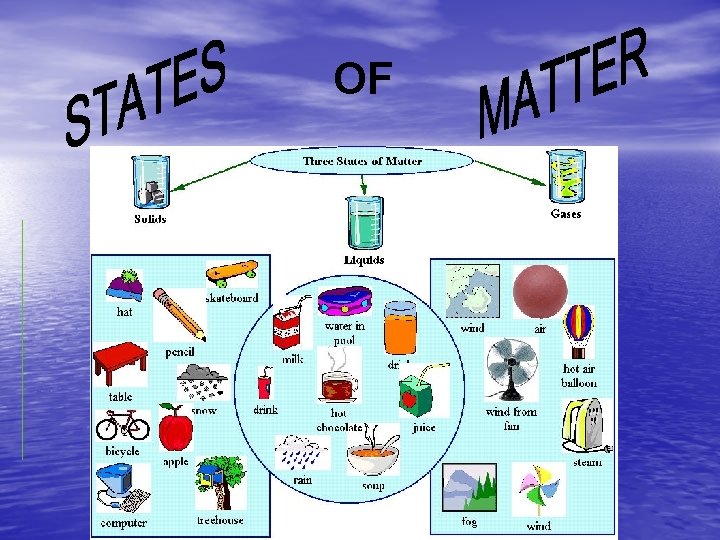
OF STATES OF MATTER The Four States of














- Slides: 29

OF

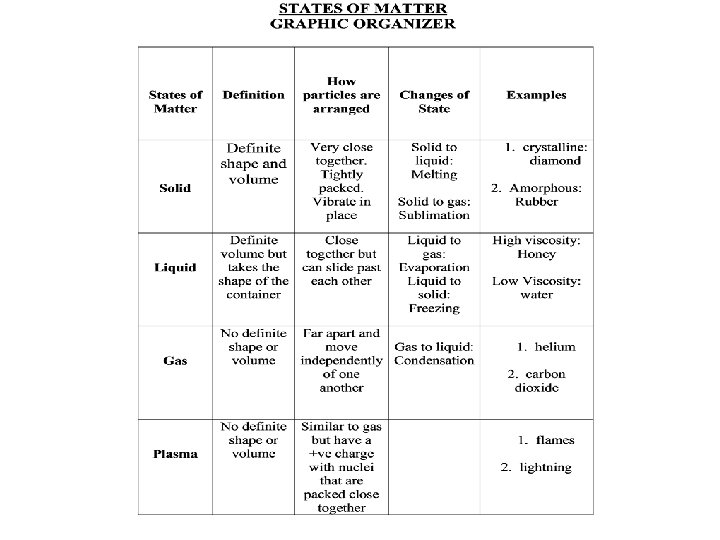
STATES OF MATTER • The Four States of Matter • Solid • Liquid • Gas • Plasma

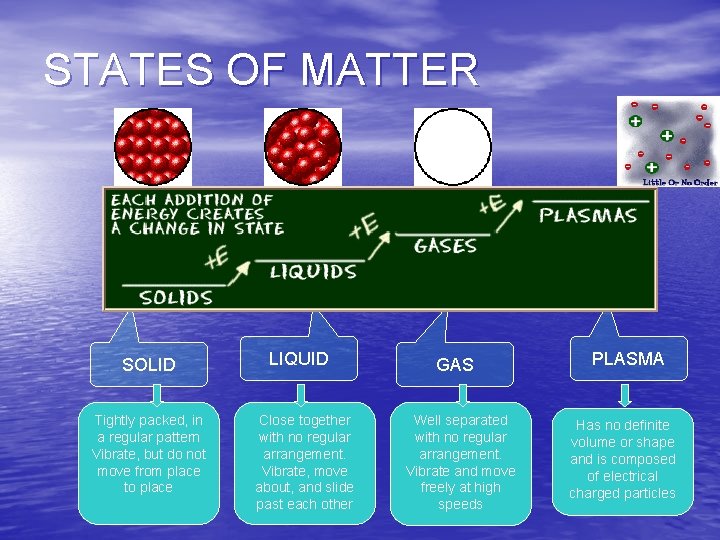
STATES OF MATTER ØBased upon particle arrangement ØBased upon energy of particles ØBased upon distance between particles

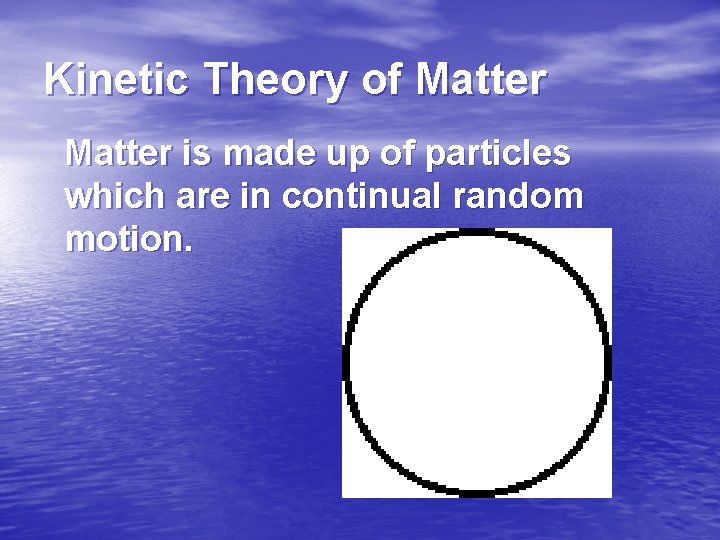
Kinetic Theory of Matter is made up of particles which are in continual random motion.

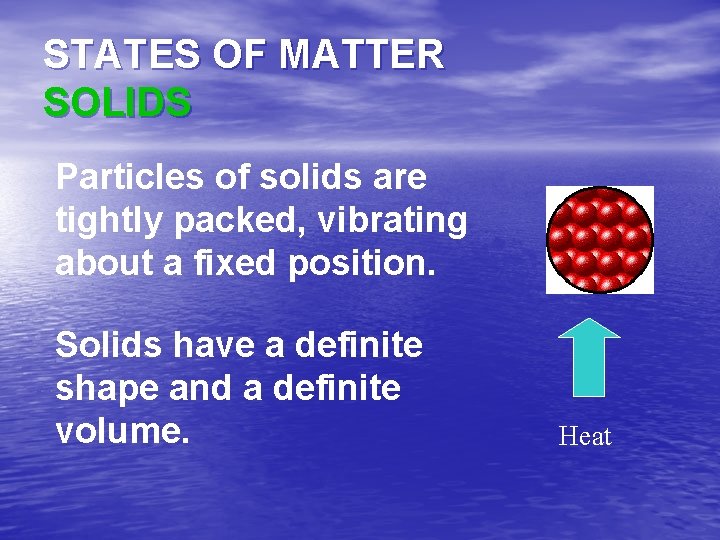
STATES OF MATTER SOLIDS Particles of solids are tightly packed, vibrating about a fixed position. Solids have a definite shape and a definite volume. Heat

Two Types of Solids Crystalline Amorphous

Crystalline Solids The molecules of crystalline solids are arranged in repeating symmetrical patterns. • Metals • Minerals such as diamonds • Salts • Ice

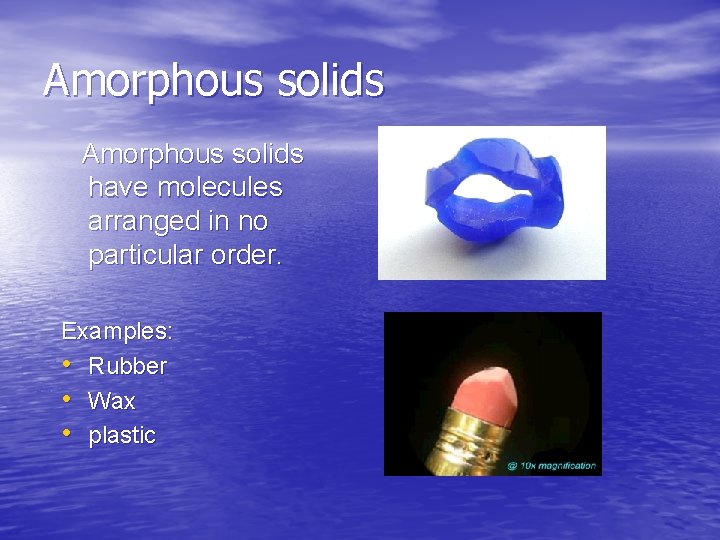
Amorphous solids have molecules arranged in no particular order. Examples: • Rubber • Wax • plastic

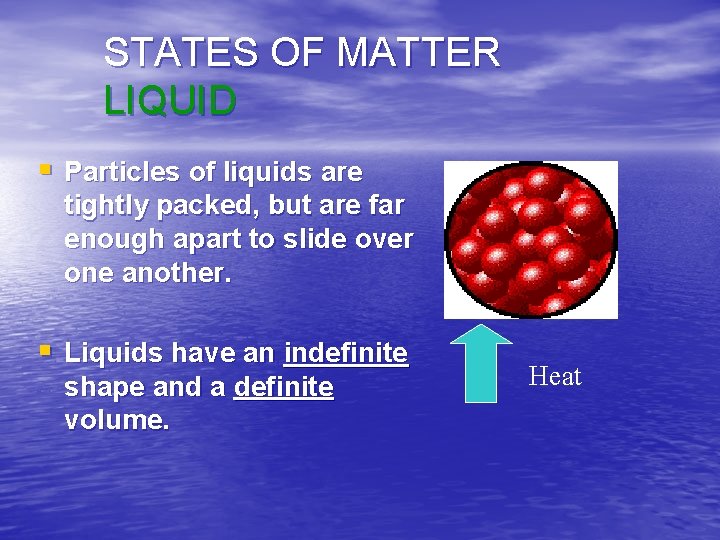
STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. Heat

Two properties of liquids • Surface tension It’s why water forms round drops • Viscosity Its why some liquids flow faster than others

Surface tension • An attractive force exists between molecules of liquids. • This force causes the liquid to form a curved surface or a round drop. • This force varies among different liquids. The greater the surface tension, the greater the curve, or the rounder the drop.

Viscosity • The force of attraction • • between liquid molecules causes the liquid to resist flowing. High viscosity liquids flow slowly examples: lava, honey Low viscosity liquids flow faster examples: water, vinegar

STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. Heat

States of Matter Can you identify the three states of matter in this picture?

But what happens if you raise the temperature to super-high levels…between 1000°C and 1, 000, 000°C ?

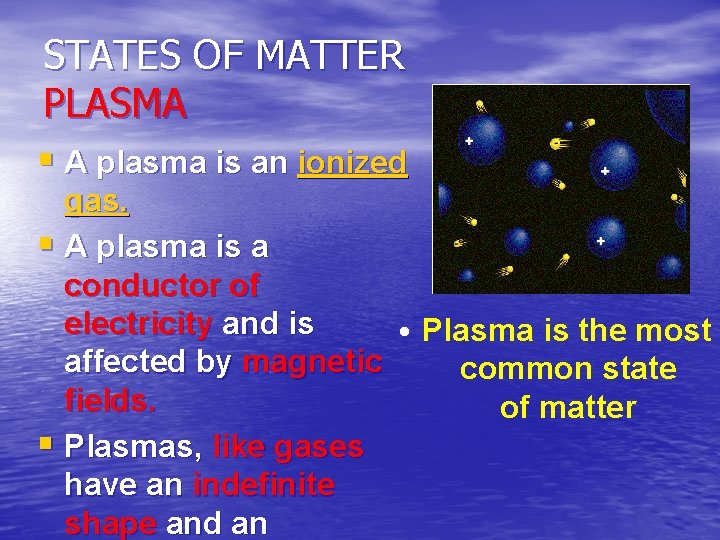
STATES OF MATTER PLASMA § A plasma is an ionized gas. § A plasma is a conductor of electricity and is • Plasma is the most affected by magnetic common state fields. of matter § Plasmas, like gases have an indefinite shape and an

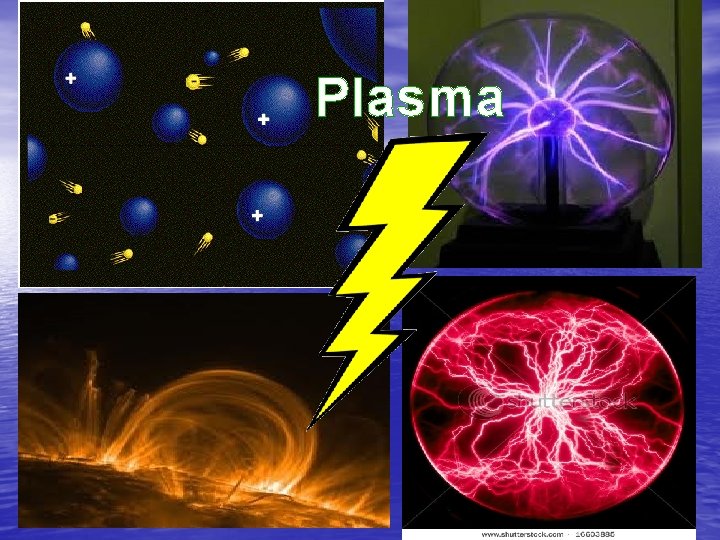
Where plasmas are found… 1. Flames

2. Lightning

3. AURORA (NORTHERN LIGHTS)

The Sun … An example of a star in its plasma state

Plasma


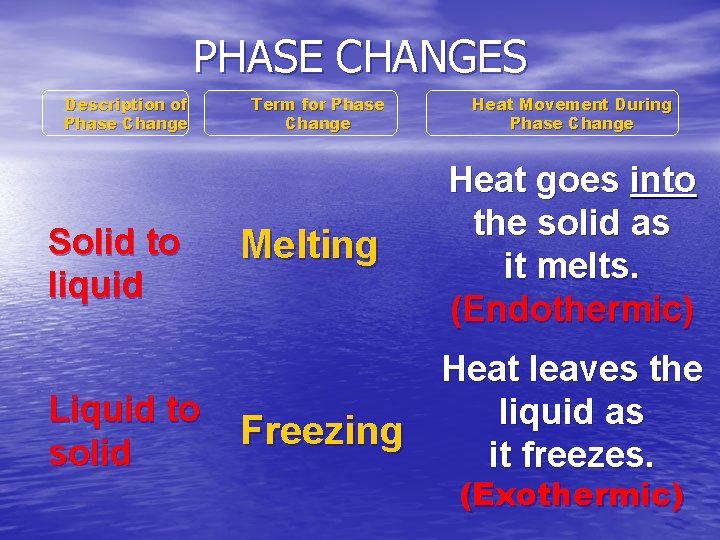
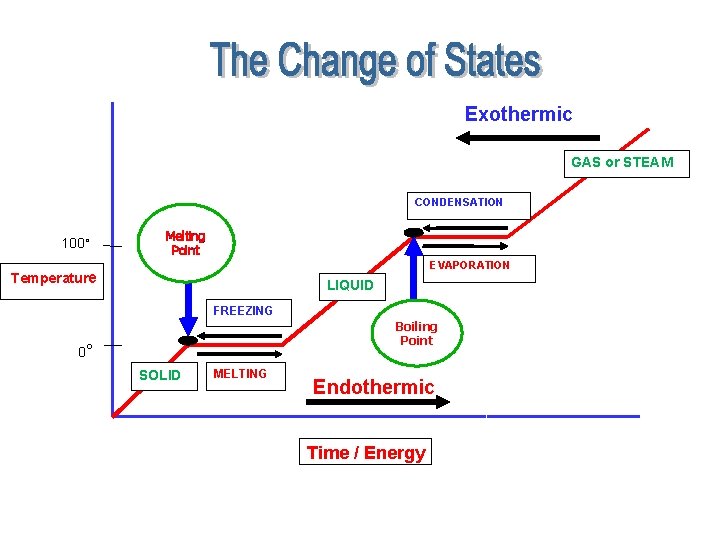
PHASE CHANGES Description of Phase Change Solid to liquid Liquid to solid Term for Phase Change Melting Heat Movement During Phase Change Heat goes into the solid as it melts. (Endothermic) Heat leaves the liquid as Freezing it freezes. (Exothermic)

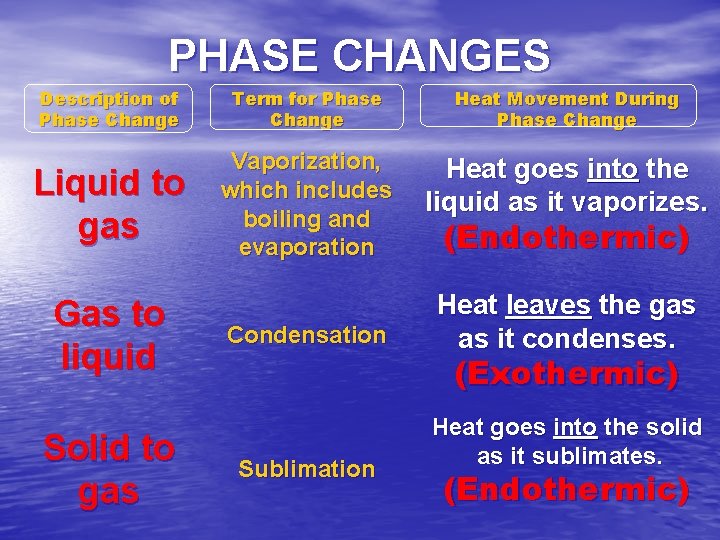
PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Liquid to gas Vaporization, which includes boiling and evaporation Heat goes into the liquid as it vaporizes. Gas to liquid Solid to gas Condensation (Endothermic) Heat leaves the gas as it condenses. (Exothermic) Sublimation Heat goes into the solid as it sublimates. (Endothermic)

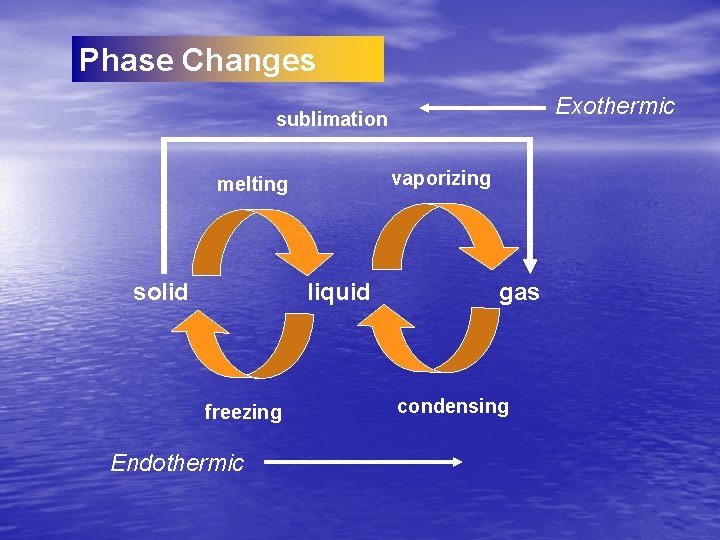
Phase Changes Exothermic sublimation vaporizing melting solid liquid freezing Endothermic gas condensing


Name _____________ Date/Period _________ Freezing Exothermic GAS or STEAM CONDENSATION 100° Melting Point EVAPORATION Temperature LIQUID FREEZING Boiling Point 0° 0° SOLID MELTING Endothermic Time / Energy

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

So…What is an “Ionized Gas”? ? An ionized atom is usually one that has lost one or more electrons! Plasma has ions and free electrons in fairly equal numbers, so generally has a neutral charge. BACK