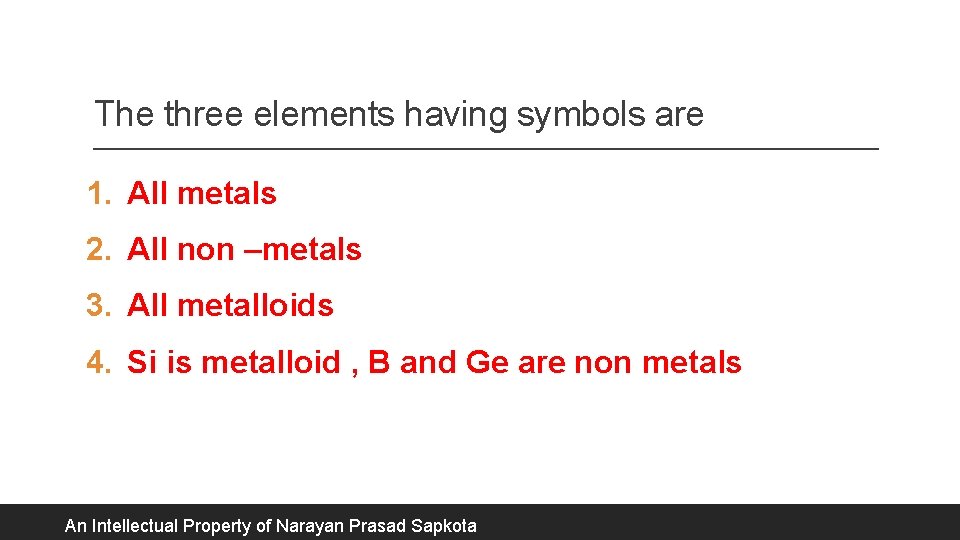
The three elements having symbols are 1. All metals 2. All non –metals 3. All metalloids 4. Si is metalloid , B and Ge are non metals An Intellectual Property of Narayan Prasad Sapkota

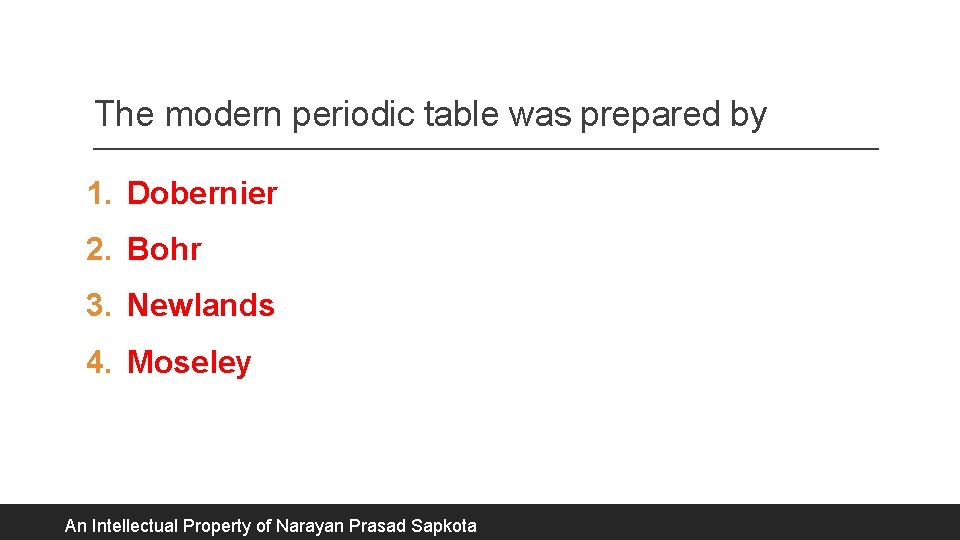
The modern periodic table was prepared by 1. Dobernier 2. Bohr 3. Newlands 4. Moseley An Intellectual Property of Narayan Prasad Sapkota

The sub atomic particle whose number in an atom always remains same and forms real basis of classification is 1. Electron 2. Proton 3. Neutron 4. Meson

The electronic configuration of the atom an element 2, 8, 4. In modern periodic table the element lies in 1. 2 nd group 2. 4 th group 3. 14 th group 4. 8 th group

The atomic number an element is 20. in modern periodic table it is placed in 1. 2 nd period 2. 3 rd period 3. 4 th period 4. 5 th period

Which of the following element will lose electron easily 1. Mg 2. Na 3. K 4. Ca

Which of the following doesnot lose electron easily 1. Na 2. F 3. Mg 4. Al

Which of the following is the valence shell of the elements of 2 nd period 1. M shell 2. K shell 3. L shell 4. N shell

According to modern periodic table elements are arranged according to 1. Increased atomic mass 2. Decreased atomic mas 3. Increased atomic number 4. Decreased atomic number

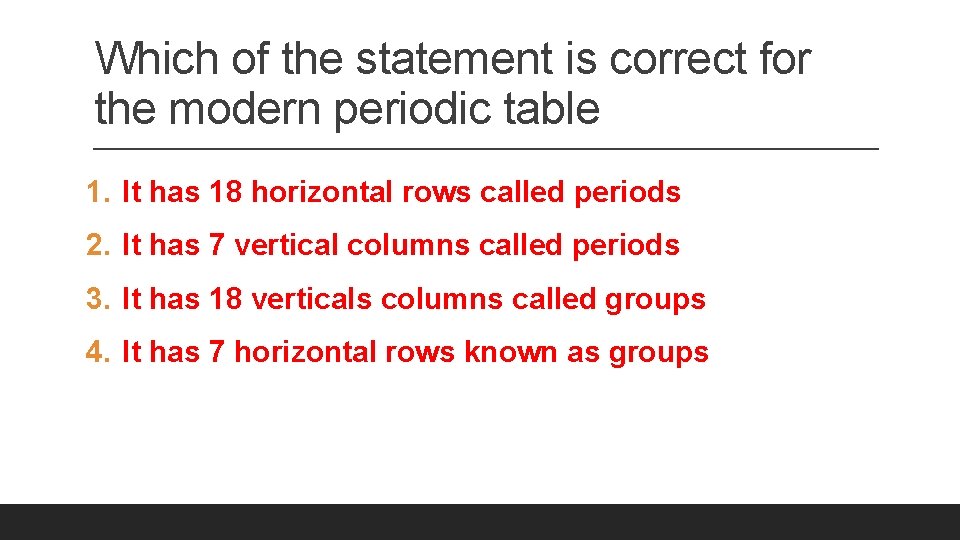
Which of the statement is correct for the modern periodic table 1. It has 18 horizontal rows called periods 2. It has 7 vertical columns called periods 3. It has 18 verticals columns called groups 4. It has 7 horizontal rows known as groups

An element which is constituent of all organic compounds belongs to 1. Group 1 2. Group 4 3. Group 15 4. Group 16

Which of the following exhibits maximum number of valence electrons 1. Na 2. Mg 3. K 4. Al

Which of the elements has largest atomic size 1. Na 2. K 3. Ca 4. Mg

Which of the following doesnot increase while moving down the group of the periodic table 1. Atomic radius 2. Valency 3. Metallic character 4. Number of shells in an element

On moving left to right in a period in the periodic table , the size of atom 1. Increases 2. Decreases 3. Doesnot change appreciably 4. First decrease then increase

Number of groups and periods respectively present in modern periodic table are 1. 16, 7 2. 6, 16 3. 18, 7 4. 18, 6

Which among the following is the most reactive halogen 1. F 2. Cl 3. Br 4. I

Which of the following is alkali metals? 1. Mg 2. Na 3. B 4. Al

Transitional elements belong to 1. S-block 2. P-block 3. D-block 4. F block

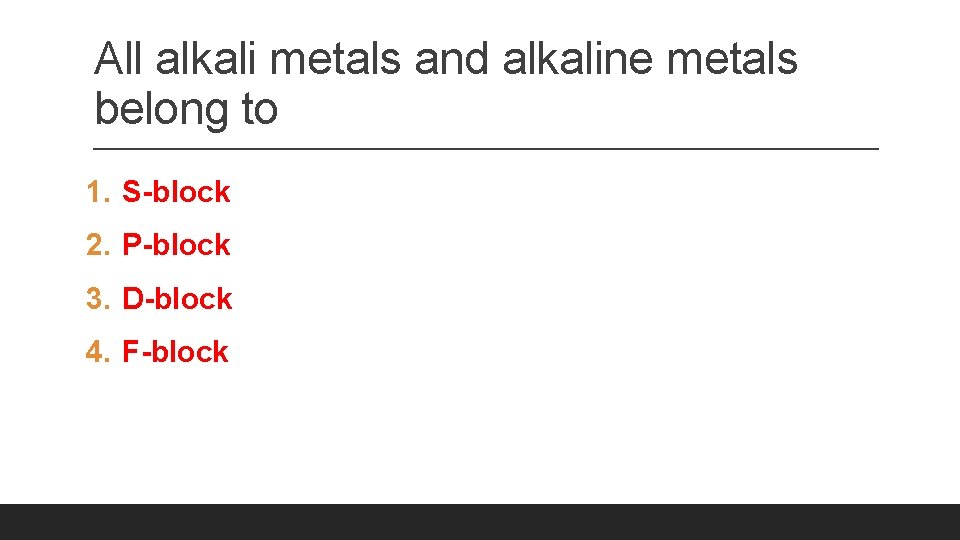
All alkali metals and alkaline metals belong to 1. S-block 2. P-block 3. D-block 4. F-block

The elements of group 18 are called 1. Alkaline earth metals 2. Alkali metals 3. Halogens 4. Inert gases

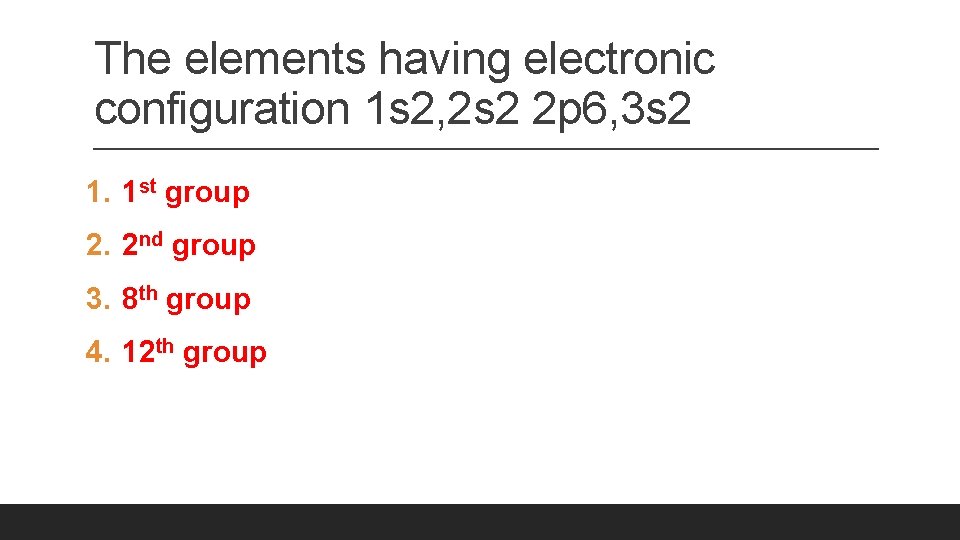
The elements having electronic configuration 1 s 2, 2 s 2 2 p 6, 3 s 2 1. 1 st group 2. 2 nd group 3. 8 th group 4. 12 th group

On moving top to bottom in the periodic table the atomic size 1. Increases 2. Decreases 3. Doesnot change 4. First increase and the decrease

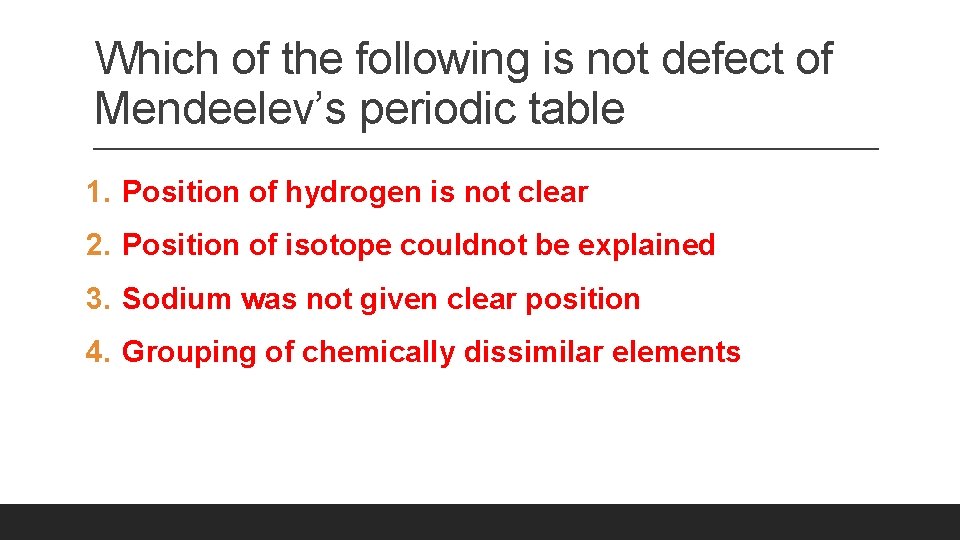
Which of the following is not defect of Mendeelev’s periodic table 1. Position of hydrogen is not clear 2. Position of isotope couldnot be explained 3. Sodium was not given clear position 4. Grouping of chemically dissimilar elements