Oncotype DX DCIS test use and clinical utility

![DCIS test utilization: Risk group defined by the test* [VALUE] (14) [VALUE] (18) [VALUE](68 DCIS test utilization: Risk group defined by the test* [VALUE] (14) [VALUE] (18) [VALUE](68](https://slidetodoc.com/presentation_image/0023d8dc10ef4a4273e33345e0f0dbc1/image-8.jpg)






- Slides: 26

Oncotype. DX DCIS test use and clinical utility: A SEER population-based study Yao Yuan, Ph. D, MPH, Alison Van Dyke, MD, Ph. D, Serban Negoita, MD, Dr. PH & Valentina Petkov, MD, MPH NCI SEER SRP 2019 NAACCR/IACR Combined Annual Conference 06/11/2019

I. Background II. Cases with linked test: • DCIS test utilization Contents • Clinical utility III. SEER data quality assessment: • Completeness and accuracy of test data items abstracted by registries 2

Multigene prognostic tests for Breast Cancer § Oncotype DX § Mamma. Print § Prosigna/PAM 50 § Endo. Predict § Breast Cancer Index § Mammostrat § IHC 4 3

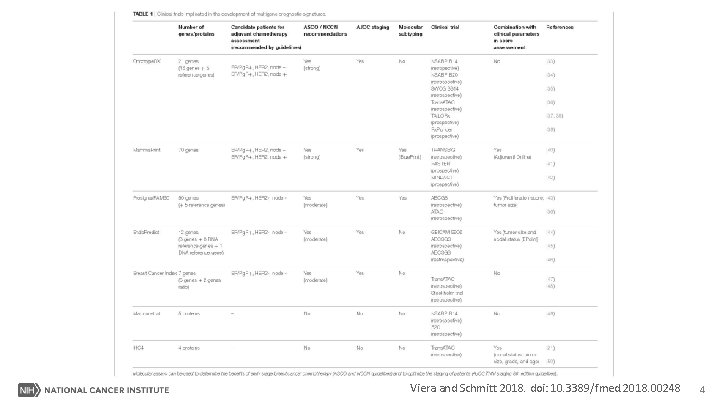
Viera and Schmitt 2018. doi: 10. 3389/fmed. 2018. 00248 4

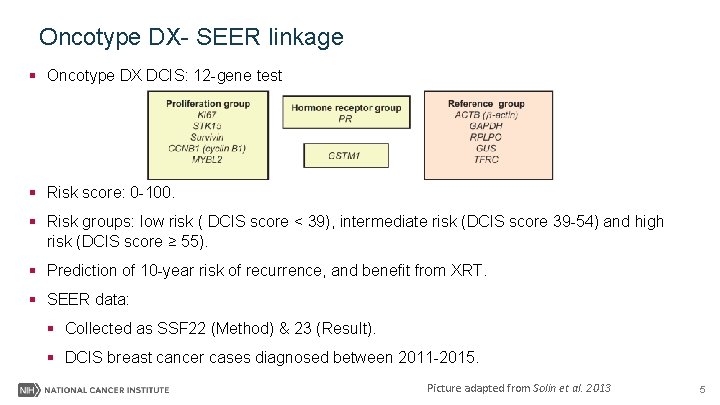
Oncotype DX- SEER linkage § Oncotype DX DCIS: 12 -gene test § Risk score: 0 -100. § Risk groups: low risk ( DCIS score < 39), intermediate risk (DCIS score 39 -54) and high risk (DCIS score ≥ 55). § Prediction of 10 -year risk of recurrence, and benefit from XRT. § SEER data: § Collected as SSF 22 (Method) & 23 (Result). § DCIS breast cancer cases diagnosed between 2011 -2015. Picture adapted from Solin et al. 2013 5

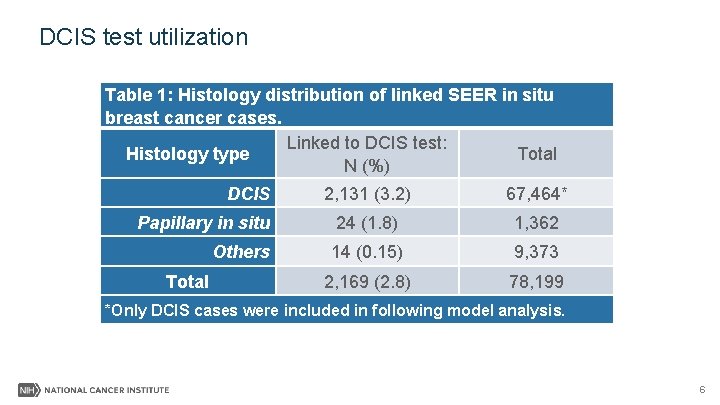
DCIS test utilization Table 1: Histology distribution of linked SEER in situ breast cancer cases. Linked to DCIS test: Histology type Total N (%) DCIS 2, 131 (3. 2) 67, 464* Papillary in situ 24 (1. 8) 1, 362 Others 14 (0. 15) 9, 373 2, 169 (2. 8) 78, 199 Total *Only DCIS cases were included in following model analysis. 6

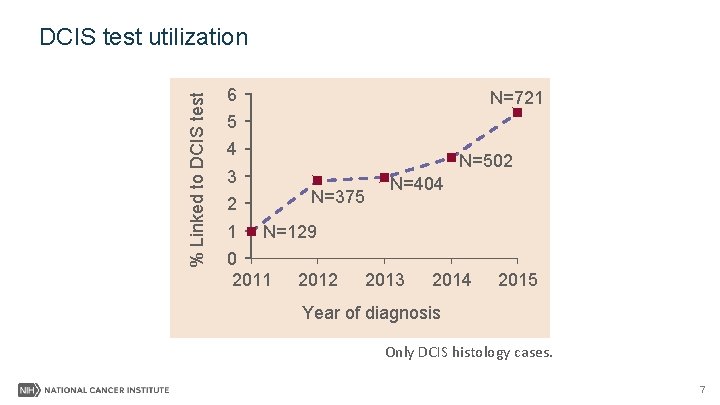
% Linked to DCIS test utilization 6 N=721 5 4 N=502 3 N=375 2 1 N=404 N=129 0 2011 2012 2013 2014 2015 Year of diagnosis Only DCIS histology cases. 7
![DCIS test utilization Risk group defined by the test VALUE 14 VALUE 18 VALUE68 DCIS test utilization: Risk group defined by the test* [VALUE] (14) [VALUE] (18) [VALUE](68](https://slidetodoc.com/presentation_image/0023d8dc10ef4a4273e33345e0f0dbc1/image-8.jpg)
DCIS test utilization: Risk group defined by the test* [VALUE] (14) [VALUE] (18) [VALUE](68 ) N=146 “Cancelled test” N=129 “Failed test” N=11 “Non-unique” 286 (13) Low 266 (12) 324(15) 1255(59) Intermediate High Low Intermediate High *DCIS histology only 8

Results I- Summary • DCIS test were performed on non-DCIS histology cases. • The proportion of DCIS patients receiving the test has been increasing since 2011. • Most of the tested cases were in low risk group. 9

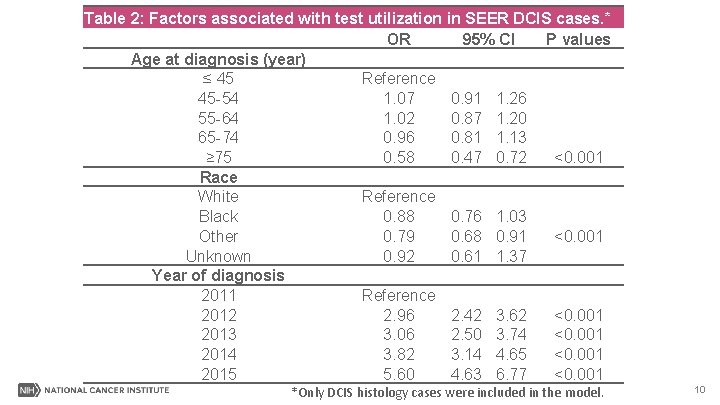
Table 2: Factors associated with test utilization in SEER DCIS cases. * OR 95% CI P values Age at diagnosis (year) ≤ 45 Reference 45 -54 1. 07 0. 91 1. 26 55 -64 1. 02 0. 87 1. 20 65 -74 0. 96 0. 81 1. 13 ≥ 75 0. 58 0. 47 0. 72 <0. 001 Race White Reference Black 0. 88 0. 76 1. 03 Other 0. 79 0. 68 0. 91 <0. 001 Unknown 0. 92 0. 61 1. 37 Year of diagnosis 2011 Reference 2012 2. 96 2. 42 3. 62 <0. 001 2013 3. 06 2. 50 3. 74 <0. 001 2014 3. 82 3. 14 4. 65 <0. 001 2015 5. 60 4. 63 6. 77 <0. 001 *Only DCIS histology cases were included in the model. 10

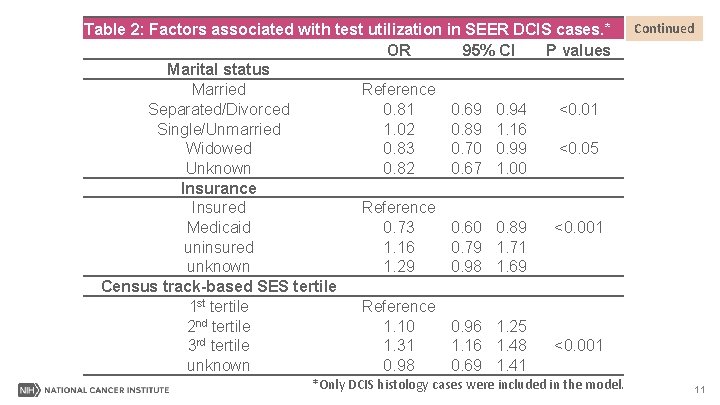
Table 2: Factors associated with test utilization in SEER DCIS cases. * OR 95% CI P values Marital status Married Reference Separated/Divorced 0. 81 0. 69 0. 94 <0. 01 Single/Unmarried 1. 02 0. 89 1. 16 Widowed 0. 83 0. 70 0. 99 <0. 05 Unknown 0. 82 0. 67 1. 00 Insurance Insured Reference Medicaid 0. 73 0. 60 0. 89 <0. 001 uninsured 1. 16 0. 79 1. 71 unknown 1. 29 0. 98 1. 69 Census track-based SES tertile 1 st tertile Reference 2 nd tertile 1. 10 0. 96 1. 25 3 rd tertile 1. 31 1. 16 1. 48 <0. 001 unknown 0. 98 0. 69 1. 41 *Only DCIS histology cases were included in the model. Continued 11

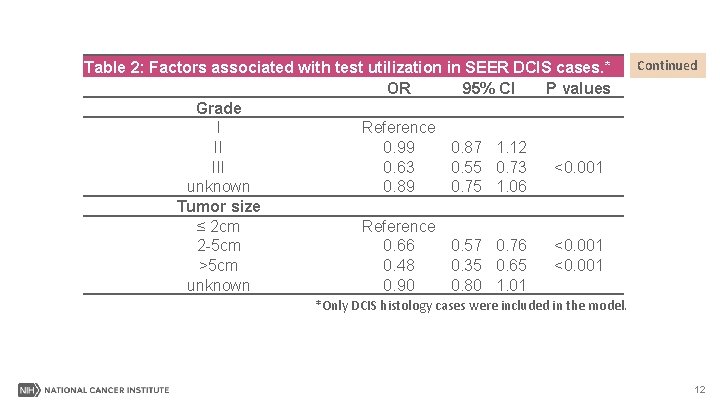
Table 2: Factors associated with test utilization in SEER DCIS cases. * OR 95% CI P values Grade I Reference II 0. 99 0. 87 1. 12 III 0. 63 0. 55 0. 73 <0. 001 unknown 0. 89 0. 75 1. 06 Tumor size ≤ 2 cm Reference 2 -5 cm 0. 66 0. 57 0. 76 <0. 001 >5 cm 0. 48 0. 35 0. 65 <0. 001 unknown 0. 90 0. 80 1. 01 Continued *Only DCIS histology cases were included in the model. 12

Results II-Summary Patients were less likely to receive the test if they were/had: • ≥ 75 years. • Larger and higher-grade tumors. • Divorced or widowed. • In Medicaid. 13

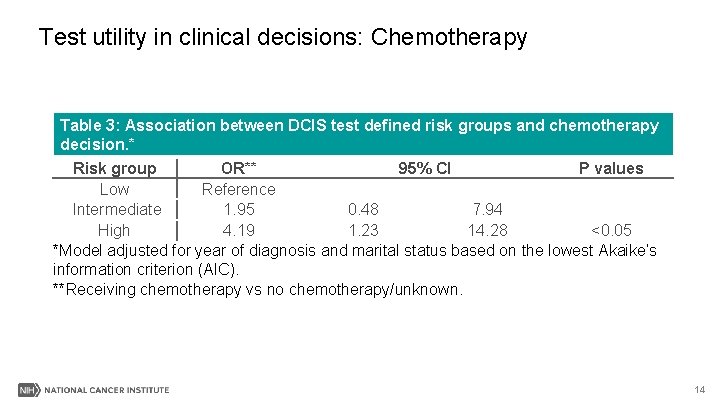
Test utility in clinical decisions: Chemotherapy Table 3: Association between DCIS test defined risk groups and chemotherapy decision. * Risk group OR** 95% CI P values Low Reference Intermediate 1. 95 0. 48 7. 94 High 4. 19 1. 23 14. 28 <0. 05 *Model adjusted for year of diagnosis and marital status based on the lowest Akaike’s information criterion (AIC). **Receiving chemotherapy vs no chemotherapy/unknown. 14

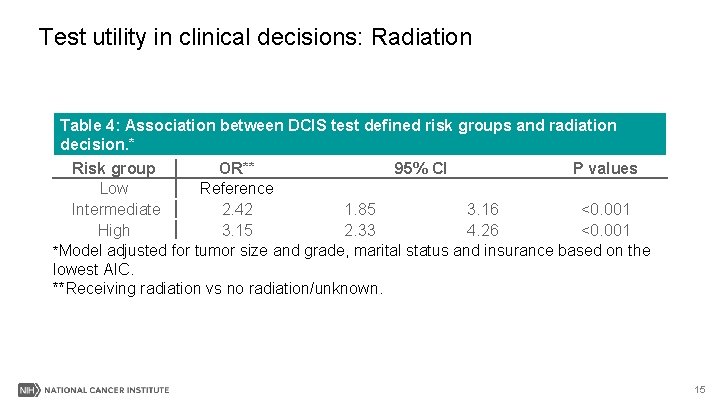
Test utility in clinical decisions: Radiation Table 4: Association between DCIS test defined risk groups and radiation decision. * Risk group OR** 95% CI P values Low Reference Intermediate 2. 42 1. 85 3. 16 <0. 001 High 3. 15 2. 33 4. 26 <0. 001 *Model adjusted for tumor size and grade, marital status and insurance based on the lowest AIC. **Receiving radiation vs no radiation/unknown. 15

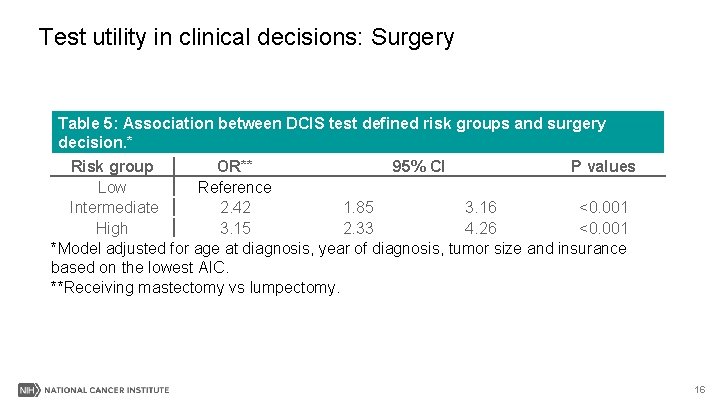
Test utility in clinical decisions: Surgery Table 5: Association between DCIS test defined risk groups and surgery decision. * Risk group OR** 95% CI P values Low Reference Intermediate 2. 42 1. 85 3. 16 <0. 001 High 3. 15 2. 33 4. 26 <0. 001 *Model adjusted for age at diagnosis, year of diagnosis, tumor size and insurance based on the lowest AIC. **Receiving mastectomy vs lumpectomy. 16

Results III-Summary § Patients in higher risk group(s) were more likely to be associated with chemotherapy, radiation or mastectomy surgery, respectively, than in low risk group. 17

Conclusions for part I: cases with linked test • Clinical adoption of the Oncotype DX DCIS test has been slowly increasing. • The association between multiple demographic factors and receiving the test indicated disparities in the US population. • Clinical factors also influenced whether patients received the test. • Oncotype DX DCIS results were associated with treatment decisions. 18

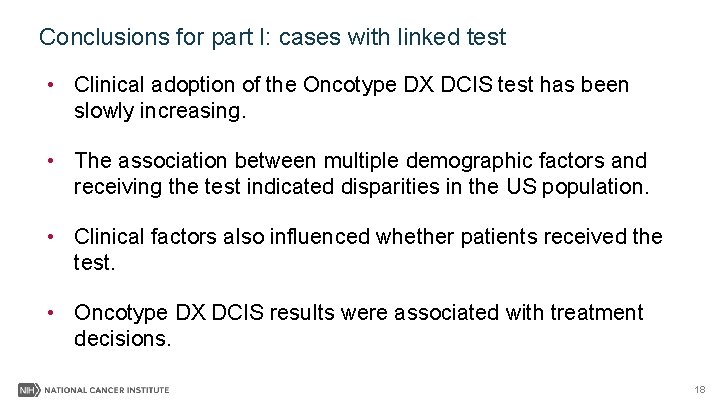
SEER data quality assessment SEER SSF 22: Multigene signature methods Table 6: Cross check SEER data and linked GHI data on Oncotype DX DCIS test. GHI data on DCIS test Test done No without result Yes reported Oncotype DX 501(30%) 39 1, 108 Mamma. Print 15 0 1 Other 197 3 3 Test performed, 50 1 2 type unknown Test not done 44, 387 154 423 Unknown Total 30, 880 95 340 76, 030 Total 292 1, 877 2169 Total 1, 648 16 203 53 44, 964 31, 315 78, 199 19

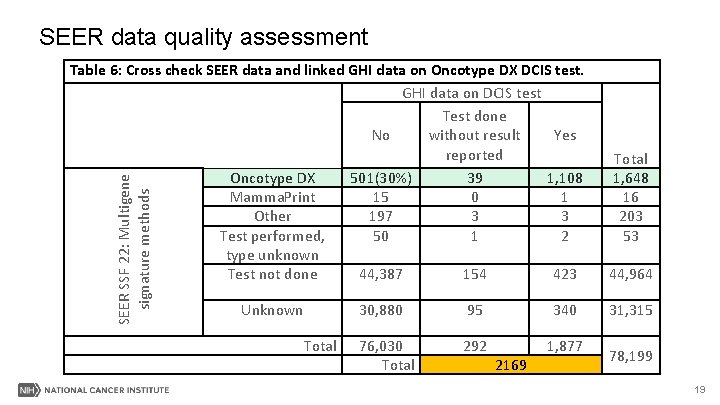
SEER data quality assessment Table 7: Abstraction errors in SEER data for DCIS test. GHI data: N(%) SEER data Total No Yes* No 75, 529 1, 022 (47%) 76, 551 Yes 501 1, 147 1, 648 76, 030 2, 169 78, 199 *Included tested cases with or without results reported. All histology included 20

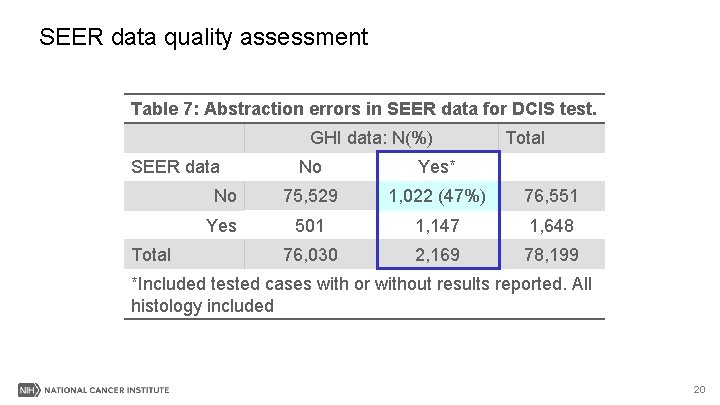
SEER data quality assessment Cases with valid test scores (0 -100) recorded in SEER N=992 Kappa=0. 96 Possibly entered in SEER as the % risk of recurrence instead of the score. 21

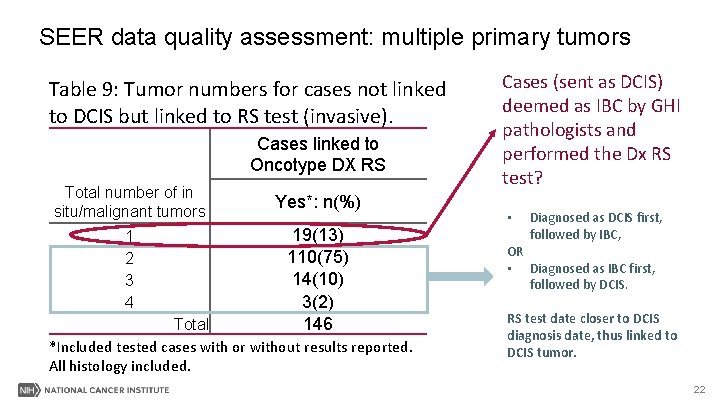
SEER data quality assessment: multiple primary tumors Table 9: Tumor numbers for cases not linked to DCIS but linked to RS test (invasive). Cases linked to Oncotype DX RS Total number of in situ/malignant tumors 1 2 3 4 Yes*: n(%) 19(13) 110(75) 14(10) 3(2) 146 Total *Included tested cases with or without results reported. All histology included. Cases (sent as DCIS) deemed as IBC by GHI pathologists and performed the Dx RS test? • Diagnosed as DCIS first, followed by IBC, OR • Diagnosed as IBC first, followed by DCIS. RS test date closer to DCIS diagnosis date, thus linked to DCIS tumor. 22

Conclusions for part II: SEER data quality assessment § SEER data items are questionable: § Tests were not captured in the registry data. § Test data were entered incorrectly. § Data linkage is important for the completeness and accuracy of registry data. 23

Acknowledgement • SEER registries participated in the linkage. • Genomic Health (GHI) provided the data. Thank you! Questions: yao. yuan@nih. gov 24

www. cancer. gov/espanol

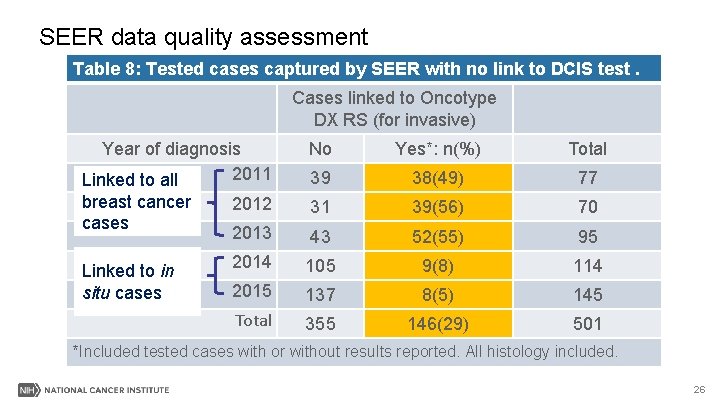
SEER data quality assessment Table 8: Tested cases captured by SEER with no link to DCIS test. Cases linked to Oncotype DX RS (for invasive) Year of diagnosis 2011 Linked to all No Yes*: n(%) Total 39 38(49) 77 breast cancer cases 2012 31 39(56) 70 2013 43 52(55) 95 2014 105 9(8) 114 2015 137 8(5) 145 Total 355 146(29) 501 Linked to in situ cases *Included tested cases with or without results reported. All histology included. 26