Oesophagus Epithelium lined muscular tube from 6 th

Oesophagus • • • Epithelium lined muscular tube from 6 th cervical – 11 th thoracic vertebra Passes through 3 regions; neck, thorax and abdomen Upper and lower sphincters Three areas of narrowing; cricoid cartilage, left main bronchus and aortic arch, diaphragmatic hiatus Orderly passage of food {solids & liquids} from mouth to stomach. Diseases are subdivided into: Congenital; oesophageal atresia, tracheoesophageal fistula, vascular ring, webs, duplication Diverticuli; Zenker’s, midoesophageal, epiphrenic Inflammatory; acid & alkaline reflux, caustic ingestion, Barrett’s oesophagus, candidiasis, Crohn’s disease

Oesophagus • Benign tumours; Leiomyoma, fibrous polyp, lipoma, haemagioma, etc • Malignant tumours; Carcinoma, leiomyosarcoma, fibrosarcoma, melanoma • Motor abnormalities; Achalasia, primary spasm, scleroderma, etc. • Miscellaneous; Foreign bodies, cartilaginous spur, presbyesophagus, acquired oesophageal web(Plummer-Vinson Syndrome)

Oesophagus • Zenker’s diverticulum. – – • Pulsion diverticulum Compressible mass in the neck Associated with gurgling sound, dysphagia, regurgitation and aspiration Diverticulectomy Upper oesophageal webs (Plummer-Vinson Syndrome) – Fissured lips(cheilosis), dry skin, smooth tongue, flat brittle nails(Koilonychia), weight loss, dsyphagia, & Fe def. Anaemia. – Women – Diagnosis with Ba. Swallow & endoscopy – Replace Fe – Bouginage

Oesophagus • Midoesophageal Webs; – Congenital or acquired in origin ie. , oesophagitis in association with Barrett’s – Long history of reflux(heartburn) & dysphagia – Ba. Swallow shows stricture in mid oesophagus often with Hiatus hernia • Lower oesophageal Webs (Schatzki’s ring) – Common xray finding – Endoscopy reveals a white membrane with a concentric opening – Treated by dilatation, electrocautery through endoscope, or by balloon tamponade

Oesophagus • Achalasia – Thomas Willis in 1674 first described it. – Other names ascribed simple ectasia, and Cardiospasm – ‘ Motility disorder characterised by absence of oesophageal peristalsis and failure of lower oesophageal sphincter to relax on swallowing’ – Most common neuroanatomic change is a decrease or loss of myenteric ganglion cells. – Incidence of 0. 4 to 0. 6 per 100, 000 & prevalence 0 f 8 per 100, 000. – Described from infancy to 9 th decade, but majority present between 20 to 40 years – Paediatric cases account for 2% - 5% of all cases – Dyspahgia for solids mainly, with variable dysphagia for liquids – Regurgitation (60%-90%), chest pain (505 -75%), weight loss, recurrent aspiration. – Distal oesophageal diverticulum may develop

Oesophagus • • Diagnosis Endoscopic finding – Dilated, patulous esophageal body with extensive mucosal friability & ulcerations – LES appears puckered and fails to open with air insufflation, but is easily traversed with minimal pressure – Presence of a hiatal hernia (4 -14%) or epiphrenic diverticulum • Manometry – Gold standard – Absent peristaltis of distal smooth muscle – LES relaxation pressures are elevated >35 mm Hg • Radiographic findings – PFA; Mediatinal widening, air-fluid level (midesophagus), absence of gastric air bubble, abnormal pulmonary marking due to repeated aspiration

Oesophagus • • • Barium swallow; dilated esophageal body tapering to a ‘Bird’s beak’ at the level of LES Esophageal emptying scans; impaired propulsion and emptying Achalasia & carcinoma; squamous cell carcinoma in approx. 5% after 20 years of diagnosis. Present a decade earlier than general population ie 40 years. Treatment Drug therapy – Smooth muscle relaxants; nitrates, calcium channel blockers – Results inconsistent & disappointing overall • Oesophageal dilatation – Began 4 centuries ago, Fabricus ab Aquadendente (1537 -1675) pushed a foreign object into stomach

Oesophagus • • Dilatation with mercury filled dilators & polyethylene balloons(pneumatic) Complications; GIT bleeding, intramural haemorrhage, perforation Response to pneumatic dilatation 32%-98%, most studies 60%-80% Remission is not durable; 60% are symptom free for 1 year, with recurrence in 50% of these patients in 5 years • • • Surgery Heller's Myotomy. Open or laparoscopic Laparoscopic technique first described in 1991, little morbidity, shorter hospital stay, and earlier return to daily activities Overall mortality rate <2% Most troublesome complication after surgery is GORD, therefore myotomy is performed in conjunction with antireflux procedure. • •

Oesophagus • Botulinum toxin – – • Potent inhibitor of acetylcholine release from presynaptic nerve terminals Short term efficacy Advantages are noninvasive, ease of administration & minimal side effects Disadvantages; need for repeated injections, lack of response in 1/3 rd of patients, diminution of response with repeat injections of Bo. Tx GORD & Hiatal Hernias – S&S; Typical, heartburn (pyrosis), regurgitation, dysphagia/odynophagia, waterbrash – Atypical; pulmonary aspiration, severe chest pain, pulmonary asthma(adult onset), chronic hoarseness, choking, difficulty initiating swallows, chronic cough

Oesophagus • Types of Hiatus hernias; – – – • • • Type 1 (Sliding) Type 1 p a postoperative hernia Type II (paraoesophageal) Type IIp a postoperative hernia Type III ( combined sliding & paraoesophageal hernia) Type IIIp a postoperative that has no sac Patient evaluation History Upper GIT series 24 hour p. H monitoring & manometry; Gold standard OGD

Oesophagus • Medical therapy – Main aims are eliminate symptoms, heal injured esophageal mucosa, and manage and/or prevent complications – Life style modifications eg, diet alterations, avoid eating 2 -3 hours before bedtime, elevation of head of the bed 6 -10 inches during sleep, sleep in in left lateral decubitus, wt. Loss, avoidance of drugs that inhibit LES pressure – PPI’s eg, omeprazole & lansoprazole – H 2 receptor antagonists eg, cimetidine, ranitidine, famotidine, nizatidine – Prokinetic agents Cisapride and Metoclopramide – Antacids – Sucralfate – Strictures Dilatation with bougies( mercury filled), wire guided dilators, hydrostatic balloons – Endoscopic procedures, endoscopic suturing device(Edno. Cinch), submucosal injection(polymethylmethacrylate, ethylene ethyl alcohol, radiofrequency energy delivery

Oesophagus • Surgical therapy – – – – Patient selection is crucial Preoperative evaluation Types of operations; Nissen’s fundoplication 1956 360 -degree wrap Toupet’s partial wrap 1963 Dor’s anterior fundoplication Mark IV operation by Skinner and Belsey Hill’s gastropexy Principles of fundoplication • Creation of fundoplication just proximal to gastroesophageal junction with fixation to esophagus • No tension and construction over a 60 F bougie inside esophagus • A total fundoplication should measure about 2 cms anteriorly • The wrapped portion of esophagus must lie below the diaphragm • The diaphragmatic hiatus must be snugged around the esophagus above the fundoplication

Oesophagus • • • Problems after surgery Dysphagia Misdiagnosed achalasia Recurrent reflux Abdominal bloating Chest pain Diarrhoea Reflux gastritis Severe fibrosis or total failure of esophageal body function

Barrett’s oesophagus • • Macroscopically seen on endoscopy as pink epithelium extending well above the squamocolumnar junction Histologically goblet cells 22. 6 per 100, 000 S&S heartburn, regurgitation, dysphagia Potential progression to carcinoma Medical treatment; PPI’s Surgical; low grade dysplasia antireflux procedure, argon plasma coagulation High grade dysplasia; thermal ablation, PDT, ultrasonic energy, oesophagectomy
Carcinoma of oesophagus • • Risk factors; alcohol, tobacco, vitamin and mineral deficiency, nitrosamines, food and water contaminants, strong family history, multiple cancers, especially head and neck cancers, corrosive esophagitis, Barrett’s esophagus, achalasia, Plummer-Vinson syndrome S&S; None, dysphagia, wt. Loss, chest pain, retrosternal pain, feeling of obstruction, etc. 5 year survival rate is 6%-9% Two histologic types; SCC & adenocarcinoma Assessment; Radiologic: plain and Barium studies, CT scan, MRI Endoscopy; rigid and flexible, endoscopic ultrasound Treatment; Oesophagectomy, 2 or 3 stage, transhiatal. Neoadjuvant chemotherapy/chemoradiation, downstage tumour, improved survival, higher pathologic response with combined chemoradiation
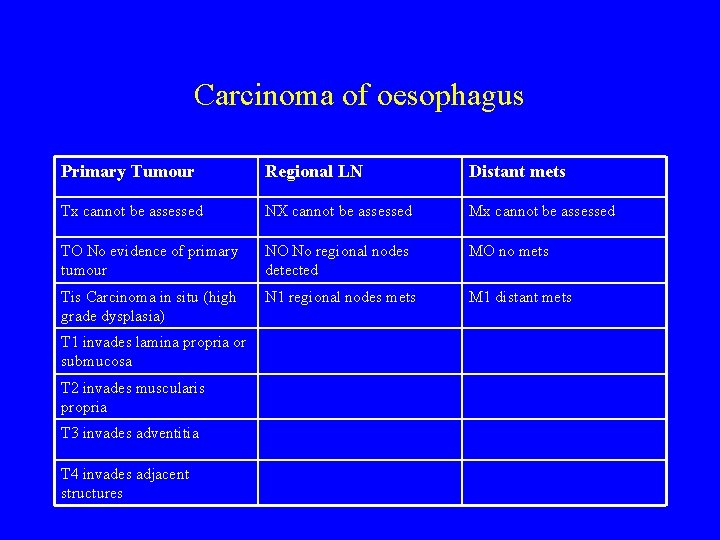
Carcinoma of oesophagus Primary Tumour Regional LN Distant mets Tx cannot be assessed NX cannot be assessed Mx cannot be assessed TO No evidence of primary tumour NO No regional nodes detected MO no mets Tis Carcinoma in situ (high grade dysplasia) N 1 regional nodes mets M 1 distant mets T 1 invades lamina propria or submucosa T 2 invades muscularis propria T 3 invades adventitia T 4 invades adjacent structures
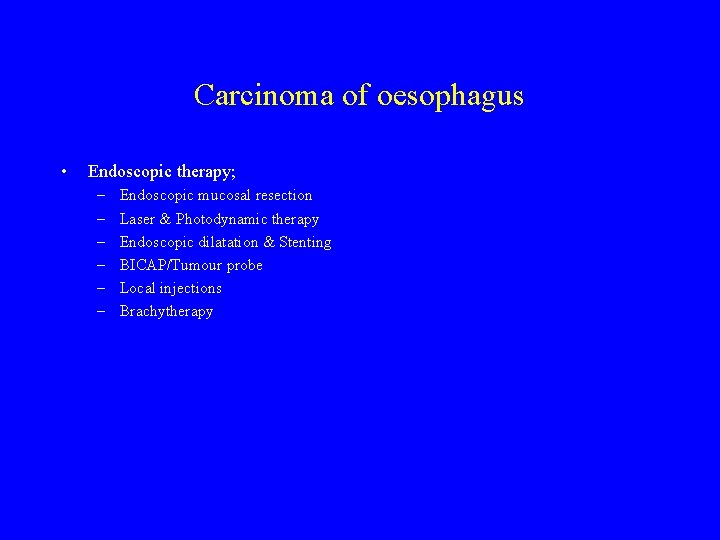
Carcinoma of oesophagus • Endoscopic therapy; – – – Endoscopic mucosal resection Laser & Photodynamic therapy Endoscopic dilatation & Stenting BICAP/Tumour probe Local injections Brachytherapy

Stomach • Benign Diseases – Chronic Gastric ulcer – Chronic Duodenal ulcer – Gastrinoma (Zollinger Ellison syndrome) • Tumours – Malignant ie. , Carcinoma of stomach • Miscellaneous gastric pathology – Gastric bezoar, Menetrier’s disease, Mallory-Weiss syndrome, gastric volvulus, etc

Stomach • • • Chronic Gastric ulcers Greater frequency in elderly Four types – Type I; lesser curve ulcers, usually antral, near antral-fundic border, occur in Patients with gastric stasis, poor emptying & low acid output – Type II; combination of type I & present or past DU. High prevalence of blood group O, high acid output – Type III; prepyloric in location, 1 -2 cms proximal to pylorus – Type IV; other areas of stomach, drug induced

Stomach • S&S and Diagnosis – Epigastric pain, relieved by eating/antacids – Sometimes worsening of pain on eating, esp, pyloric ulcers. – Ba. Studies, OGD & biopsy • Treatment – – – Antacids Surgical options Type I ulcers; antrectomy and Billroth anastomosis, vagotomy & pyloroplasty Type II & III; similar to DU Type IV; stress ulceration, curling’s & cushing ulcers, if bleeding point easily identified, truncal vagotomy, pyloroplasty & oversewing of bleeding ulcer. Otherwise gastrectomy in seriously ill Patients. For less seriously ill patients, vagotomy & distal gatrectomy

Stomach • • • Chronic DU Younger age group Men more often than women Epigastric or RUQ pain Ba. Series and endoscopy Treatment – Antacids, H 2 receptors blockers, PPI’s, H pylori treatment
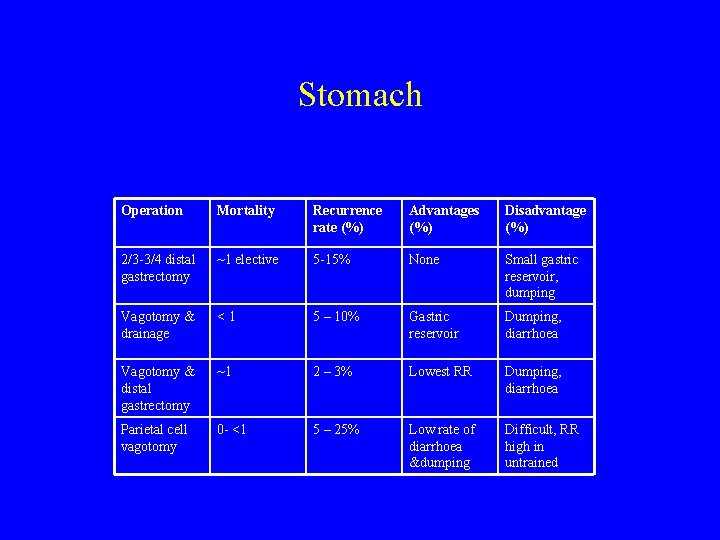
Stomach Operation Mortality Recurrence rate (%) Advantages (%) Disadvantage (%) 2/3 -3/4 distal gastrectomy ~1 elective 5 -15% None Small gastric reservoir, dumping Vagotomy & drainage <1 5 – 10% Gastric reservoir Dumping, diarrhoea Vagotomy & distal gastrectomy ~1 2 – 3% Lowest RR Dumping, diarrhoea Parietal cell vagotomy 0 - <1 5 – 25% Low rate of diarrhoea &dumping Difficult, RR high in untrained

Stomach • Complications of peptic ulcers – Perforation – Haemorrhage – Pyloric obstruction • Complication of gastric surgery – – Mortality Recurrent ulcers Gastrojujenocolic fistula Postgastrectomy syndromes • Dumping • Diarrhoea, afferent loop syndrome, efferent loop syndrome, alkaline gastritis, weight loss, anaemia, bone disease, TB, gastric cancer

Gastric carcinoma • Risk factors – – – • Polycyclic hydrocarbons, dimethylnitrosamines Miners, metal workers, rubber workers Helicobacter pylori Gastric polyps Pernicious anaemia, atrophic gastritis, gastric ulcer, Pathology – 95% adenocarcinomas – Two types; intestinal & diffuse

Stomach • S&S – – • • • Vague epigastric discomfort Wt. Loss, anaemia, anorexia, vomiting Virchow’s node Sister Mary Joseph nodule, ascites, jaundice, liver or pelvic mass Preoperative evaluation Endoscopy CT scanning Endoscopic ultrasound CEA levels Laparoscopy
Carcinoma of Stomach • • • Surgical treatment Overall 5 year survival rate 10 – 21% in western series Japanese series 50% • • • Adjuvant therapy VASAG Thiotepa & fluxuridine no survival benefit GITSG 5 -FU and methyl CCNU • • Neoadjuvant therapy Response rates vary from 21 – 31% clinical response rate to complete response rate of 0 -15%
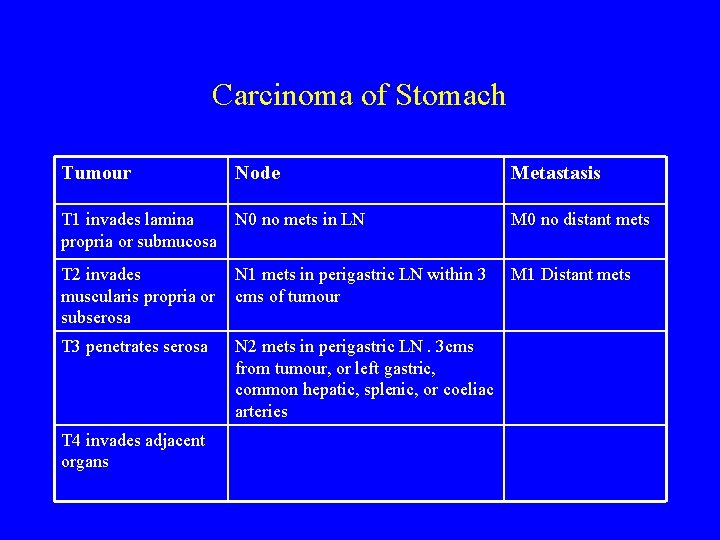
Carcinoma of Stomach Tumour Node Metastasis T 1 invades lamina propria or submucosa N 0 no mets in LN M 0 no distant mets T 2 invades muscularis propria or subserosa N 1 mets in perigastric LN within 3 cms of tumour M 1 Distant mets T 3 penetrates serosa N 2 mets in perigastric LN. 3 cms from tumour, or left gastric, common hepatic, splenic, or coeliac arteries T 4 invades adjacent organs
Carcinoma of Stomach • Surgical options – Proximal tumours – Total or proximal subtotal gastrectomy with Roux –N- reconstruction – Mid-body tumours – Total gastrectomy – Distal tumours – Distal subtotal gastrectomy with or without regional lymphadenectomy – Splenectomy
- Slides: 28