OFFICE OF CLINICAL RESEARCH The UF CTSI is







- Slides: 11

OFFICE OF CLINICAL RESEARCH The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

For up-to-date information: OCR http: //clinicalresearch. ctsi. ufl. edu The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

(1) Minimize Study Start-Up Time (2) Reduce Administrative Burden (3) Improve Financial Performance (1) Customer Satisfaction (2) Research Advocacy (3) Transparency (4) Efficiency (1) Electronic Submission (2) On. Core (3) Facilitative Process (4) Elimination of Redundant Submissions and Signatures The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH. OCR GOALS

OCR SERVICES On. Core Training and Management of the clinical trials management system, including building study records Study Navigation Intake process determination of needed services and supports Clinical Trials Budgeting Budget negotiation strategy, service, and budget review Assessment of qualifying status, completion of billing plan, Research Billing Compliance documentation in On. Core and Epic Contracting Service-oriented contracting officers that focus entirely on clinical research Clinicaltrials. gov Registration, maintenance and results reporting for investigator. Integrations with Clinical Applications Sponsor Invoicing initiated clinical trials Integrations with UF Health (Epic), Investigational Drug Service (Vestigo) and Electronic Data Capture (REDCap) Monitoring invoiceable study activity, sponsor invoicing, accounts receivable collection, and financial reconciliation The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

CLINICAL TRIALS REPORTING New opportunities for portfolio-based clinical trials reporting: § Number of studies § Enrollment tracking § Budgeting and financial performance

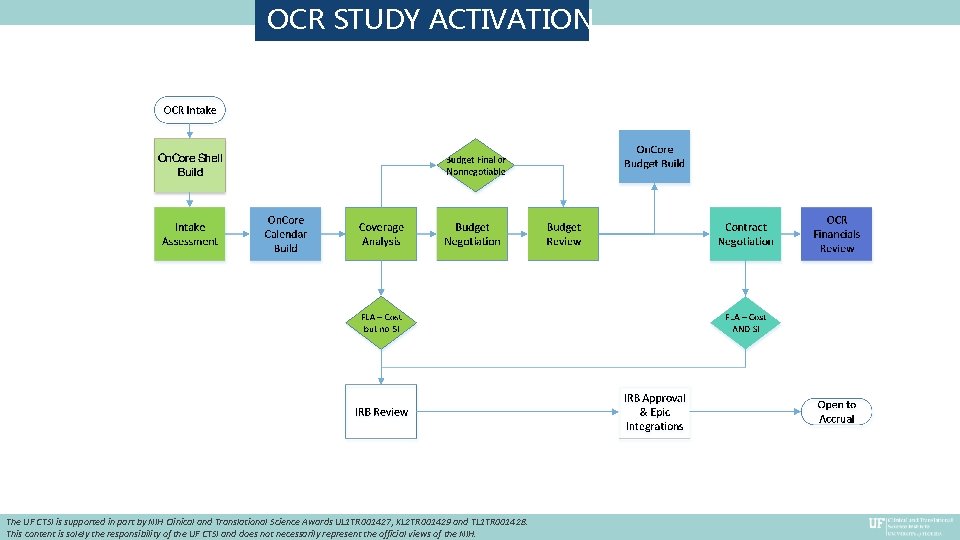
OCR STUDY ACTIVATION The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.

Thank you Katie Eddleton Associate Director, Operations Office of Clinical Research keddleton@ufl. edu The UF CTSI is supported in part by NIH Clinical and Translational Science Awards UL 1 TR 001427, KL 2 TR 001429 and TL 1 TR 001428. This content is solely the responsibility of the UF CTSI and does not necessarily represent the official views of the NIH.
 Ctsi
Ctsi Academic research building uf
Academic research building uf Readyset ohsu
Readyset ohsu Society of clinical research associates
Society of clinical research associates Dr charlotte lemech
Dr charlotte lemech Research design in clinical psychology
Research design in clinical psychology Pi clinical research consultancy
Pi clinical research consultancy Good documentation practices in clinical research
Good documentation practices in clinical research Clinical research statistician
Clinical research statistician Diabetic retinopathy clinical research network
Diabetic retinopathy clinical research network Kavi institute of clinical research
Kavi institute of clinical research Aros klinik
Aros klinik