On Surrogate Endpoints in HIV Vaccine Efficacy Trials






- Slides: 24

On Surrogate Endpoints in HIV Vaccine Efficacy Trials FDA/Industry Statistics Workshop, Sept 18 -19, 2003 “Statistics: From Theory to Regulatory Acceptance” Steven Self, Peter Gilbert, Michael Hudgens FHCRC/UW

Outline 1. HIV Vaccine Trials: Current Status 2. Clinical Endpoints in Vaccine Trials 3. Endpoints in HIV Vaccine Trials 4. A Simulation Approach 1. Goal 2. Approach 3. Example 5. Conclusions/Discussion

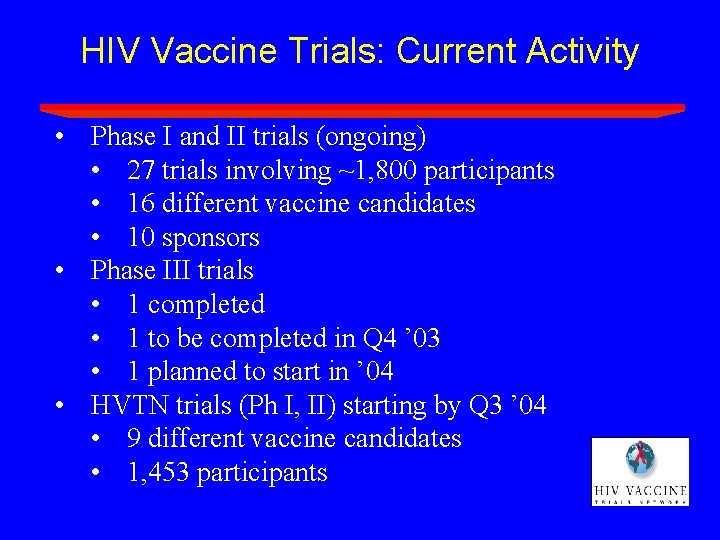
HIV Vaccine Trials: Current Activity • Phase I and II trials (ongoing) • 27 trials involving ~1, 800 participants • 16 different vaccine candidates • 10 sponsors • Phase III trials • 1 completed • 1 to be completed in Q 4 ’ 03 • 1 planned to start in ’ 04 • HVTN trials (Ph I, II) starting by Q 3 ’ 04 • 9 different vaccine candidates • 1, 453 participants

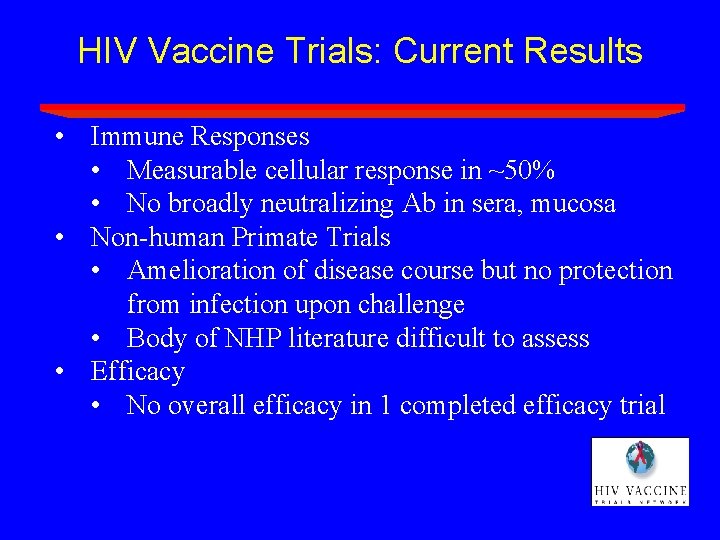
HIV Vaccine Trials: Current Results • Immune Responses • Measurable cellular response in ~50% • No broadly neutralizing Ab in sera, mucosa • Non-human Primate Trials • Amelioration of disease course but no protection from infection upon challenge • Body of NHP literature difficult to assess • Efficacy • No overall efficacy in 1 completed efficacy trial

HIV Vaccine Trials: Summary • Immune correlate of protection unknown • Many candidate vaccines but full range of desired immune responses poorly covered • Multiple efficacy trials will be required* • Plan for long-term, iterative development program* * Klausner et. al. (2003) Science

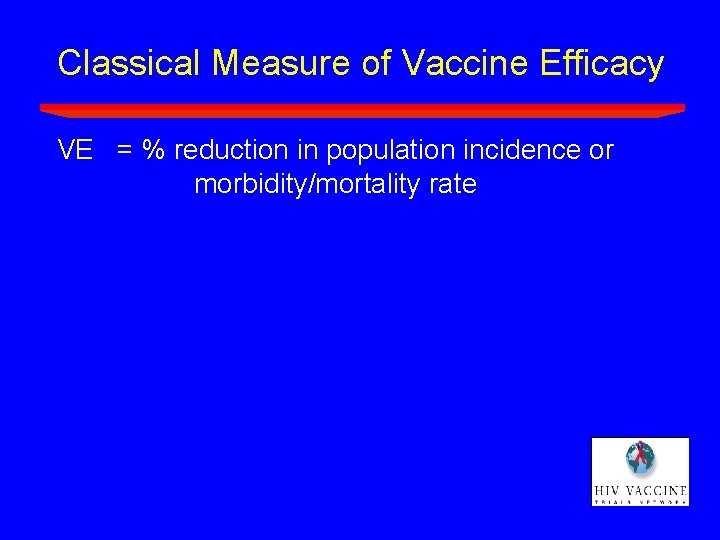
Classical Measure of Vaccine Efficacy VE = % reduction in population incidence or morbidity/mortality rate

Classical Endpoint for Vaccine Efficacy • • Clinically significant morbidity and mortality Pathogen specificity Standard of care For treatable infections: – Prevent/delay constellation of signs/symptoms sufficient to trigger treatment initiation (save cost/toxicity assoc with treatment) – Interact w/ treatment to improve risk/benefit profile of vaccine/tmt vs tmt alone

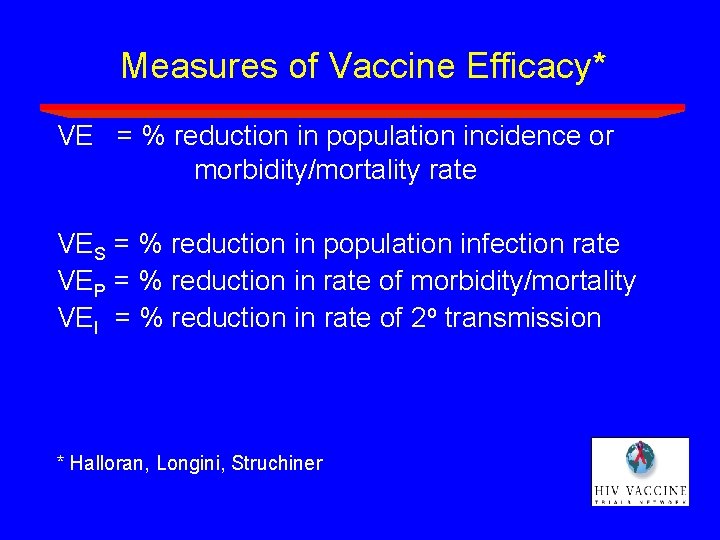
Measures of Vaccine Efficacy* VE = % reduction in population incidence or morbidity/mortality rate VES = % reduction in population infection rate VEP = % reduction in rate of morbidity/mortality VEI = % reduction in rate of 2 o transmission * Halloran, Longini, Struchiner

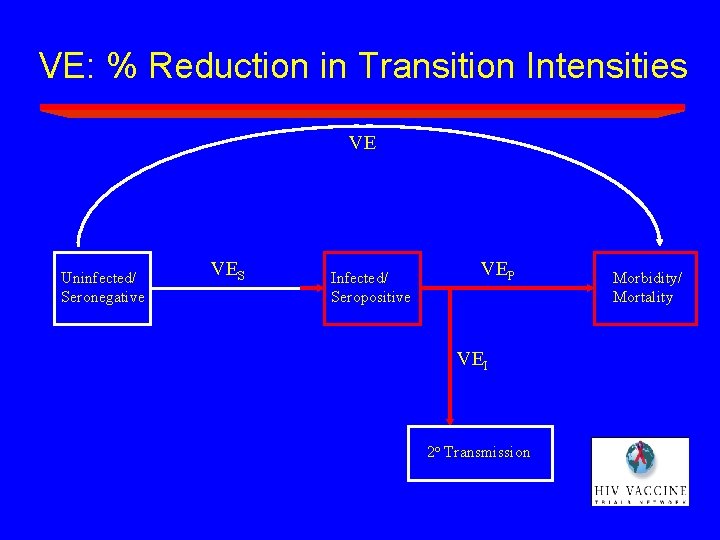
VE: % Reduction in Transition Intensities VE Uninfected/ Seronegative VES Infected/ Seropositive VEP VEI 2 o Transmission Morbidity/ Mortality

Endpoints in HIV Vaccine Efficacy Trials – “Infection” Endpoint (A biomarker-based surrogate) • • Operationally: presence of Ab and detectable HIV RNA Aligned with one primary objective of HIV vaccine Acceptable by all However captures only one aspect of potential vaccine effects on clinical outcomes

Endpoints in HIV Vaccine Efficacy Trials “Post-infection” Endpoints: Some Issues • Long-term FU required for morbidity/mortality endpoints esp with ARV treatment • Complicated dynamical process likely dominated by treatment effects • Uncertainty of optimal treatment initiation triggers • Variability in treatment initiation • Analytics – Key biomarker trajectories “dependently censored” by treatment initiation – Conditional vs unconditional analyses – Combination of analyses

Post-Infection Endpoints: Current Approach · Provide treatment within trial – standardized treatment initiation guidelines (e. g. DHHS, UNAIDS) – standardized treatment monitoring/management · Develop complementary array of endpoints to cover key aspects of post-infection outcomes – Early Endpoints - pre-ART – Mid-term Endpoints - peri-ART – Long-term Endpoints - post-ART · “Reasonable conservatism” for interpretation of vaccine effects on surrogates

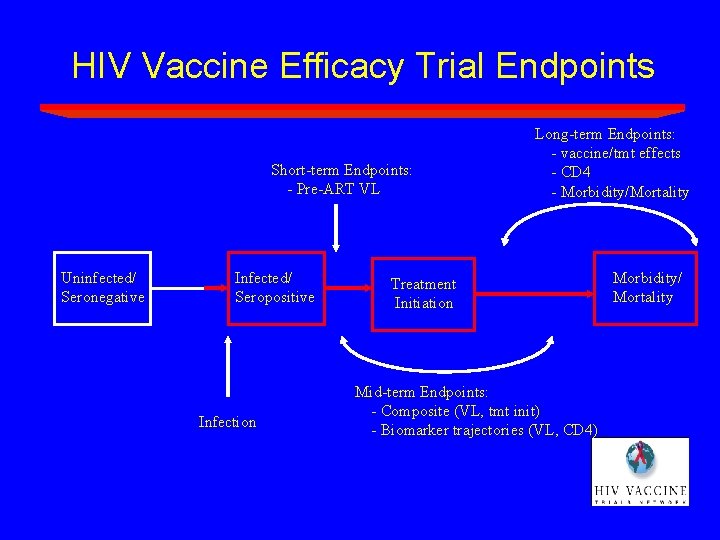
HIV Vaccine Efficacy Trial Endpoints Short-term Endpoints: - Pre-ART VL Uninfected/ Seronegative Infected/ Seropositive Infection Long-term Endpoints: - vaccine/tmt effects - CD 4 - Morbidity/Mortality Treatment Initiation Mid-term Endpoints: - Composite (VL, tmt init) - Biomarker trajectories (VL, CD 4) Morbidity/ Mortality

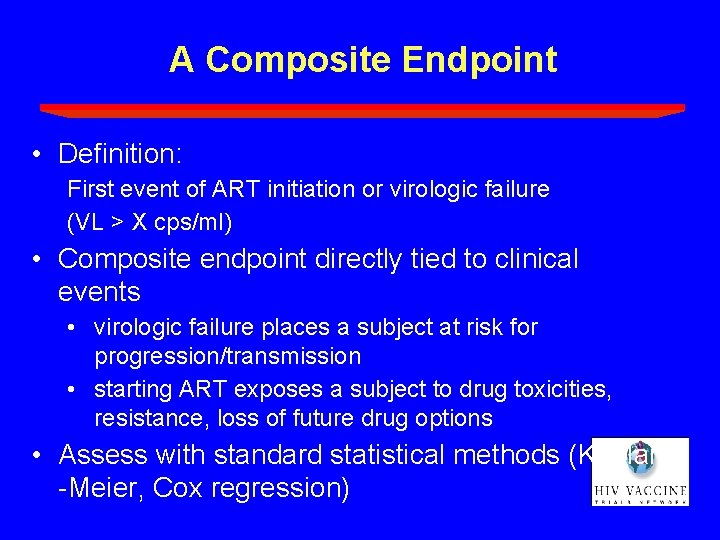
A Composite Endpoint • Definition: First event of ART initiation or virologic failure (VL > X cps/ml) • Composite endpoint directly tied to clinical events • virologic failure places a subject at risk for progression/transmission • starting ART exposes a subject to drug toxicities, resistance, loss of future drug options • Assess with standard statistical methods (Kaplan -Meier, Cox regression)

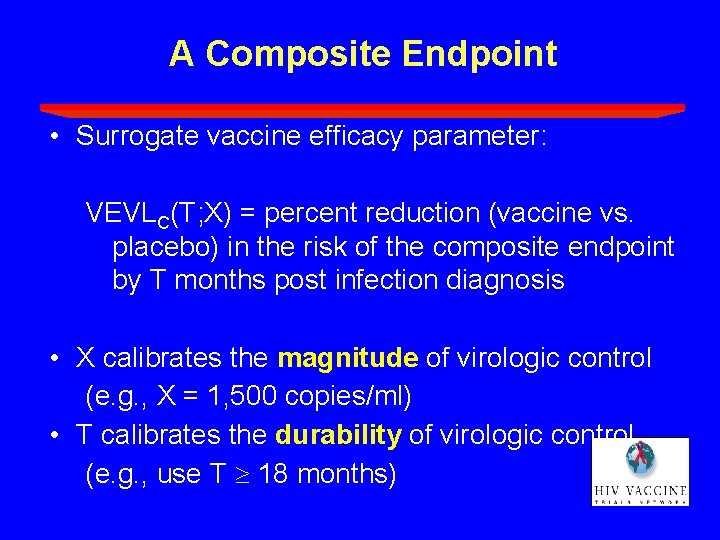
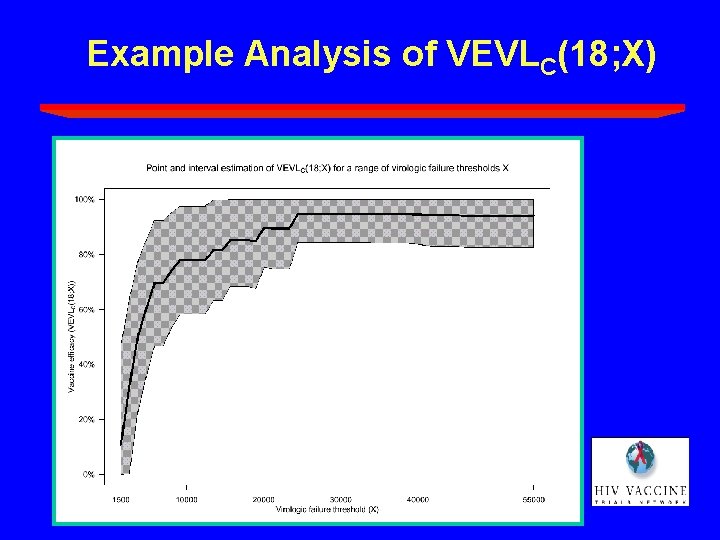
A Composite Endpoint • Surrogate vaccine efficacy parameter: VEVLC(T; X) = percent reduction (vaccine vs. placebo) in the risk of the composite endpoint by T months post infection diagnosis • X calibrates the magnitude of virologic control (e. g. , X = 1, 500 copies/ml) • T calibrates the durability of virologic control (e. g. , use T 18 months)


Example Analysis of VEVLC(18; X)

A Numerical Study*: Goal · Provide an approach to facilitate the discussion of how to use surrogate endpoints – specific to trial design – specific to particular surrogate endpoints – accommodate statistical uncertainties – accommodate model uncertainties with desired degree of conservatism * Gilbert et al (2003) JID

A Numerical Study: Approach · adopt empirically-based joint model of biomarker process and clinical outcomes as “true” prediction model* · modify model to incorporate degrees of “reasonable conservatism” – proportion vaccine effect explained** (attenuate log RR relating surrogate to clinical outcome by f percent ) – selection bias for conditional analyses*** (attenuate observed vaccine effect on surrogate outcome) · RCT simulation to identify minimum observed effects on specific surrogate endpoints that would generate 95% prediction intervals for VE parameters exceeding 40%, say * Albert et al (1998) Stat in Med ** Freedman et al (1992) Stat in Med *** Hudgens et al (2003) Stat in Med; Gilbert et al (2003) Biometrics

Numerical Study: An Example • Question: • What inference on VEVLC(18; X) “reasonably” predicts a clinically significant VEP? • Numerical study based on the following predictions: • from the MACS*: Predicted(VEP) = VEVLC(18; X) for X 5, 000 -10, 000 cps/ml * Albert et al (1998) Stat in Med

Hypothetical Efficacy Trial The numerical study is based on the following hypothetical trial:

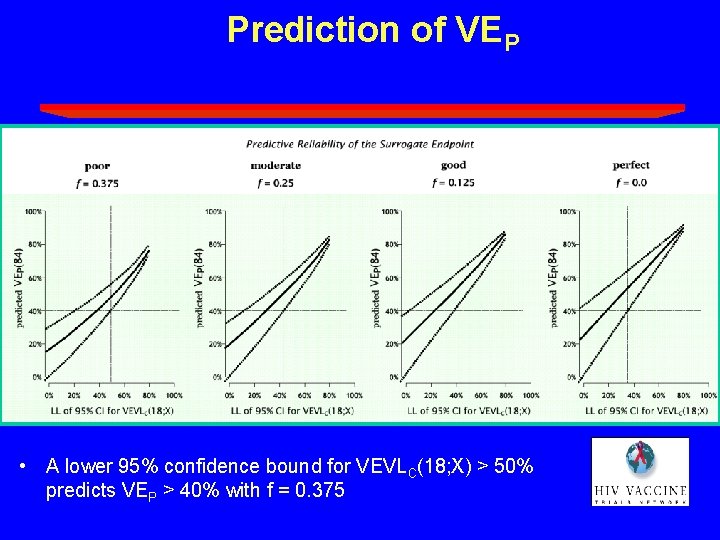
Prediction of VEP • A lower 95% confidence bound for VEVLC(18; X) > 50% predicts VEP > 40% with f = 0. 375

Summary/Conclusions · Use of surrogate endpoints in HIV vaccine efficacy trials is question of how not whether · A framework is proposed to help interpret observed effects on surrogate endpoints that is – specific to particular trial designs/endpoints – captures relevant aspects of magnitude and durability of effect on surrogates – uses available empirical information relating biomarkers to clinical outcomes – is tunable with respect to degree of conservatism w/r/t use of empirical information – flexible to evolve with development program

Summary/Conclusions · HIV vaccines showing strong and durable effects on post-infection endpoints should be licensed – use of standardized ART guidelines important – use simulation studies to assist in building agreement about defining “sufficiently strong” and “sufficiently durable” – design trials to detect significant levels of either VES or VEVLC(T; X) – use supporting data on other endpoints · Long-term follow-up needed – for assessing VE and VEP directly – better understanding of surrogate endpoints