Oleh Sri Juari Santosa Jurusan Kimia FMIPA UGM



![Hydrotalcite (layered double hydroxide anionic clay) 1) General formula: [M 2+1 -x. M 3+x(OH)2]x+(An-)x/n. Hydrotalcite (layered double hydroxide anionic clay) 1) General formula: [M 2+1 -x. M 3+x(OH)2]x+(An-)x/n.](https://slidetodoc.com/presentation_image_h/fb786f0ff327a4026e70511f28f5a33b/image-7.jpg)















- Slides: 35

Oleh Sri Juari Santosa Jurusan Kimia FMIPA UGM

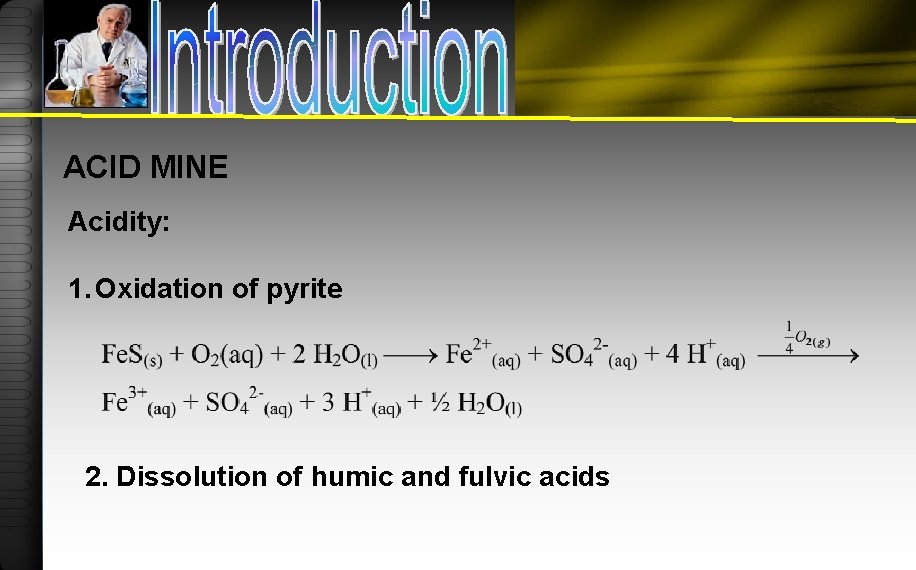
ACID MINE Acidity: 1. Oxidation of pyrite 2. Dissolution of humic and fulvic acids

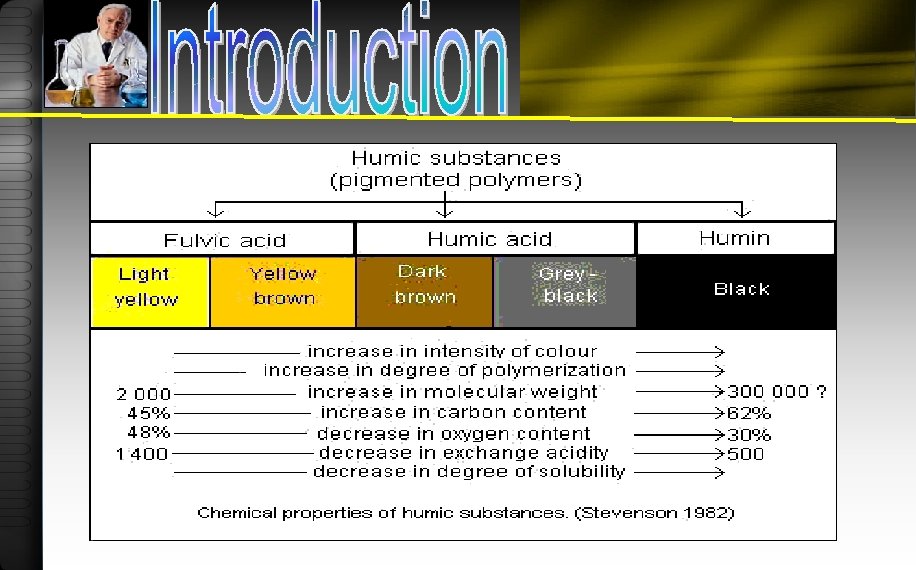
Humic Substances are traditionally fractionated according to their solubilities ØFulvic acids: are those organic materials that are soluble in water at all p. H values ØHumic acids: are those materials that are insoluble at acidic p. H values (p. H < 2) but are soluble at higher p. H values ØHumin: is the fraction that is insoluble in water at all p. H values


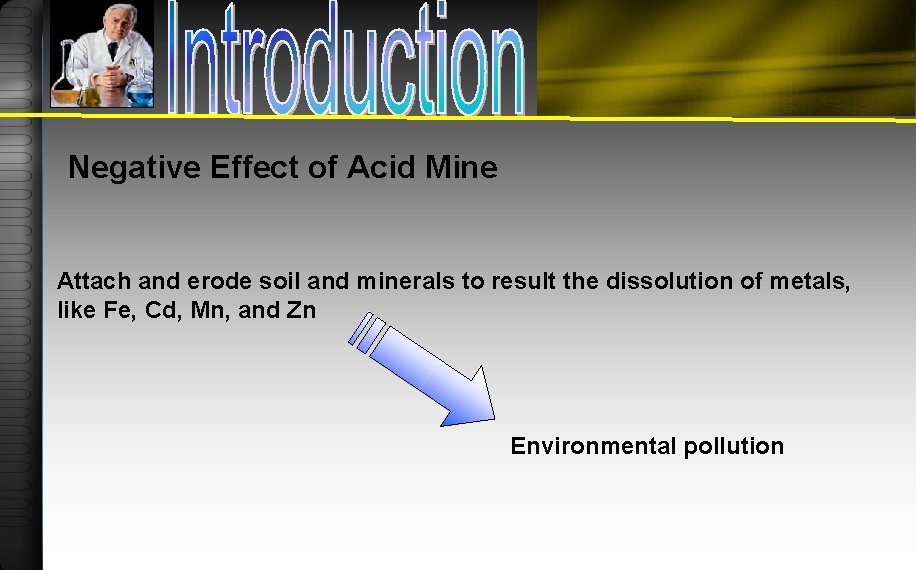
Negative Effect of Acid Mine Attach and erode soil and minerals to result the dissolution of metals, like Fe, Cd, Mn, and Zn Environmental pollution

Utilization of Hydrotalcite to remediate acid mine Study on the ability of Mg/Al hydrotalcite in neutralize the acidity through exchanging its interlayer hydroxide (OH-), carbonate (CO 32 -), and bicarbonate (HCO 3 -) anions with polluting anions of SO 42 -, humic acid (HA), and fulvic acid (FA) in aqueous solution
![Hydrotalcite layered double hydroxide anionic clay 1 General formula M 21 x M 3xOH2xAnxn Hydrotalcite (layered double hydroxide anionic clay) 1) General formula: [M 2+1 -x. M 3+x(OH)2]x+(An-)x/n.](https://slidetodoc.com/presentation_image_h/fb786f0ff327a4026e70511f28f5a33b/image-7.jpg)
Hydrotalcite (layered double hydroxide anionic clay) 1) General formula: [M 2+1 -x. M 3+x(OH)2]x+(An-)x/n. y. H 2 O where: M 2+ is divalent cation M 3+ is trivalent cation An- is interlayer anion (1 -x/x) varies from 1 to 5 2) Structure:

No Name Empirical formula Crystal system 1 Barbertonite Not Radioactive Mg 6 Cr 2(CO 3)(OH)16· 4(H 2 O) Molecular Weight = 654. 01 gm Hexagonal Dihexagonal Dipyramidal 2 Bechererite Not Radioactive Zn 4. 5 Cu 1. 5 Zn 2(OH)132(S 0. 75)2(Si 0. 25)2(O 3)2(OH) Trigonal Pyramidal 3 Carbonatecyanotrichite Not Radioactive Cu 4 Al 2(CO 3)0. 66(SO 4)0. 34(OH)12· 2(H 2 O) Molecular Weight = 620. 53 gm Orthorhombic Widowmaker mine, Fry Canyon, San Juan Co. , Utah, USA 4 Caresite Not Radioactive Fe 2+4 Al 2(CO 3)(OH)12· 3(H 2 O) Molecular Weight = 595. 49 gm Trigonal Trapezohedra l Poudrette quarry, Mt Saint. Hilaire, Rouville Co. , Québec, Canada 5 Carrboydite Not Radioactive Ni 10 Cu 4 Al 9(SO 4)4(CO 3)2(OH)43· 7(H 2 O) Molecular Weight = 2, 445. 61 gm Hexagonal Carr-Boyd Nickel Mine, Western Australia, Australia 6 Chalcoalumite Not Radioactive Cu. Al 4(SO 4)(OH)12· 3(H 2 O) Molecular Weight = 525. 67 gm Monoclinic – Sphenoidal Lavender Pit, Bisbee, Cohchise County, Arizona, USA 7 Charmarite-2 H & Charmarite-3 T Not Radioactive Mn 2+4 Al 2(CO 3)(OH)12· 3(H 2 O) Molecular Weight = 591. 86 gm Hexagonal – Trapezohedra l Molecular Weight = 933. 60 gm Image Origin Nevada Creek, Tasmania, Australia Color: Light green Color: Orange brown, Pale blue, Colorless Tonopah-Belmont mine, Osborne silver-gold district, Maricopa County, Arizona USA Mt. St. -Hilaire, QUE, Canada

8 Chlormagaluminite Not Radioactive Mg 3. 5 Fe 2+0. 5 Al 2(OH)12 Cl(CO 3)0. 5· 2(H 2 O) Molecular Weight = 472. 53 gm Hexagonal Color: Colorless, Yellow brown 9 Cyanotrichite Not Radioactive Cu 4 Al 2(SO 4)(OH)12· 2(H 2 O) Molecular Weight = 644. 33 gm Orthorhombic Grandview mine, Grand Canyon, Coconino County, Arizona, USA 10 Glaucocerinite Not Radioactive Zn 3 Cu 2 Al 3(SO 4)1. 5(OH)16· 9(H 2 O) Molecular Weight = 982. 56 gm Trigonal Mina Serpieri, Laurium, Attiki, Greece 11 Hydrombobomkulite Not Radioactive Ni 0. 75 Cu 0. 25 Al 4(NO 3)1. 5(SO 4)0. 5(OH)12· 14(H 2 O) Molecular Weight = 765. 17 gm Monoclinic 12 Hydrowoodwardite Not Radioactive Cu 0. 5 Al 0. 5(OH)2(SO 4)0. 25· 0. 75(H 2 O) Molecular Weight = 116. 81 gm Trigonal – Hexagonal Scalenohedral St Christoph Mine, Bärenhecke, Glashütte, Erzgebirge, Saxony, Germany 13 Manasseite Not Radioactive Mg 6 Al 2(CO 3)(OH)16· 4(H 2 O) Molecular Weight = 603. 98 gm Hexagonal – Dihexagonal Dipyramidal Jacupiranga mine, Jacupiranga, Sao Paolo, Brazil 14 Mbobomkulite Not Radioactive Ni 0. 75 Cu 0. 25 Al 4(NO 3)1. 5(SO 4)0. 5(OH)12· 3(H 2 O) Molecular Weight = 567. 00 gm Monoclinic – Sphenoidal. H Jomac Uranium mine, White Canyon, San Juan Co. , Utah, USA Color: Sky blue Kapaev explosion pipe, Angara River, southern Siberian Platform, Russia Mbobo Mkulu cave, Nelspruit district, South Africa

15 Nickelalumite Not Radioactive Ni 0. 7 Cu 0. 3 Al 4(SO 4)1. 5(NO 3)(OH)12· 3(H 2 O) Molecular Weight = 632. 31 gm Monoclinic – Sphenoidal Color: Sky blue Mbobo Mkulu cave, eastern Transvaal, South Africa 16 Quintinite-2 H Not Radioactive Mg 4 Al 2(OH)12(CO 3)· 4(H 2 O) Molecular Weight = 487. 34 gm Hexagonal – Trapezohedral Jacupiranga Mine, São Paulo, Southeast Region, Brazil 17 Quintinite-3 T Not Radioactive Mg 4 Al 2(OH)12(CO 3)· 4(H 2 O) Molecular Weight = 487. 34 gm Trigonal – Trapezohedral Jacupiranga Mine, São Paulo, Southeast Region, Brazil 18 Spangolite Not Radioactive Cu 6 Al(SO 4)(OH)12 Cl· 3(H 2 O) Molecular Weight = 797. 91 gm Trigonal – Ditrigonal Pyramidal Blanchard mine, Bingham, Socorro County, New Mexico, USA 19 Woodwardite Not Radioactive Cu 4 Al 2(SO 4)(OH)12· 3(H 2 O) Molecular Weight = 662. 34 gm Hexagonal Green River Formation, Uintah Co. , Utah, USA 20 Zaccagnaite Not Radioactive Zn 3. 9 Al 2. 1(OH)12(CO 3)· 3(H 2 O) Molecular Weight = 629. 83 gm Hexagonal – Dihexagonal Dipyramidal Hilarion Mine, Agios Konstantinos, Lavrion District, Attikí Prefecture, Greece 21 Zincaluminite Not Radioactive Zn 6 Al 6(SO 4)2(OH)26· 5(H 2 O) Molecular Weight = 1, 278. 62 gm Hexagonal Kamariza Mine, Laurium, Attiki, Greece

22 Zincowoodwardite Not Radioactive Zn 0. 47 Al 0. 38(OH)2(SO 4)0. 18(H 2 O)0. 6 Molecular Weight = 103. 10 gm Trigonal Rhombohedr al Hilarion Mine, Agios Konstantinos, Lavrion District, Attikí Prefecture, Greece 23 Zincowoodwardite-1 T Not Radioactive Zn 0. 66 B 0. 3 Al 0. 33(OH)2(SO 4)0. 155· 0. 96(H 2 O) Molecular Weight = 121. 50 gm Trigonal Rhombohedr al Color: Pale blue, Bluish white Laurion (Lavrion, Laurium), Greece 24 Zincowoodwardite-3 R Not Radioactive Zn 0. 5 B 0. 33 Al 0. 33(OH)2(SO 4)0. 2· 0. 59(H 2 O) Molecular Weight = 109. 02 gm Trigonal Hexagonal Scalenohedra l Color: Pale bluish white, Bluish white Laurion (Lavrion, Laurium), Greece

Synthesis of Mg/Al hydrotalcite 50 m. L mixed solution of Mg 2+ and Al 3+ p. H Na. OH 0, 5 M

Synthesis of Mg/Al hydrotalcite (continued) 120 o. C 7. 0 p. H 0 XRD IR

Stability Test 3; 5; 7; 9; p. H 11; 13 50 m. L Aqua dest 0. 1 g Mg/Al hydrotalcit e Shaker bath, 2 hours

Stability Test (countinued) 12 0 mg o. C 198

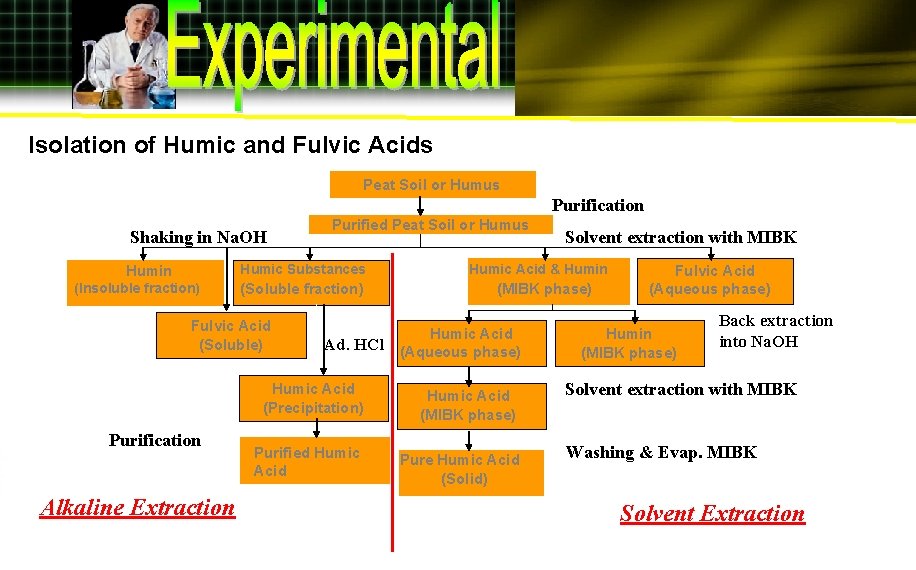
Isolation of Humic and Fulvic Acids Peat Soil or Humus Purification Shaking in Na. OH Purified Peat Soil or Humus Humic Substances Humin (Insoluble fraction) (Soluble fraction) Fulvic Acid (Soluble) Purification Alkaline Extraction Ad. HCl Solvent extraction with MIBK Humic Acid & Humin (MIBK phase) Humic Acid (Aqueous phase) Humic Acid (Precipitation) Humic Acid (MIBK phase) Purified Humic Acid Pure Humic Acid (Solid) Fulvic Acid (Aqueous phase) Humin (MIBK phase) Back extraction into Na. OH Solvent extraction with MIBK Washing & Evap. MIBK Solvent Extraction

Effect of Medium Acidity on the Adsorption 3; 5; 7; 9; 11; 13 p. H 50 m. L Humic acid 150 mg/L 0. 1 g Mg/Al hydrotalcite Shaker bath, 2 hours

Effect of Medium Acidity on the Adsorption (continued) separati on filtrate UV-Vis 400 nm Aads Vs p. H

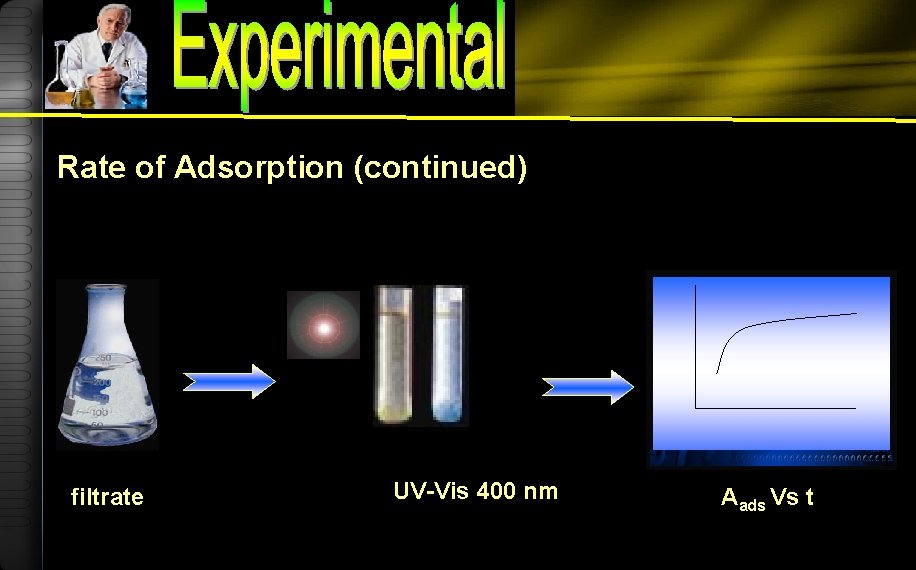
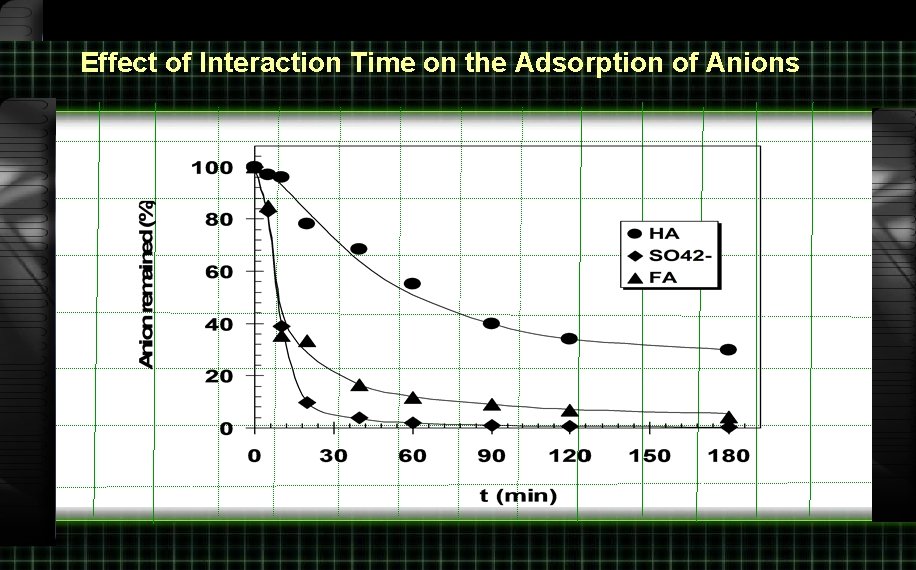
Rate of Adsorption 9. 00 10, 20, 40, 60, 180, 300 minut e 50 m. L Humic acid 150 mg/L 0. 1 g Mg/Al hydrotalcite Shaker bath

Rate of Adsorption (continued) filtrate UV-Vis 400 nm Aads Vs t

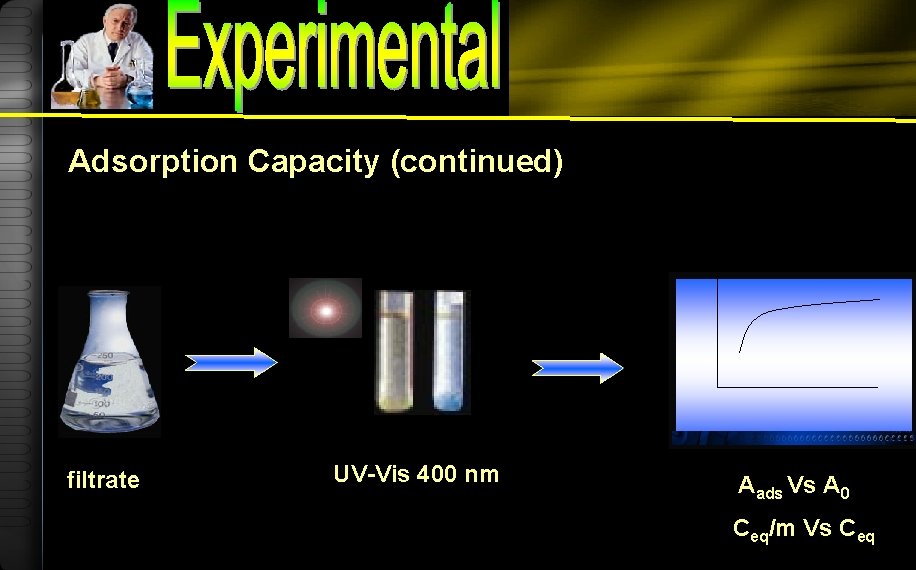
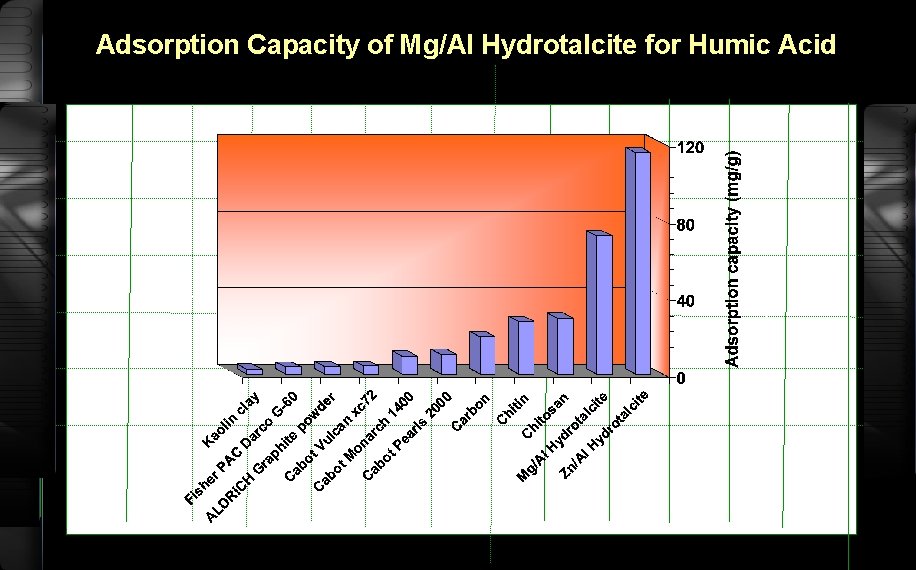
Adsorption Capacity 120, 150, 180, 210, 240 t optimum ppm 50 m. L Humic acid minute 0. 1 g Mg/Al hydrotalcite Shaker bath

Adsorption Capacity (continued) filtrate UV-Vis 400 nm Aads Vs A 0 Ceq/m Vs Ceq

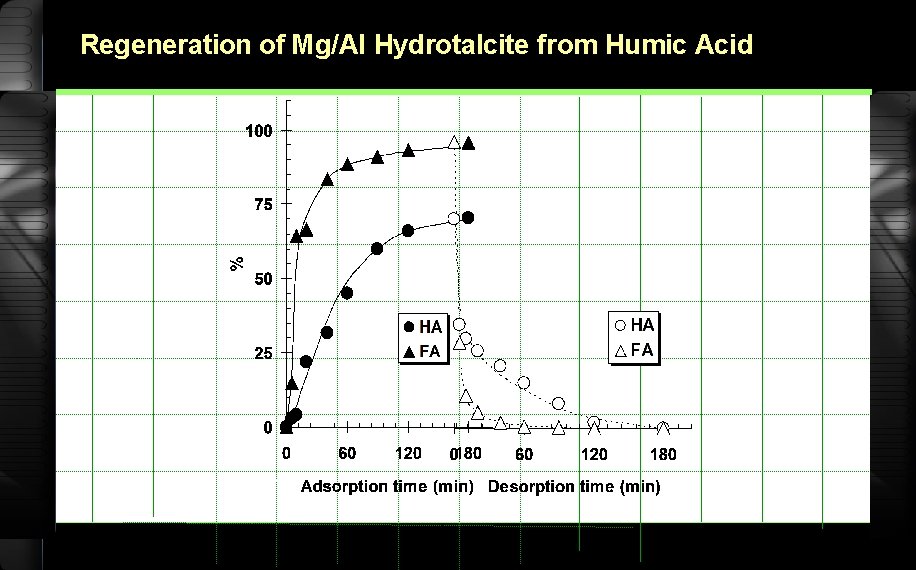
Desorption 5, 15, 30, 60, 120 minute 0. 01 g humic acid + Mg/Al hydrotalcite 25 m. L Na. OH 0. 5 M Shaker bath separation

Desorption (continued) filtrate UV-Vis 400 nm % Elution Vs t


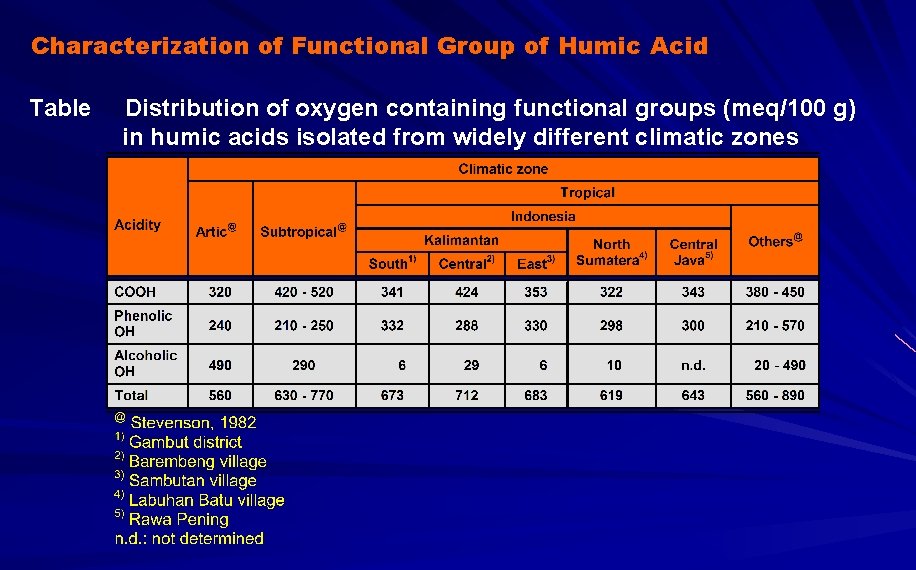
Characterization of Functional Group of Humic Acid Table Distribution of oxygen containing functional groups (meq/100 g) in humic acids isolated from widely different climatic zones

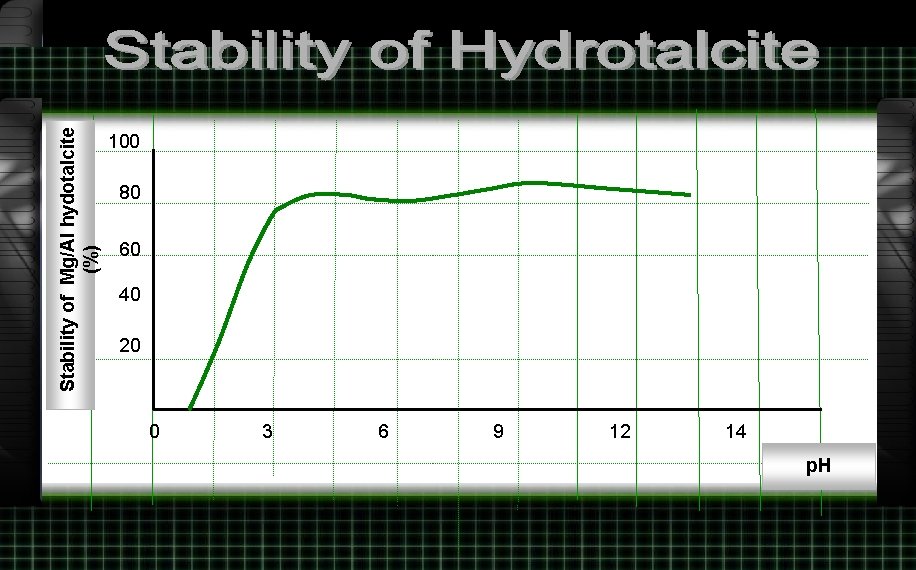
Stability of Mg/Al hydotalcite (%) 100 80 60 40 20 0 3 6 9 12 14 p. H

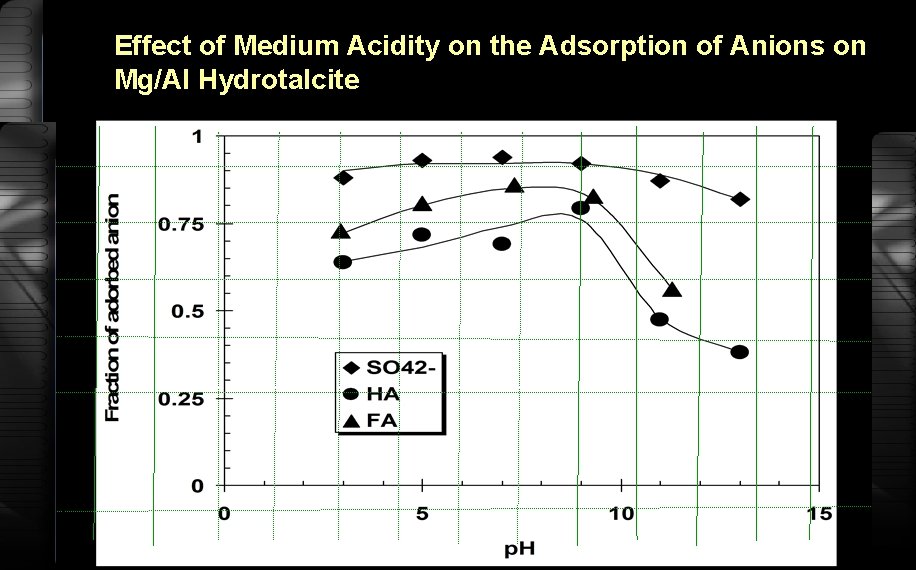
Effect of Medium Acidity on the Adsorption of Anions on Mg/Al Hydrotalcite

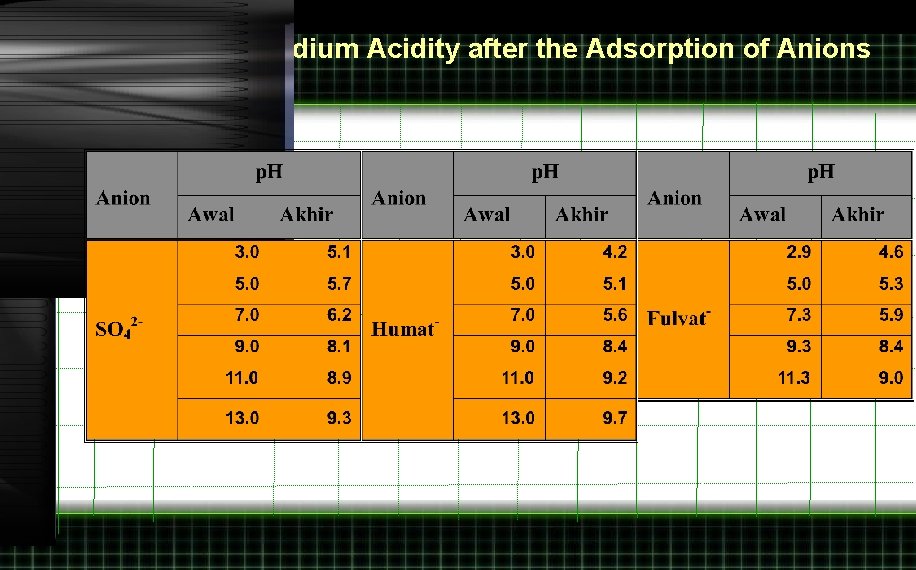
The Change of Medium Acidity after the Adsorption of Anions

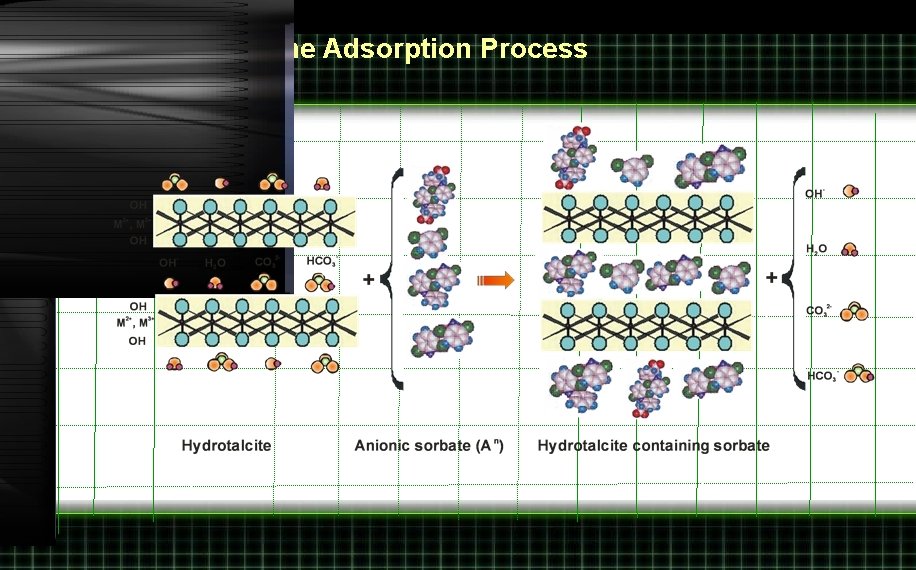
Interpretation of the Adsorption Process

Effect of Interaction Time on the Adsorption of Anions

Adsorption Capacity of Mg/Al Hydrotalcite for Humic Acid

Regeneration of Mg/Al Hydrotalcite from Humic Acid

Mg/Al hydrotalcite is a very potential adsorbent for the remediation of acid mine since: 1. Easy to be synthesized 2. Highly stable at wide range of medium acidity 3. Highly adsorb anions occurring in acid mine 4. Increasing the p. H of medium to close to neutral 5. Easy to be regenerated Implication: 1. Dissolution of metals from soil and minerals in acid mine may be minimized 2. The desorption product may be used to ameliorant

 Sri juari santosa
Sri juari santosa Sri rama sri rama sri manoharama
Sri rama sri rama sri manoharama Dekan fmipa unej
Dekan fmipa unej Made hery santosa
Made hery santosa Maliki heru santosa
Maliki heru santosa Dwi andreas santosa
Dwi andreas santosa Langgeng wahyu santosa
Langgeng wahyu santosa Zulaela ugm
Zulaela ugm Mun ugm
Mun ugm Architecury
Architecury Indwiani astuti
Indwiani astuti Contoh indikator kegiatan
Contoh indikator kegiatan Joko waluyo ugm
Joko waluyo ugm Abdul rohman ugm
Abdul rohman ugm Sps ugm
Sps ugm Matkul matematika ugm
Matkul matematika ugm úgm
úgm Ugm logo
Ugm logo Internet ugm
Internet ugm Animasixxx
Animasixxx Jurusan psikologi di medan
Jurusan psikologi di medan Jurusan it
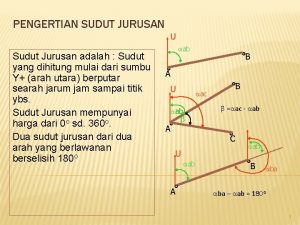
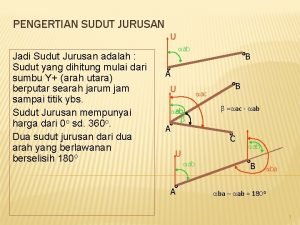
Jurusan it Sudut jurusan
Sudut jurusan Jurusan akamigas balongan
Jurusan akamigas balongan Jurusan akuntansi upi
Jurusan akuntansi upi Lobbying public relations
Lobbying public relations Jurusan penelitian dan evaluasi pendidikan
Jurusan penelitian dan evaluasi pendidikan Smkn 2 trenggalek
Smkn 2 trenggalek Jurusan digital forensik
Jurusan digital forensik Albiq
Albiq Jurusan event management
Jurusan event management Sudut jurusan adalah
Sudut jurusan adalah Apakah khodam itu musyrik
Apakah khodam itu musyrik Sistem informasi universitas gunadarma
Sistem informasi universitas gunadarma Jurusan agribisnis di medan
Jurusan agribisnis di medan Proteksi tanaman unand
Proteksi tanaman unand