Oncotype DX a Genomic Approach to Breast Cancer

Oncotype DX a Genomic Approach to Breast Cancer

Pathology: 20 th and 21 st Century Size Age Phenotype Nodal status Protein/Gene “Genomic Profiling”

Role of Traditional Markers in ER+ EBC • Markers are used to determine diagnosis, estimate prognosis and to inform treatment decisions • Some markers (tumour grade, nodal status and genomic tests) are prognostic; some are predictive of treatment benefit. Some are both predictive and prognostic (ER, PR, HER 2 and Oncotype DX® assay) • Standardisation and / or reproducibility of a test may present a challenge • Variability in interpretation of results • Despite the use of many markers, a large proportion of patients are classified as intermediate risk and there is a population in whom treatment decisions are not clear Cianfrocca and Goldstein. Oncologist. 2004; 9(6): 606 -616; Lonning PE. Ann Oncol. 2007; 18(suppl 8): viii 3 -viii 7

What are Genetics and Genomics? Genetics Genomics • Genes (units of heredity) carry the instructions for making proteins, which direct the activities of cells and functions of the body • Study of all of a person's genes (the genome), interactions of the genes with each other and with the environment • Study of a single gene, its roles in inheritance and its effects • Examples of genetic disorders include cystic fibrosis, Huntington's disease, and in oncology; BRCA • Study of complex diseases such as heart disease, asthma, diabetes, and cancer because these diseases are typically caused more by a combination of genetic and environmental factors than by individual genes

Oncotype DX® Breast Test • It is a genomic test of the expression of 21 genes (16 tumor genes and 5 housekeeping genes) • Utilizes RT-PCR technology on FFPE tissue • Quantitatively predicts the likelihood of breast cancer recurrence in women with newly diagnosed, early stage invasive breast cancer • Assesses the likely benefit from both hormonal therapy and chemotherapy • Is the only multi-parameter gene expression assay to show clinical utility in breast cancer • Is recommended by clinical practice guidelines (St Gallen, ESMO, ASCO, NCCN) and NICE Harris L, et al. J Clin Oncol. 2007; 33(25): 5287 -5312. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 2. 2008. Available at: http: //www. nccn. org/professionals/physician_gls/PDF/breast. pdf. Accessed December 8, 2008. 5

Oncotype DX® Breast Test Genes 16 CANCER RELATED GENES Estrogen Proliferation HER 2 Invasion Ki-67 STK 15 Survivin Cyclin B 1 MYBL 2 GRB 7 HER 2 Stromelysin 3 Cathepsin L 2 ER PR Bcl 2 SCUBE 2 Others CD 68 GSTM 1 BAG 1 5 REFERENCE GENES Beta-actin Paik et al. N Engl J Med. 2004; 351: 2817 -2826. GAPDH RPLPO GUS TFRC 6

The centralisation of the Oncotype DX testing allows >97% reliability The GHI lab has processed >400, 000 tests from >70 countries ORDER ENTRY Online or Fax INTAKE Phone Benefits Investigation Order Entry Patient Information Retrieval PATHOLOGY ANALYTICAL LABORATORY REPORT FULFILLMENT Fax Request Fed. EX Specimen Retrieval Fed. Ex Pathology Review Extraction Quantitation Results Generation Report Delivery g. DNA Detection Specimen Accessioning Histopath Reverse Transcription Billing QPCR Online 10 -14 working days *Anderson JM et al, 2009. MATERIAL RETURN Materials Return

Automation is Central to Laboratory Processes 8
The Oncotype DX® Breast Test Results • Quantifies expression of a panel of genes which is expressed as a Recurrence Score® • The Recurrencence Score® is a continuous value ranging from 0 to 100; and classifies patients into 3 risk categories: – Low (<18) – minimal benefit from adjuvant chemotherapy 1, 2 – Intermediate (18 -30) - ? 1, 2 – High (≥ 31) - significant benefit from adjuvant chemotherapy 1, 2 1. 2. Paik et al. J Clin Oncol. 2006; , Albain et al. Lancet Oncol 2010

The Precision of the Oncotype DX® Recurrence Score® Defines Individual Biology for ER Positive Breast Cancer Distant Recurrence at 10 Years CONTINUOUS BIOLOGY RS 30 = 20% risk of distant recurrence at 10 years Recurrence Score LOW RECURRENCE SCORE DISEASE Indolent Hormone Therapy Sensitive Minimal, If Any, Chemotherapy Benefit Paik S, et al. N Engl J Med. 2004; 351: 2817; Paik S, et al. J Clin Oncol. 2006; 24: 3726; Habel LA, et al. Breast Cancer Res. 2006; 8: R 25. HIGH RECURRENCE SCORE DISEASE Aggressive Hormone Therapy Insensitive Large Chemotherapy Benefit

Oncotype DX® Clinical Validation: NSABP B-14 • Objective: Prospectively validate the Recurrence Score® result as a predictor of distant recurrence in nodenegative, ER+ patients Placebo—not eligible Randomized Registered Tamoxifen—eligible • Multicenter study with prespecified 21 -gene assay, algorithm, endpoints, analysis plan 11 Paik S, et al. N Engl J Med. 2004; 351: 2817 -2826.

Proportion without distant recurrence Oncotype DX® Clinical Validation: NSABP B-14, Distant Recurrence Distant recurrence over time 100% 10 -Year rate of recurrence = 6. 8%* 90% 95% CI: 4. 0%, 9. 6% 80% 10 -Year rate of recurrence = 14. 3% 70% 95% CI: 8. 3%, 20. 3% 10 -Year rate of recurrence = 30. 5%* 60% 95% CI: 23. 6%, 37. 4% 50% 40% All Patients, n = 668 30% RS < 18, n = 338 20% RS 18 -30, n = 149 10% RS ≥ 31, n = 181 P < 0. 001 0% 0 2 RS, Recurrence Score® result 4 6 8 10 14 16 Years *10 -Year distant recurrence comparison between low- and high-risk groups: P < 0. 001 Paik S, et al. N Engl J Med. 2004; 351: 2817 -2826. 12 12
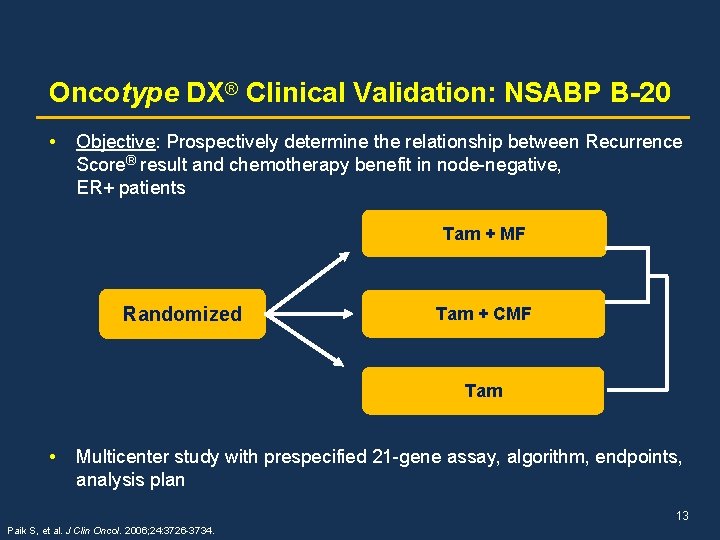
Oncotype DX® Clinical Validation: NSABP B-20 • Objective: Prospectively determine the relationship between Recurrence Score® result and chemotherapy benefit in node-negative, ER+ patients Tam + MF Randomized Tam + CMF Tam • Multicenter study with prespecified 21 -gene assay, algorithm, endpoints, analysis plan 13 Paik S, et al. J Clin Oncol. 2006; 24: 3726 -3734.
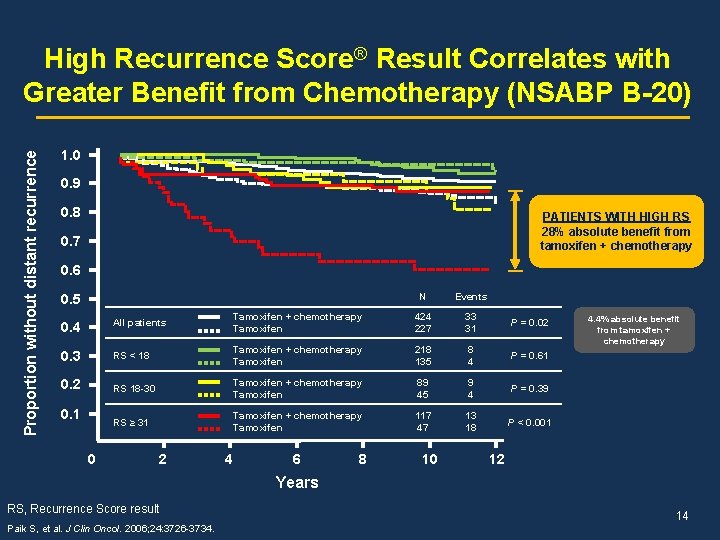
Proportion without distant recurrence High Recurrence Score® Result Correlates with Greater Benefit from Chemotherapy (NSABP B-20) 1. 0 0. 9 0. 8 PATIENTS WITH HIGH RS 28% absolute benefit from tamoxifen + chemotherapy 0. 7 0. 6 0. 5 N Events 0. 4 All patients Tamoxifen + chemotherapy Tamoxifen 424 227 33 31 P = 0. 02 0. 3 RS < 18 Tamoxifen + chemotherapy Tamoxifen 218 135 8 4 P = 0. 61 0. 2 RS 18 -30 Tamoxifen + chemotherapy Tamoxifen 89 45 9 4 P = 0. 39 RS ≥ 31 Tamoxifen + chemotherapy Tamoxifen 117 47 13 18 P < 0. 001 0. 1 0 2 4 6 8 10 4. 4% absolute benefit from tamoxifen + chemotherapy 12 Years RS, Recurrence Score result Paik S, et al. J Clin Oncol. 2006; 24: 3726 -3734. 14
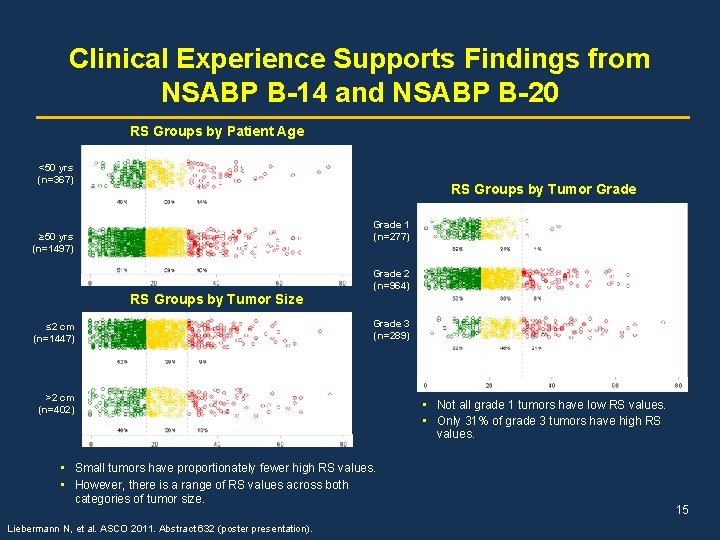
Clinical Experience Supports Findings from NSABP B-14 and NSABP B-20 RS Groups by Patient Age <50 yrs (n=367) RS Groups by Tumor Grade 1 (n=277) ≥ 50 yrs (n=1497) RS Groups by Tumor Size ≤ 2 cm (n=1447) Grade 2 (n=964) Grade 3 (n=289) >2 cm (n=402) • Small tumors have proportionately fewer high RS values. • However, there is a range of RS values across both categories of tumor size. Liebermann N, et al. ASCO 2011. Abstract 632 (poster presentation). • Not all grade 1 tumors have low RS values. • Only 31% of grade 3 tumors have high RS values. 15
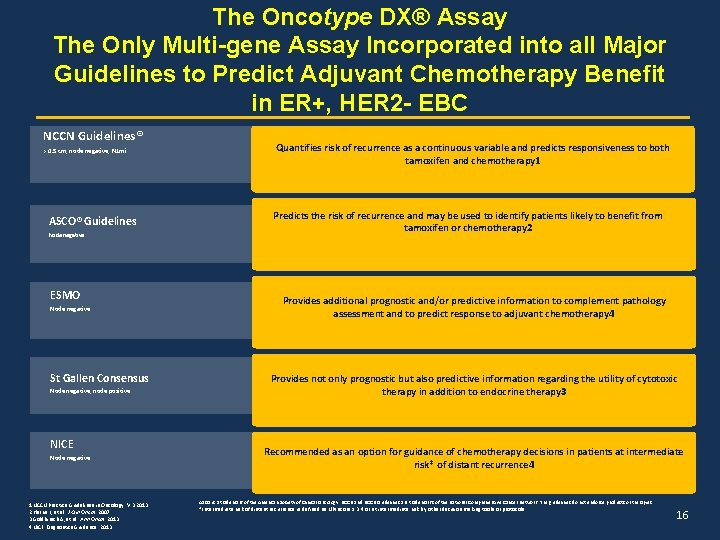
The Oncotype DX® Assay The Only Multi-gene Assay Incorporated into all Major Guidelines to Predict Adjuvant Chemotherapy Benefit in ER+, HER 2 - EBC NCCN Guidelines® > 0. 5 cm, node negative, N 1 mi ASCO® Guidelines Node negative ESMO Node negative St Gallen Consensus Node negative, node positive NICE Node negative 1 NCCN Practice Guidelines in Oncology. V. 3. 2013. 2 Harris L, et al. J Clin Oncol. 2007. 3 Goldhirsch A, et al. Ann Oncol. 2013. 4 NICE Diagnostics Guidance 2013. Quantifies risk of recurrence as a continuous variable and predicts responsiveness to both tamoxifen and chemotherapy 1 Predicts the risk of recurrence and may be used to identify patients likely to benefit from tamoxifen or chemotherapy 2 Provides additional prognostic and/or predictive information to complement pathology assessment and to predict response to adjuvant chemotherapy 4 Provides not only prognostic but also predictive information regarding the utility of cytotoxic therapy in addition to endocrine therapy 3 Recommended as an option for guidance of chemotherapy decisions in patients at intermediate risk* of distant recurrence 4 ASCO is a trademark of the American Society of Clinical Oncology. NCCN and NCCN Guidelines are trademarks of the National Comprehensive Cancer Network. The guidelines do not endorse products or therapies. *Intermediate risk of distant recurrence is defined as NPI score ≥ 3. 4 or at intermediate risk by other decision making tools or protocols 16

Oncotype DX test funding in England • Recommended by NICE – Oncotype DX is recommended as an option for guiding adjuvant chemotherapy decisions for people with oestrogen receptor positive (ER+), lymph node negative (LN−) and human epidermal growth factor receptor 2 negative (HER 2−) early breast cancer if: • The person is assessed as being at intermediate risk and • Information on the biological features of the cancer provided by Oncotype DX is likely to help in predicting the course of the disease and would therefore help when making the decision about prescribing chemotherapy • Progressing through NHS England, policy document out for comment by 14 March – Intermediate risk defined as an OS benefit of >3% with Predict tool – Registration of patients and collection of information required by NHS England 17

Which Patients may Benefit from the Oncotype DX® Test? Clinical indication NICE guidance Use of the Oncotype DX® breast cancer assay in the N+ setting validated for post-menopausal patients ER: Oestrogen receptor HER 2: Human Epidermal Growth Factor Receptor 2 NICE guidance DG 10. http: //guidance. NICE. org. UK/DG 10 Accessed 14 Jan 2014
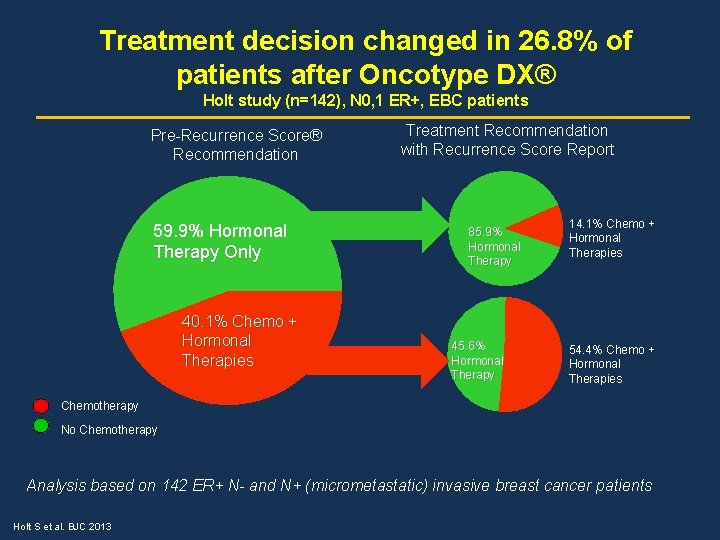
Treatment decision changed in 26. 8% of patients after Oncotype DX® Holt study (n=142), N 0, 1 ER+, EBC patients Pre-Recurrence Score® Recommendation 59. 9% Hormonal Therapy Only 40. 1% Chemo + Hormonal Therapies Treatment Recommendation with Recurrence Score Report 85. 9% Hormonal Therapy 45. 6% Hormonal Therapy 14. 1% Chemo + Hormonal Therapies 54. 4% Chemo + Hormonal Therapies Chemotherapy No Chemotherapy Analysis based on 142 ER+ N- and N+ (micrometastatic) invasive breast cancer patients Holt S et al. BJC 2013
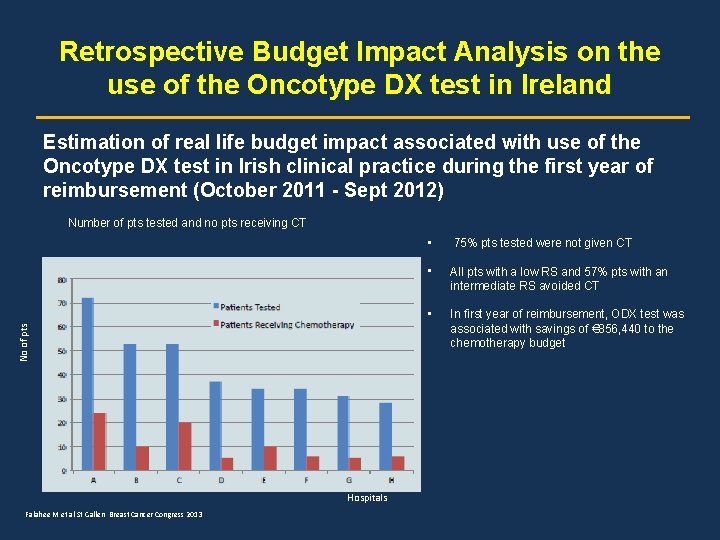
Retrospective Budget Impact Analysis on the use of the Oncotype DX test in Ireland Estimation of real life budget impact associated with use of the Oncotype DX test in Irish clinical practice during the first year of reimbursement (October 2011 - Sept 2012) No of pts Number of pts tested and no pts receiving CT Hospitals Falahee M et al St Gallen Breast Cancer Congress 2013 • 75% pts tested were not given CT • All pts with a low RS and 57% pts with an intermediate RS avoided CT • In first year of reimbursement, ODX test was associated with savings of € 856, 440 to the chemotherapy budget

Conclusions • Oncotype DX® is a consistent and effective test that compliments current decision making tools – Recommended in all major international treatment guidelines • The Recurrence Score® cannot be predicted by standard clinicopathological data • 31. 9% change in treatment recommendations • Oncotype DX® was shown to be consistently cost effective across different countries and is expected to generate cost savings

Conclusions • Despite the use of traditional markers, a proportion of patients are classified as intermediate risk and in whom treatment decisions are not clear • Recurrence Score® results reflects individual tumor biology • The risk of distant recurrence or chemotherapy benefit can't be accurately predicted by relying on conventional tools alone • Oncotype DX the only assay that has been demonstrated to be predictive of likelihood of benefit from chemotherapy allowing chemotherapy to be given to those most likely to benefit 2, 3 (Level I Evidence) • Only assay incorporated into ASCO®, NCCN® , ESMO, St Gallen and NICE guidelines • Oncotype DX® was shown to be consistently cost effective across and is expected to generate cost savings ASCO is a trademark of the American Society of Clinical Oncology and NCCN is a trademark of the National Comprehensive Cancer Network. ASCO and NCCN do not endorse any therapy or product. 1. Harris L, et al. J Clin Oncol. 2007; 33(25): 5287 -5312. 2. Paik S, et al. J Clin Oncol. 2006; 24: 3726 -3734; , 3. Albain et al. Lancet Oncol 2010; 11: 55 - 65 4. NCCN Practice Guidelines in Oncology. V. 3. 2013. 5. Goldhirsch A, et al. Ann Oncol. 2013. 6. NICE Diagnostics Guidance DG 10 2013.

Patient Cases
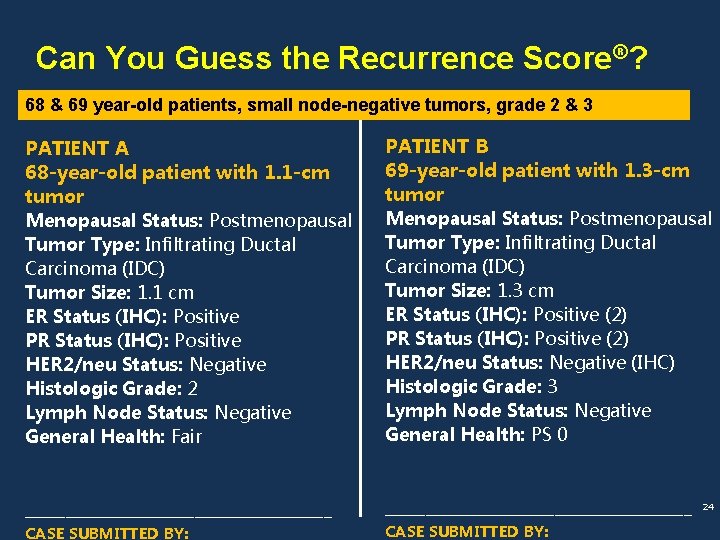
Can You Guess the Recurrence Score®? 68 & 69 year-old patients, small node-negative tumors, grade 2 & 3 PATIENT A 68 -year-old patient with 1. 1 -cm tumor PATIENT B 69 -year-old patient with 1. 3 -cm tumor Menopausal Status: Postmenopausal Tumor Type: Infiltrating Ductal Carcinoma (IDC) Tumor Size: 1. 1 cm ER Status (IHC): Positive PR Status (IHC): Positive HER 2/neu Status: Negative Histologic Grade: 2 Lymph Node Status: Negative General Health: Fair Menopausal Status: Postmenopausal Tumor Type: Infiltrating Ductal Carcinoma (IDC) Tumor Size: 1. 3 cm ER Status (IHC): Positive (2) PR Status (IHC): Positive (2) HER 2/neu Status: Negative (IHC) Histologic Grade: 3 Lymph Node Status: Negative General Health: PS 0 ______________________________________ CASE SUBMITTED BY: 24
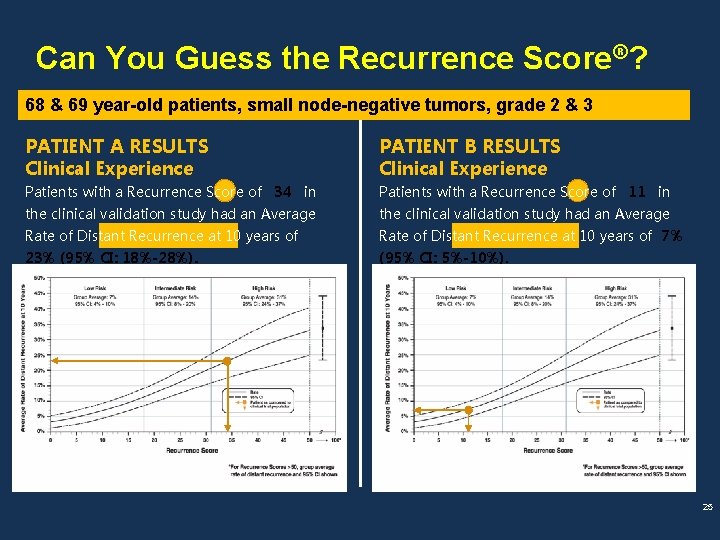
Can You Guess the Recurrence Score®? 68 & 69 year-old patients, small node-negative tumors, grade 2 & 3 PATIENT A RESULTS Clinical Experience PATIENT B RESULTS Clinical Experience Patients with a Recurrence Score of 34 in Patients with a Recurrence Score of 11 in the clinical validation study had an Average Rate of Distant Recurrence at 10 years of 7% 23% (95% CI: 18%-28%). (95% CI: 5%-10%). 25
Can You Guess the Recurrence Score®? 45 & 46 year-old patients, small node-negative tumors, grade 2 & 3 PATIENT A 45 -year-old patient with 0. 9 -cm tumor Menopausal Status: Premenopausal Tumor Type: Infiltrating Ductal Carcinoma (IDC) Tumor Size: 0. 9 cm ER Status (IHC): Positive (99%) PR Status (IHC): Positive (13%) HER 2/neu Status: Negative (1. 7 by FISH) Ki-67: 38% Histologic Grade: 2 Lymph Node Status: Negative (0/2 SLNs) PATIENT B 46 -year-old patient with 0. 7 -cm tumor Menopausal Status: Premenopausal Tumor Type: Infiltrating Ductal Carcinoma (IDC) Tumor Size: 0. 7 cm ER Status (IHC): Positive (91%) PR Status (IHC): Positive (99%) HER 2/neu Status: Negative (0. 7 by FISH) Ki-67: 35% Histologic Grade: 3 Lymph Node Status: Negative ______________________________________ CASE SUBMITTED BY: 26
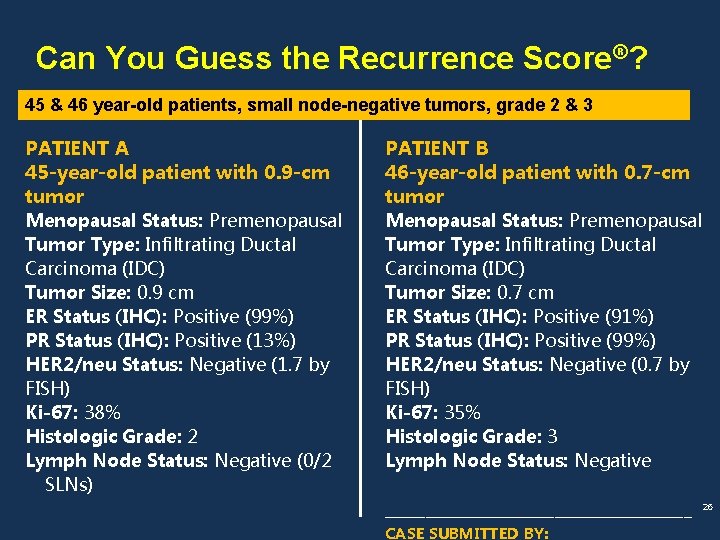
Can You Guess the Recurrence Score®? 45 & 46 year-old patients, small node-negative tumors, grade 2 & 3 PATIENT A RESULTS Clinical Experience PATIENT B RESULTS Clinical Experience Patients with a Recurrence Score of 15 in Patients with a Recurrence Score of 35 in the clinical validation study had an Average Rate of Distant Recurrence at 10 years of 10% (95% CI: 7%-12%). 24% (95% CI: 18%-30%). 27

Questions
The Oncotype DX® Report Provides Valuable Information Along a Continuum of ER+ Breast Cancer • The Oncotype DX report provides valuable information on: – Node-negative prognosis – Node-negative predicted chemotherapy benefit – Quantitative data on ER/PR/HER 2 • Node-positive report contains an additional page with prognosis and predicted chemo benefit information specific to node-positive patients 29

The Oncotype DX® Breast Cancer Assay • • • Quantitatively predicts the likelihood of breast cancer recurrence and assesses the benefit from both hormonal therapy and chemotherapy (Level I Evidence) High and low Recurrence Score® results reflect different intrinsic tumor biology You cannot predict the risk of distant recurrence or chemotherapy benefit by relying on clinical and pathological variables Changes treatment decisions based on traditional measures 37% of time, sparing patients the negative health and QOL impact of unnecessary chemotherapy and resulting in cost savings Only assay incorporated into ASCO®, NCCN® and St Gallen’s clinical practice guidelines Longest history of commercial genomic assays with over 200, 000 patients tested worldwide 30 ASCO is a trademark of the American Society of Clinical Oncology and NCCN is a trademark of the National Comprehensive Cancer Network. ASCO and NCCN do not endorse any therapy or product.
- Slides: 30