Official Prescription Program 2 Prescription Drug Reform Act


























- Slides: 28


Official Prescription Program 2

Prescription Drug Reform Act 2012

Why Electronic Prescribing?

Electronic Prescribing

Electronic Prescribing • • 6

Electronic Prescribing Exceptions • Technological or electrical failure; • Use of EPCS would impact the patient’s medical condition (up to 5 day supply); • Issued by a practitioner to be dispensed outside of New York State; • Veterinarians; • Practitioners who have received a waiver from the Department of Health. 7

Electronic Prescribing Waivers • Practitioners may apply for a waiver from the requirement to electronically prescribe controlled substances. • Waivers will be granted upon a proper showing of economic hardship, technological limitations outside of the practitioner’s control or other exceptional circumstances. • By statute, waivers are good for one year, after which a practitioner may apply for a renewal. 8

Electronic Prescribing for Controlled Substances (EPCS) DEA Rule Overview

EPCS—DEA RULE Background • http: //www. deadiversion. usdoj. gov/fed_regs/rules/2010/fr 0331. pdf

EPCS—DEA Rule Background

EPCS—Practitioner Requirements

EPCS—DEA Rule Institutional Practitioner • 21 CFR Part 1311

EPCS—Pharmacy Application Requirements

EPCS—Pharmacy Application Requirements

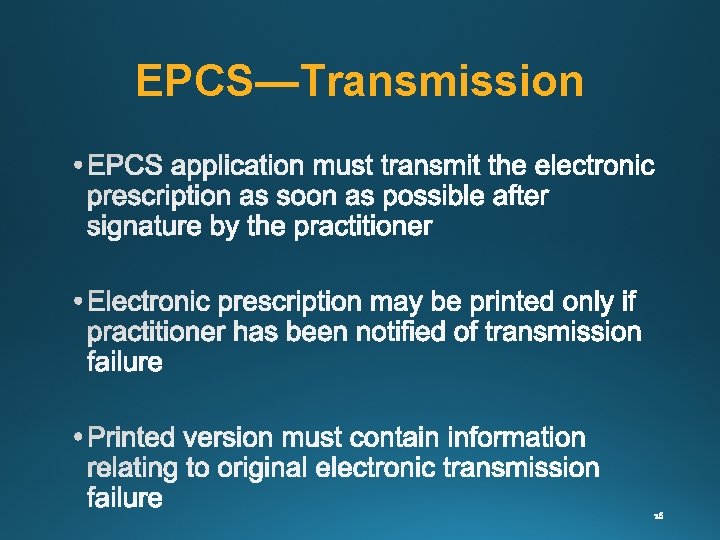
EPCS—Transmission

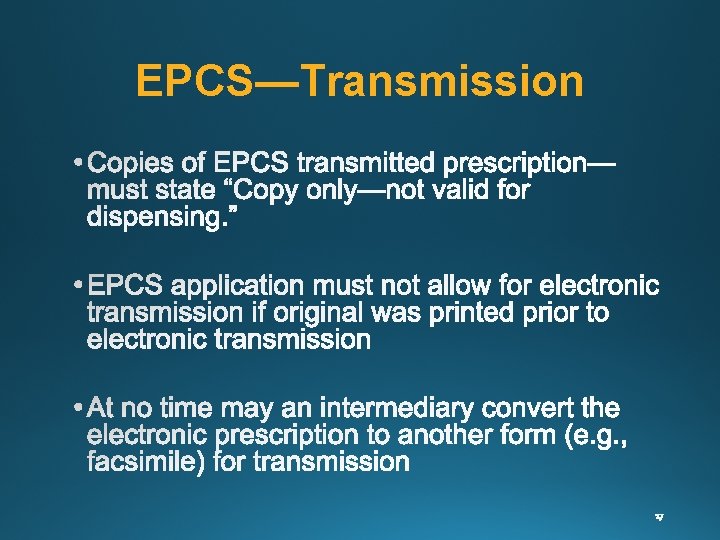
EPCS—Transmission

Electronic Prescribing NYS Regulations

Electronic Prescribing NYS Regulations

Electronic Prescribing for Controlled Substances (EPCS) • Prescribing and dispensing application must meet security standards set forth by the DEA for EPCS. • Certified computer application must be registered with BNE. • Two-factor authentication for prescribers to ensure prescription integrity and non-repudiation. • http: //www. deadiversion. usdoj. gov/fed_regs/rules/ 2010/fr 0331. pdf 20

EPCS—NYS Regulations

EPCS—NYS Regulations

EPCS—NYS Regulations

EPCS—NYS Regulations

What’s Next

EPCS—Take Aways

Regulations http: //www. deadiversion. usdoj. gov/fed_regs/rules/2010/fr 03 31. pdf http: //www. health. ny. gov/professionals/narcotic/electroni c_prescribing/ New York State Regulations related to Electronic Prescribing of Controlled Substances

Bureau of Narcotic Enforcement narcotic@health. state. ny. us 28