OE PREAWARD AND CLINICAL RESEARCH UPDATE 1 COMMITTEE







- Slides: 9

OE PRE-AWARD AND CLINICAL RESEARCH UPDATE 1 COMMITTEE ON RESEARCH MEETING DECEMBER 17, 2012 Susanne Hildebrand-Zanki - AVC, Research

Agenda 2 Progress to Date Evaluation Plans UCSF Pre-Award Priorities for FY 12 -13 Status on Clinical Research Infrastructure Workplan Questions

Progress as of December, 2012 3 4 teams went live in August 2012; all ten teams in the Research Management Services (RMS) structure are now in operation Reorganization of Contracts and Grants completed in November 2012 Sponsored Research Advisory Board is meeting monthly

4 Sponsored Research Advisory Board Provide input on service levels and service delivery Advise on new and/or anticipated customer needs and requirements Provide input on budget Ensure alignment with UCSF-wide goals

UCSF HR Priorities FY 12 -13 5 Customer Service and SLAs Development of Program Evaluation with Claire Brindis Comprehensive and coordinated OE assessment Performance Metrics Refinement of Satisfaction Survey RMS Process and Procedure Refinement Internal between C&G and RMS External between RMS and our customers Clear delineation of pre- and post-award responsibilities Enabling technologies (CACTAS and e. Proposal) Reporting

6 UCSF HR Priorities FY 12 -13 cont’d Contracts and Grants Process Refinements Clear award set up back log Develop and deploy SLAs Strengthen communication with PIs and departments

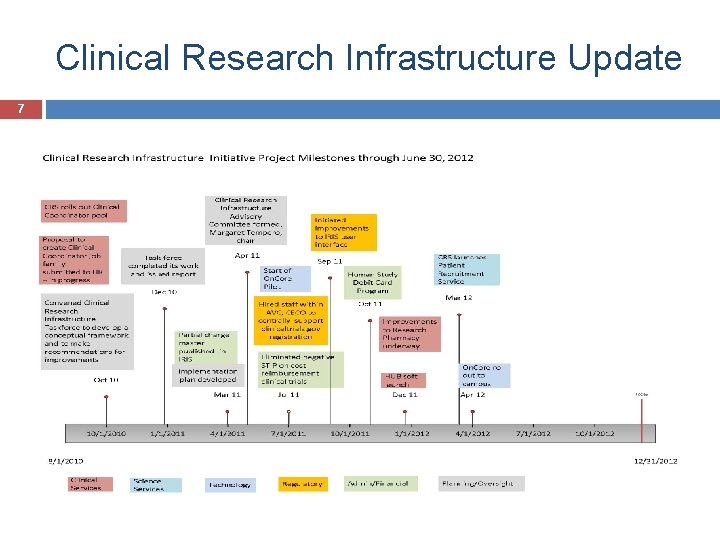
Clinical Research Infrastructure Update 7

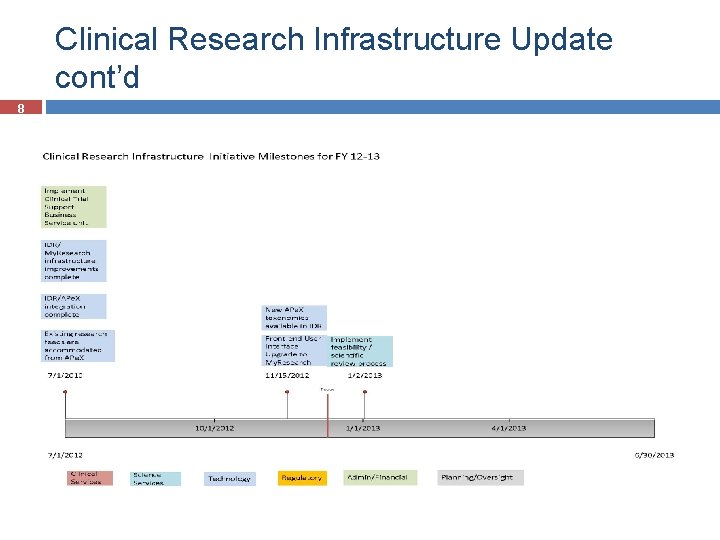
Clinical Research Infrastructure Update cont’d 8

9 Questions?