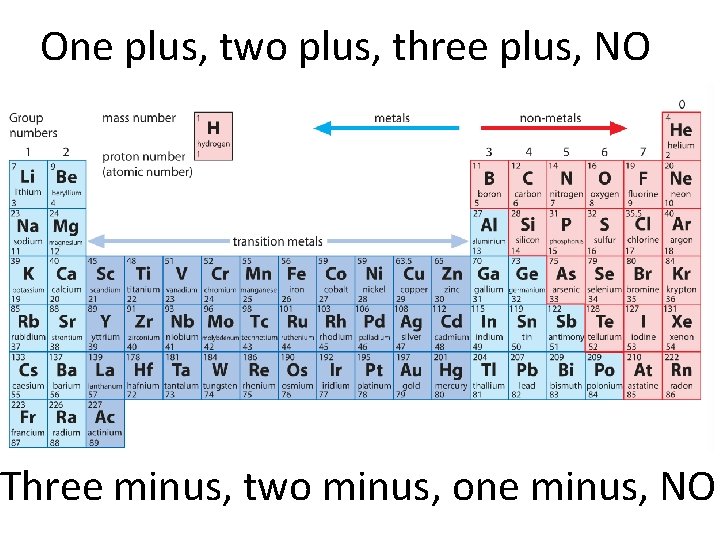
One plus two plus three plus NO Three






- Slides: 22

One plus, two plus, three plus, NO Three minus, two minus, one minus, NO

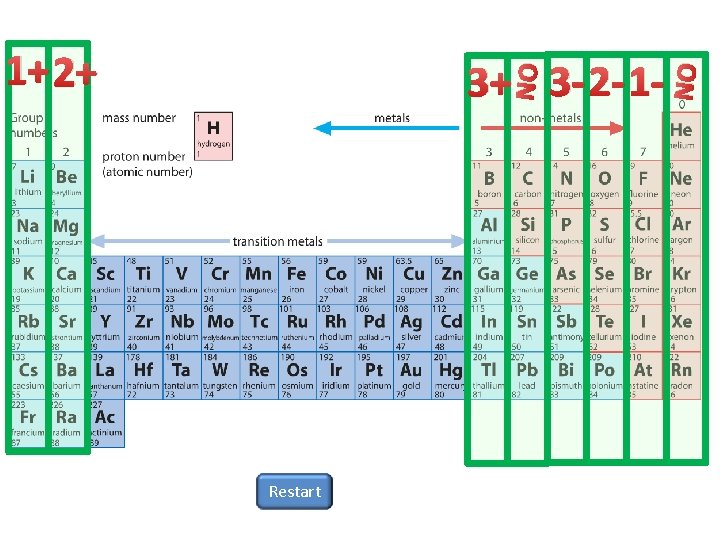
1+ 2+ Restart NO NO 3+ 3 -2 -1 -

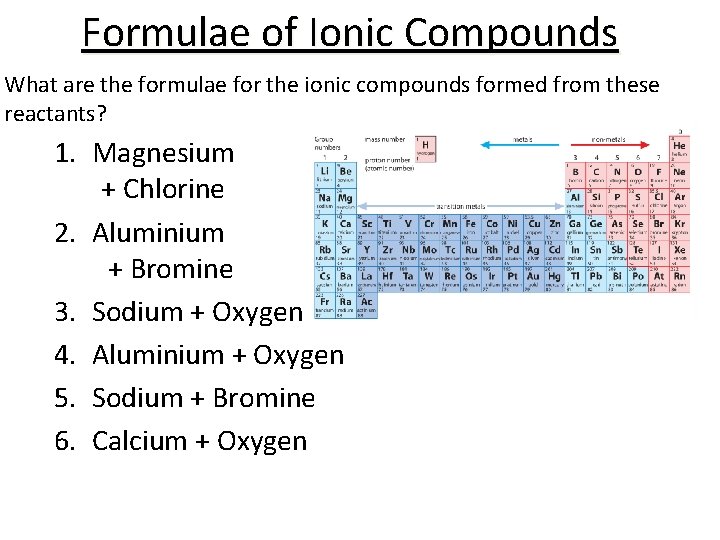
Formulae of Ionic Compounds What are the formulae for the ionic compounds formed from these reactants? 1. Magnesium + Chlorine 2. Aluminium + Bromine 3. Sodium + Oxygen 4. Aluminium + Oxygen 5. Sodium + Bromine 6. Calcium + Oxygen

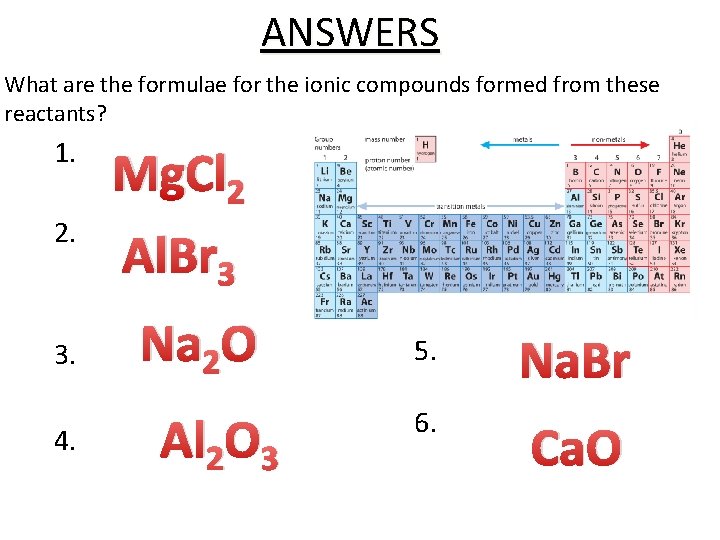
ANSWERS What are the formulae for the ionic compounds formed from these reactants? 1. Magnesium + Chlorine 2 2. Aluminium + Bromine 3 Mg. Cl Al. Br Na O 3. Sodium +2 Oxygen Al 2 O 3 4. Aluminium + Oxygen Na. Br 5. Sodium + Bromine 6. Calcium + Oxygen Ca. O

Iron Man 2 Please insert picture of Iron Man SPOT THE MISTAKE!!! http: //www. youtube. com/watch? v=1 B 2 A-Ge. Yci 0

Iron Man 2 TASK: INSERT PICTURE OF IRON MAN In iron man 2, Tony Stark is injected with ‘lithium dioxide’. • What would the formula for lithium dioxide be? • Why is this a mistake? SPOT THE MISTAKE!!!

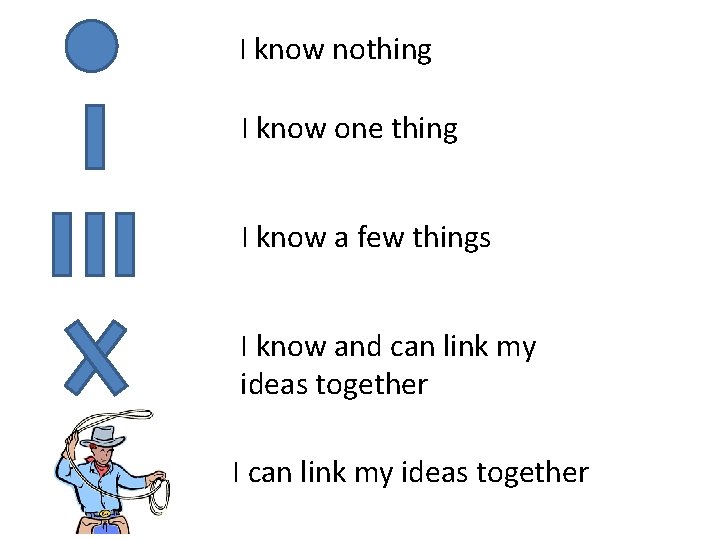
I know nothing I know one thing I know a few things I know and can link my ideas together I can link my ideas together

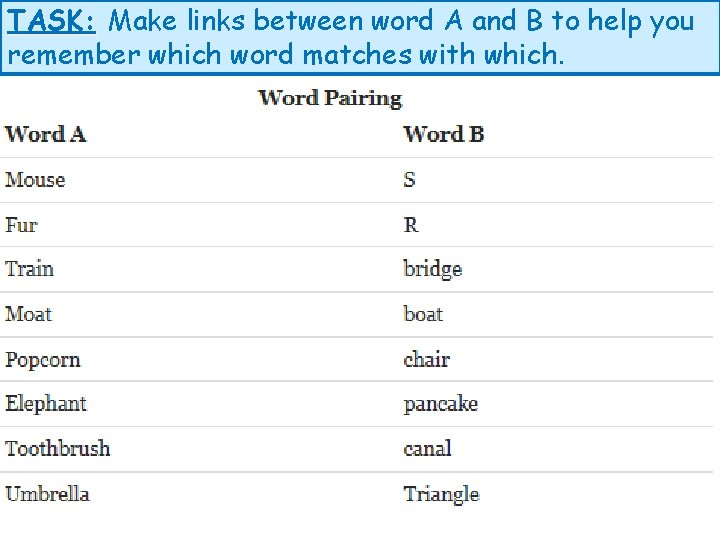
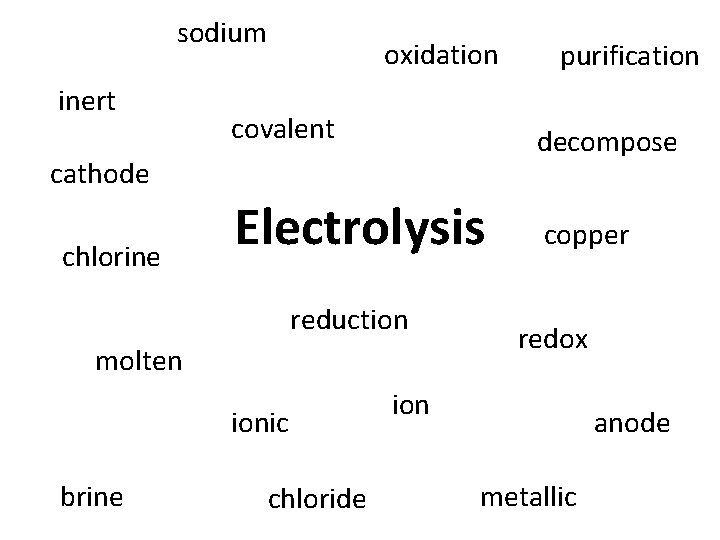
TASK: Make links between word A and B to help you remember which word matches with which.


sodium inert oxidation covalent decompose Electrolysis copper cathode chlorine reduction molten ionic brine purification chloride redox ion anode metallic

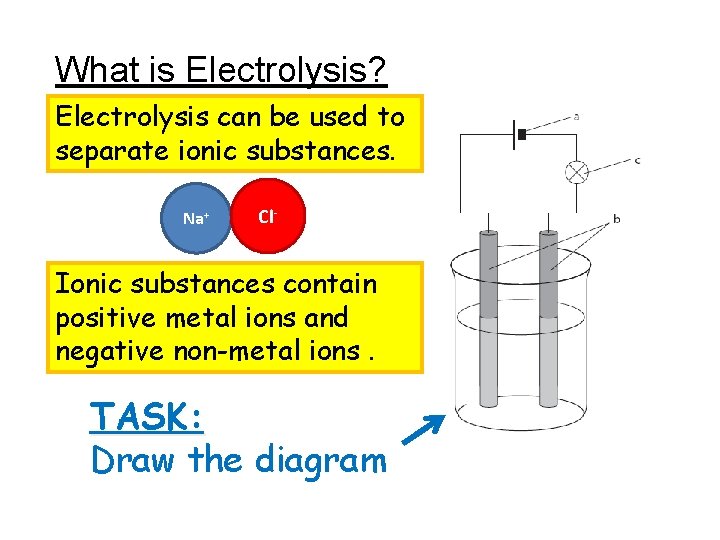
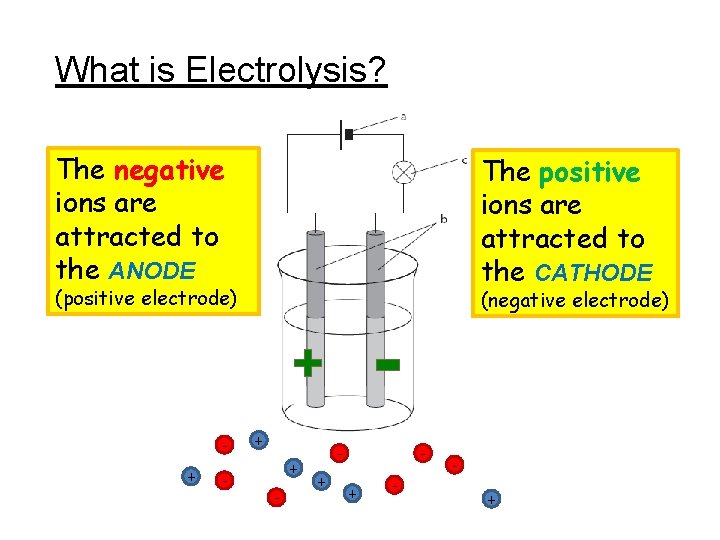
What is Electrolysis? Electrolysis can be used to separate ionic substances. Na+ Cl- Ionic substances contain positive metal ions and negative non-metal ions. TASK: Draw the diagram

What is Electrolysis? The negative ions are attracted to the ANODE The positive ions are attracted to the CATHODE (positive electrode) - + + - - + (negative electrode) + - +

What happens at the electrodes? Oxidation Is Loss Reduction Is Gain OILRIG

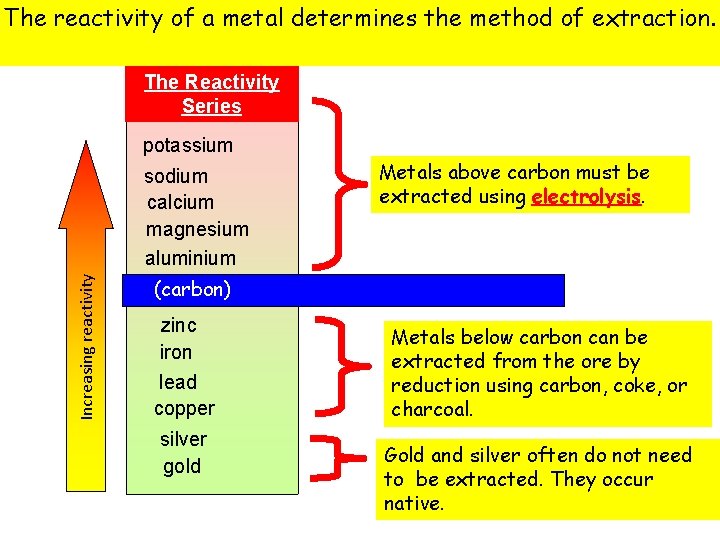
The reactivityprocesses of a metal determines the method of extraction. Extraction The Reactivity Series potassium Increasing reactivity sodium calcium magnesium aluminium Metals above carbon must be extracted using electrolysis. (carbon) zinc iron lead copper silver gold Metals below carbon can be extracted from the ore by reduction using carbon, coke, or charcoal. Gold and silver often do not need to be extracted. They occur native.

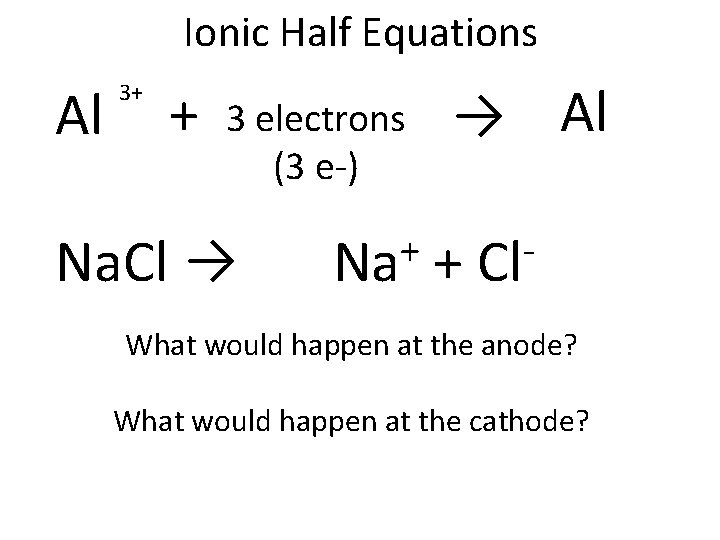
Ionic Half Equations Al 3+ + 3 electrons (3 e-) Na. Cl → + Na → Al + Cl What would happen at the anode? What would happen at the cathode?

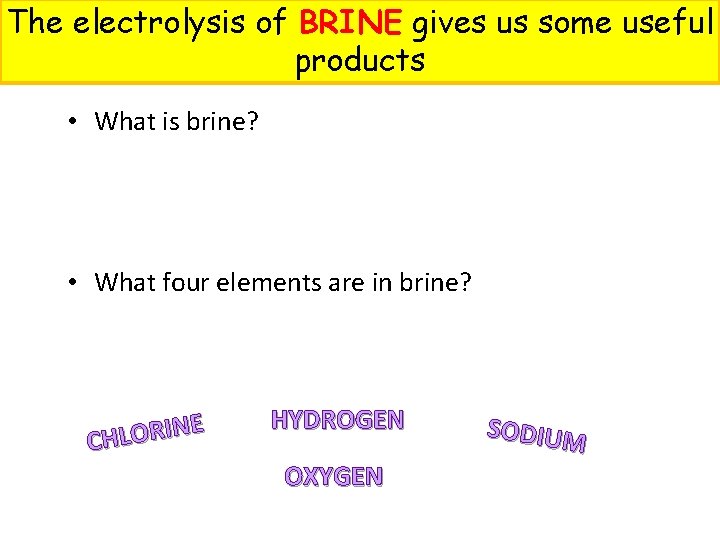
The electrolysis of BRINE gives us some useful products • What is brine? • What four elements are in brine? E N I R O L CH HYDROGEN OXYGEN SODIUM

n n e V s m a r Diag Famous People Austrians Horrible

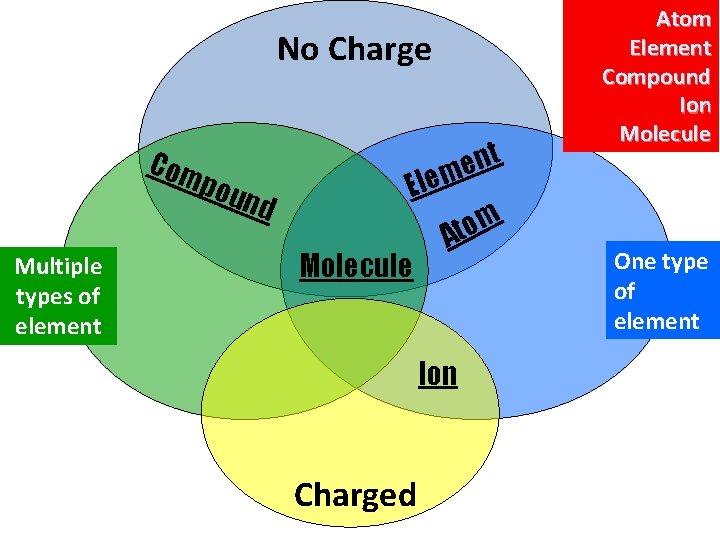
TASK: • Define the keywords below • Create a Venn diagram to show the similarities between these keywords. • Use the textbook to help you. Atom Element Compound Molecule Ion

No Charge Com pou Multiple types of element nd t n e em El Molecule m o t A Ion Charged Atom Element Compound Ion Molecule One type of element

I know nothing I know one thing I know a few things I know and can link my ideas together I can link my ideas together

sodium inert oxidation covalent decompose Electrolysis copper cathode chlorine reduction molten ionic brine purification chloride redox ion anode metallic
